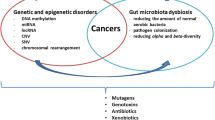
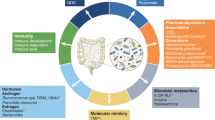
Abstract
A healthy cell can turn into a cancerous cell, which requires a complex mechanism, such as genetic and epigenetic modification. This leads to an increase in cell size and growth, differentiation, and uncontrolled proliferation and eventually becomes an immortal cell. These processes are mainly influenced by “reprogramming of cellular metabolism” and this sets up the cancer cells to evade the natural destruction process by lymphocytes (T and B), macrophages, and natural killer cells. Over the last decade, researchers have explored the role of microbiota in regulating cancer immunometabolism, suggesting an unprecedented role in cancer progression and regression. In particular, some microbes act as a probiotic—a live microorganism that produces beneficial effects when administered, mainly Lactobacillus and Bifidobacterium spp. A new addition to this list is Akkermansia muciniphila (A. muciniphila). In this review, we emphasize the importance of A. muciniphila and the personalized therapy approach in the crosstalk of immuno-oncology and metabolism.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100(1):57–70
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Guo C et al (2019) Immunometabolism: a new target for improving cancer immunotherapy. Adv Cancer Res 143:195–253
Cantor JR, Sabatini DM (2012) Cancer cell metabolism: one hallmark, many faces. Cancer Discov 2(10):881–898
Romero-Garcia S et al (2011) Tumor cell metabolism: an integral view. Cancer Biol Ther 12(11):939–948
O’Neill LA, Kishton RJ, Rathmell J (2016) A guide to immunometabolism for immunologists. Nat Rev Immunol 16(9):553–565
O’Neill LA, Pearce EJ (2016) Immunometabolism governs dendritic cell and macrophage function. J Exp Med 213(1):15–23
Renner K et al (2015) Metabolic plasticity of human T cells: preserved cytokine production under glucose deprivation or mitochondrial restriction, but 2-deoxy-glucose affects effector functions. Eur J Immunol 45(9):2504–2516
Warburg O (1956) On the origin of cancer cells. Science 123(3191):309–314
Busk M et al (2011) Inhibition of tumor lactate oxidation: consequences for the tumor microenvironment. Radiother Oncol 99(3):404–411
Voelxen NF et al (2018) Comparative metabolic analysis in head and neck cancer and the normal gingiva. Clin Oral Investig 22(2):1033–1043
Almuhaideb A, Papathanasiou N, Bomanji J (2011) 18F-FDG PET/CT imaging in oncology. Ann Saudi Med 31(1):3–13
Chang CH et al (2015) Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162(6):1229–1241
Cascone T et al (2018) Increased tumor glycolysis characterizes immune resistance to adoptive T cell therapy. Cell Metab 27(5):977–987.e4
Szablewski L (2013) Expression of glucose transporters in cancers. Biochim Biophys Acta 1835(2):164–169
Macheda ML, Rogers S, Best JD (2005) Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol 202(3):654–662
Medina RA, Owen GI (2002) Glucose transporters: expression, regulation and cancer. Biol Res 35(1):9–26
Furuta E et al (2010) Metabolic genes in cancer: their roles in tumor progression and clinical implications. Biochim Biophys Acta 1805(2):141–152
Mates JM et al (2009) Glutamine homeostasis and mitochondrial dynamics. Int J Biochem Cell Biol 41(10):2051–2061
Vettore L, Westbrook RL, Tennant DA (2020) New aspects of amino acid metabolism in cancer. Br J Cancer 122(2):150–156
Eagle H (1955) Nutrition needs of mammalian cells in tissue culture. Science 122(3168):501–514
Wise DR, Thompson CB (2010) Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci 35(8):427–433
Bolzoni M et al (2016) Dependence on glutamine uptake and glutamine addiction characterize myeloma cells: a new attractive target. Blood 128(5):667–679
Furusawa A et al (2018) Ovarian cancer therapeutic potential of glutamine depletion based on GS expression. Carcinogenesis 39(6):758–766
Chiu M et al (2018) Oligodendroglioma cells lack glutamine synthetase and are auxotrophic for glutamine, but do not depend on glutamine anaplerosis for growth. Int J Mol Sci 19(4)
Wang JJ, Lei KF, Han F (2018) Tumor microenvironment: recent advances in various cancer treatments. Eur Rev Med Pharmacol Sci 22(12):3855–3864
Helmy KY et al (2013) Cancer immunotherapy: accomplishments to date and future promise. Ther Deliv 4(10):1307–1320
Korgaonkar N, Yadav KS (2019) Understanding the biology and advent of physics of cancer with perspicacity in current treatment therapy. Life Sci 239:117060
Xing M (2013) Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer 13(3):184–199
Reifenberger G et al (2017) Advances in the molecular genetics of gliomas—implications for classification and therapy. Nat Rev Clin Oncol 14(7):434–452
Lemjabbar-Alaoui H et al (2015) Lung cancer: biology and treatment options. Biochim Biophys Acta 1856(2):189–210
Ahdoot M et al (2020) MRI-targeted, systematic, and combined biopsy for prostate cancer diagnosis. N Engl J Med 382(10):917–928
Sheill G et al (2020) Preoperative exercise to improve fitness in patients undergoing complex surgery for cancer of the lung or oesophagus (PRE-HIIT): protocol for a randomized controlled trial. BMC Cancer 20(1):321
Bregni G et al (2020) Adjuvant chemotherapy for rectal cancer: current evidence and recommendations for clinical practice. Cancer Treat Rev 83:101948
Turgeon GA et al (2019) Radiotherapy and immunotherapy: a synergistic effect in cancer care. Med J Aust 210(1):47–53
Marcello M et al (2020) Relationships between rectal and perirectal doses and rectal bleeding or tenesmus in pooled voxel-based analysis of 3 randomised phase III trials. Radiother Oncol 150:281–292
Kauff DW et al (2020) Fecal incontinence after total mesorectal excision for rectal cancer-impact of potential risk factors and pelvic intraoperative neuromonitoring. World J Surg Oncol 18(1):12
Lohse I, Brothers SP (2020) Pathogenesis and treatment of pancreatic cancer related pain. Anticancer Res 40(4):1789–1796
Franzosa EA et al (2019) Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat Microbiol 4(2):293–305
Hu S et al (2021) Whole exome sequencing analyses reveal gene-microbiota interactions in the context of IBD. Gut 70(2):285–296
Regnier M et al (2021) Gut microbiome, endocrine control of gut barrier function and metabolic diseases. J Endocrinol 248(2):R67–R82
Tripathi A et al (2018) The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol 15(7):397–411
Jobin C (2018) Precision medicine using microbiota. Science 359(6371):32–34
Alexander JL et al (2017) Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat Rev Gastroenterol Hepatol 14(6):356–365
Gopalakrishnan V et al (2018) The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell 33(4):570–580
Elkrief A et al (2019) The intimate relationship between gut microbiota and cancer immunotherapy. Gut Microbes 10(3):424–428
Appelbe OK et al (2017) Radiation-enhanced delivery of systemically administered amphiphilic-CpG oligodeoxynucleotide. J Control Release 266:248–255
Panebianco C, Andriulli A, Pazienza V (2018) Pharmacomicrobiomics: exploiting the drug-microbiota interactions in anticancer therapies. Microbiome 6(1):92
Iida N et al (2013) Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 342(6161):967–970
Li B, Chan HL, Chen P (2019) Immune checkpoint inhibitors: basics and challenges. Curr Med Chem 26(17):3009–3025
Hodi FS et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363(8):711–723
Rittmeyer A et al (2017) Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389(10066):255–265
Gopalakrishnan V et al (2018) Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359(6371):97–103
Temraz S et al (2019) Gut microbiome: a promising biomarker for immunotherapy in colorectal Cancer. Int J Mol Sci 20(17)
Shuwen H et al (2020) Effects of postoperative adjuvant chemotherapy and palliative chemotherapy on the gut microbiome in colorectal cancer. Microb Pathog 149:104343
Zhang M et al (2020) The gut microbiome can be used to predict the gastrointestinal response and efficacy of lung cancer patients undergoing chemotherapy. Ann Palliat Med 9(6):4211–4227
Mini E et al (2006) Cellular pharmacology of gemcitabine. Ann Oncol 17(Suppl 5):v7–v12
Vande Voorde J, Balzarini J, Liekens S (2014) Mycoplasmas and cancer: focus on nucleoside metabolism. EXCLI J 13:300–322
Geller LT et al (2017) Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 357(6356):1156–1160
Ahlmann M, Hempel G (2016) The effect of cyclophosphamide on the immune system: implications for clinical cancer therapy. Cancer Chemother Pharmacol 78(4):661–671
Viaud S et al (2013) The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 342(6161):971–976
Daillere R et al (2016) Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity 45(4):931–943
Jang SE et al (2013) Lactobacillus casei HY7213 ameliorates cyclophosphamide-induced immunosuppression in mice by activating NK, cytotoxic T cells and macrophages. Immunopharmacol Immunotoxicol 35(3):396–402
Jang SE et al (2013) Lactobacillus plantarum HY7712 ameliorates cyclophosphamide-induced immunosuppression in mice. J Microbiol Biotechnol 23(3):414–421
Maroof H et al (2012) Lactobacillus acidophilus could modulate the immune response against breast cancer in murine model. J Clin Immunol 32(6):1353–1359
Petty RD, Cassidy J (2004) Novel fluoropyrimidines: improving the efficacy and tolerability of cytotoxic therapy. Curr Cancer Drug Targets 4(2):191–204
Mindt S et al (2019) Therapeutic drug monitoring (TDM) of 5-fluorouracil (5-FU): new preanalytic aspects. Clin Chem Lab Med 57(7):1012–1016
Garcia-Gonzalez AP et al (2017) Bacterial metabolism affects the C. elegans response to cancer chemotherapeutics. Cell 169(3):431–441.e8
Hashemi Goradel N et al (2019) Fusobacterium nucleatum and colorectal cancer: a mechanistic overview. J Cell Physiol 234(3):2337–2344
Chen Y et al (2020) Fusobacterium nucleatum promotes metastasis in colorectal cancer by activating autophagy signaling via the upregulation of CARD3 expression. Theranostics 10(1):323–339
Luo K et al (2019) Fusobacterium nucleatum, the communication with colorectal cancer. Biomed Pharmacother 116:108988
Li M, Chen WD, Wang YD (2020) The roles of the gut microbiota-miRNA interaction in the host pathophysiology. Mol Med 26(1):101
Neradil J, Pavlasova G, Veselska R (2012) New mechanisms for an old drug; DHFR- and non-DHFR-mediated effects of methotrexate in cancer cells. Klin Onkol 25 Suppl 2:2S87–2S92
Zhou B et al (2018) Induction and amelioration of methotrexate-induced gastrointestinal toxicity are related to immune response and gut microbiota. EBioMedicine 33:122–133
Frank M et al (2015) TLR signaling modulates side effects of anticancer therapy in the small intestine. J Immunol 194(4):1983–1995
Nishida A et al (2018) Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol 11(1):1–10
Hills RD Jr et al (2019) Gut microbiome: profound implications for diet and disease. Nutrients 11(7):1613
Di Marzo V, Silvestri C (2019) Lifestyle and metabolic syndrome: contribution of the Endocannabinoidome. Nutrients 11(8):1956
Reid G (2016) Probiotics: definition, scope and mechanisms of action. Best Pract Res Clin Gastroenterol 30(1):17–25
Goldin BR, Gorbach SL (1980) Effect of lactobacillus acidophilus dietary supplements on 1,2-dimethylhydrazine dihydrochloride-induced intestinal cancer in rats. J Natl Cancer Inst 64(2):263–265
Slizewska K, Markowiak-Kopec P, Slizewska W (2020) The role of probiotics in cancer prevention. Cancers (Basel) 13(1):20
Daisley BA et al (2020) Abiraterone acetate preferentially enriches for the gut commensal Akkermansia muciniphila in castrate-resistant prostate cancer patients. Nat Commun 11(1):4822
Chen Z et al (2020) Akkermansia muciniphila enhances the antitumor effect of cisplatin in Lewis lung cancer mice. J Immunol Res 2020:2969287
Maldonado Galdeano C et al (2019) Beneficial effects of probiotic consumption on the immune system. Ann Nutr Metab 74(2):115–124
Cerdo T et al (2019) The role of probiotics and prebiotics in the prevention and treatment of obesity. Nutrients 11(3):635
Naghmouchi K et al (2020) Lactobacillus fermentum: a bacterial species with potential for food preservation and biomedical applications. Crit Rev Food Sci Nutr 60(20):3387–3399
Arena MP et al (2018) Immunobiosis and probiosis: antimicrobial activity of lactic acid bacteria with a focus on their antiviral and antifungal properties. Appl Microbiol Biotechnol 102(23):9949–9958
Degnan FH (2008) The US Food and Drug Administration and probiotics: regulatory categorization. Clin Infect Dis 46(Suppl 2):S133–S136; discussion S144-51
Gorska A et al (2019) Probiotic bacteria: a promising tool in cancer prevention and therapy. Curr Microbiol 76(8):939–949
Wada M et al (2010) Effects of the enteral administration of Bifidobacterium breve on patients undergoing chemotherapy for pediatric malignancies. Support Care Cancer 18(6):751–759
Asadollahi P et al (2020) Anti-cancer effects of Bifidobacterium species in colon cancer cells and a mouse model of carcinogenesis. PLoS One 15(5):e0232930
Sanctis VDE et al (2019) Lactobacillus brevis CD2 for prevention of Oral mucositis in patients with head and neck tumors: a multicentric randomized study. Anticancer Res 39(4):1935–1942
Gui QF et al (2015) Well-balanced commensal microbiota contributes to anti-cancer response in a lung cancer mouse model. Genet Mol Res 14(2):5642–5651
Routy B et al (2018) Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359(6371):91–97
Naito Y, Uchiyama K, Takagi T (2018) A next-generation beneficial microbe: Akkermansia muciniphila. J Clin Biochem Nutr 63(1):33–35
Wang L et al (2020) A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8(+) T cells in mice. Gut 69(11):1988–1997
Davani-Davari D et al (2019) Prebiotics: definition, types, sources, mechanisms, and clinical applications. Foods 8(3):92
Kumar V et al (2012) Dietary roles of non-starch polysaccharides in human nutrition: a review. Crit Rev Food Sci Nutr 52(10):899–935
Schroeder BO et al (2018) Bifidobacteria or fiber protects against diet-induced microbiota-mediated colonic mucus deterioration. Cell Host Microbe 23(1):27–40.e7
Sebastian C, Mostoslavsky R (2014) Untangling the fiber yarn: butyrate feeds Warburg to suppress colorectal cancer. Cancer Discov 4(12):1368–1370
Sasidharan BK et al (2019) A phase 2 randomized controlled trial of oral resistant starch supplements in the prevention of acute radiation proctitis in patients treated for cervical cancer. J Cancer Res Ther 15(6):1383–1391
Bruno-Barcena JM, Azcarate-Peril MA (2015) Galacto-oligosaccharides and colorectal cancer: feeding our intestinal probiome. J Funct Foods 12:92–108
Watson H et al (2018) A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut 67(11):1974–1983
Garcia-So J et al (2019) Omega-3 fatty acids suppress Fusobacterium nucleatum-induced placental inflammation originating from maternal endothelial cells. JCI Insight 4(3):e125436
Segura Munoz RR et al (2020) Stearidonic-enriched soybean oil modulates obesity, glucose metabolism, and fatty acid profiles independently of Akkermansia muciniphila. Mol Nutr Food Res 64(17):e2000162
Wegh CAM et al (2019) Postbiotics and their potential applications in early life nutrition and beyond. Int J Mol Sci 20(19):4673
Rad AH et al (2021) Molecular mechanisms of postbiotics in colorectal cancer prevention and treatment. Crit Rev Food Sci Nutr 61(11):1787–1803
Whitford EJ et al (2009) Effects of Streptococcus thermophilus TH-4 on intestinal mucositis induced by the chemotherapeutic agent, 5-fluorouracil (5-FU). Cancer Biol Ther 8(6):505–511
Prisciandaro LD et al (2011) Probiotic factors partially improve parameters of 5-fluorouracil-induced intestinal mucositis in rats. Cancer Biol Ther 11(7):671–677
An J, Ha EM (2016) Combination therapy of Lactobacillus plantarum supernatant and 5-Fluouracil increases chemosensitivity in colorectal cancer cells. J Microbiol Biotechnol 26(8):1490–1503
Zhao Y et al (2021) Dual gate-controlled therapeutics for overcoming bacterium-induced drug resistance and potentiating cancer immunotherapy. Angew Chem Int Ed Engl 60(25):14013–14021
Flieger D et al (2007) Phase II clinical trial for prevention of delayed diarrhea with cholestyramine/levofloxacin in the second-line treatment with irinotecan biweekly in patients with metastatic colorectal carcinoma. Oncology 72(1–2):10–16
Vetizou M et al (2015) Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350(6264):1079–1084
Bobay LM, Ochman H (2018) Biological species in the viral world. Proc Natl Acad Sci U S A 115(23):6040–6045
Fluckiger A et al (2020) Cross-reactivity between tumor MHC class I-restricted antigens and an enterococcal bacteriophage. Science 369(6506):936–942
Derrien M et al (2004) Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol 54(Pt 5):1469–1476
Ouwerkerk JP et al (2016) Akkermansia glycaniphila sp. Nov., an anaerobic mucin-degrading bacterium isolated from reticulated python faeces. Int J Syst Evol Microbiol 66(11):4614–4620
Ley RE et al (2006) Microbial ecology: human gut microbes associated with obesity. Nature 444(7122):1022–1023
Salzman NH et al (2002) Analysis of 16S libraries of mouse gastrointestinal microflora reveals a large new group of mouse intestinal bacteria. Microbiology (Reading) 148(Pt 11):3651–3660
Sonoyama K et al (2009) Response of gut microbiota to fasting and hibernation in Syrian hamsters. Appl Environ Microbiol 75(20):6451–6456
Zhang T et al (2019) Akkermansia muciniphila is a promising probiotic. Microb Biotechnol 12(6):1109–1125
Derrien M, Belzer C, de Vos WM (2017) Akkermansia muciniphila and its role in regulating host functions. Microb Pathog 106:171–181
Brahe LK et al (2015) Specific gut microbiota features and metabolic markers in postmenopausal women with obesity. Nutr Diabetes 5:e159
Collado MC et al (2010) Effect of mother’s weight on infant’s microbiota acquisition, composition, and activity during early infancy: a prospective follow-up study initiated in early pregnancy. Am J Clin Nutr 92(5):1023–1030
Hanninen A et al (2018) Akkermansia muciniphila induces gut microbiota remodelling and controls islet autoimmunity in NOD mice. Gut 67(8):1445–1453
Plovier H et al (2017) A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med 23(1):107–113
Li J et al (2016) Akkermansia Muciniphila protects against atherosclerosis by preventing metabolic Endotoxemia-induced inflammation in Apoe−/− mice. Circulation 133(24):2434–2446
van der Lugt B et al (2019) Akkermansia muciniphila ameliorates the age-related decline in colonic mucus thickness and attenuates immune activation in accelerated aging Ercc1 (−/Delta7) mice. Immun Ageing 16:6
Olivier-Van Stichelen S, Rother KI, Hanover JA (2019) Maternal exposure to non-nutritive sweeteners impacts Progeny’s metabolism and microbiome. Front Microbiol 10:1360
Zhao S et al (2017) Akkermansia muciniphila improves metabolic profiles by reducing inflammation in chow diet-fed mice. J Mol Endocrinol 58(1):1–14
Grander C et al (2018) Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut 67(5):891–901
Kim S et al (2020) Akkermansia muciniphila prevents fatty liver disease, decreases serum triglycerides, and maintains gut homeostasis. Appl Environ Microbiol 86(7)
Ottman N et al (2017) Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS One 12(3):e0173004
Bedarf JR et al (2017) Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naive Parkinson’s disease patients. Genome Med 9(1):39
de la Cuesta-Zuluaga J et al (2017) Metformin is associated with higher relative abundance of mucin-degrading Akkermansia muciniphila and several short-chain fatty acid-producing microbiota in the gut. Diabetes Care 40(1):54–62
Panebianco C et al (2018) Influence of gemcitabine chemotherapy on the microbiota of pancreatic cancer xenografted mice. Cancer Chemother Pharmacol 81(4):773–782
Ramakrishna C et al (2019) Dominant role of the gut microbiota in chemotherapy induced neuropathic pain. Sci Rep 9(1):20324
Su H et al (2020) Andrographolide exerts antihyperglycemic effect through strengthening intestinal barrier function and increasing microbial composition of Akkermansia muciniphila. Oxid Med Cell Longev 2020:6538930
Wang L et al (2019) Puerarin prevents high-fat diet-induced obesity by enriching Akkermansia muciniphila in the gut microbiota of mice. PLoS One 14(6):e0218490
Fujisaka S et al (2020) Bofutsushosan improves gut barrier function with a bloom of Akkermansia muciniphila and improves glucose metabolism in mice with diet-induced obesity. Sci Rep 10(1):5544
Chen ML et al (2016) Resveratrol attenuates trimethylamine-N-oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. MBio 7(2):e02210–e02215
Shin NR et al (2014) An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 63(5):727–735
Everard A et al (2013) Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A 110(22):9066–9071
Ganesh BP et al (2013) Commensal Akkermansia muciniphila exacerbates gut inflammation in Salmonella typhimurium-infected gnotobiotic mice. PLoS One 8(9):e74963
Farhana L et al (2018) Gut microbiome profiling and colorectal cancer in African Americans and Caucasian Americans. World J Gastrointest Pathophysiol 9(2):47–58
Lapidot Y et al (2020) Alterations in the gut microbiome in the progression of cirrhosis to hepatocellular carcinoma. mSystems 5(3):e00153–e00120
Snider EJ et al (2019) Alterations to the esophageal microbiome associated with progression from Barrett’s esophagus to esophageal adenocarcinoma. Cancer Epidemiol Biomark Prev 28(10):1687–1693
Jiang H et al (2019) Intestinal flora disruption and novel biomarkers associated with nasopharyngeal carcinoma. Front Oncol 9:1346
Fruge AD et al (2020) Fecal Akkermansia muciniphila is associated with body composition and microbiota diversity in overweight and obese women with breast cancer participating in a presurgical weight loss trial. J Acad Nutr Diet 120(4):650–659
Howe C et al (2018) Differential expression of tumor-associated genes and altered gut microbiome with decreased Akkermansia muciniphila confer a tumor-preventive microenvironment in intestinal epithelial Pten-deficient mice. Biochim Biophys Acta Mol Basis Dis 1864(12):3746–3758
Dingemanse C et al (2015) Akkermansia muciniphila and Helicobacter typhlonius modulate intestinal tumor development in mice. Carcinogenesis 36(11):1388–1396
Weir TL et al (2013) Stool microbiome and metabolome differences between colorectal cancer patients and healthy adults. PLoS One 8(8):e70803
Kang CS et al (2013) Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLoS One 8(10):e76520
Meng X et al (2020) Akkermansia muciniphila aspartic protease Amuc_1434* inhibits human colorectal cancer LS174T cell viability via TRAIL-mediated apoptosis pathway. Int J Mol Sci 21(9):3385
Salgia NJ et al (2020) Stool microbiome profiling of patients with metastatic renal cell carcinoma receiving anti-PD-1 immune checkpoint inhibitors. Eur Urol 78(4):498–502
Huang K et al (2015) Biochemical characterisation of the neuraminidase pool of the human gut symbiont Akkermansia muciniphila. Carbohydr Res 415:60–65
Ottman N et al (2017) Genome-scale model and omics analysis of metabolic capacities of Akkermansia muciniphila reveal a preferential mucin-degrading lifestyle. Appl Environ Microbiol 83(18):e01014–e01017
Santoni M et al (2018) Re: gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Eur Urol 74(4):521–522
Hinnebusch BF et al (2002) The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation. J Nutr 132(5):1012–1017
Park J et al (2015) Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol 8(1):80–93
Wang Y et al (2021) Sini decoction ameliorates colorectal cancer and modulates the composition of gut microbiota in mice. Front Pharmacol 12:609992
Dizman N et al (2021) Randomized trial assessing impact of probiotic supplementation on gut microbiome and clinical outcome from targeted therapy in metastatic renal cell carcinoma. Cancer Med 10(1):79–86
Teng L et al (2020) HYR-2 plays an anti-lung cancer role by regulating PD-L1 and Akkermansia muciniphila. Pharmacol Res 160:105086
Xu X et al (2020) Gut microbiome influences the efficacy of PD-1 antibody immunotherapy on MSS-type colorectal cancer via metabolic pathway. Front Microbiol 11:814
Zhou X et al (2020) Effect and mechanism of vitamin D on the development of colorectal cancer based on intestinal flora disorder. J Gastroenterol Hepatol 35(6):1023–1031
Sfanos KS et al (2018) Compositional differences in gastrointestinal microbiota in prostate cancer patients treated with androgen axis-targeted therapies. Prostate Cancer Prostatic Dis 21(4):539–548
Jones GR, Molloy MP (2021) Metformin, microbiome and protection against colorectal cancer. Dig Dis Sci 66(5):1409–1414
Lim SH, Dutta D, Moore J (2019) Rifaximin for sickle cell disease. Am J Hematol 94(12):E325–E328
Grajeda-Iglesias C et al (2021) Oral administration of Akkermansia muciniphila elevates systemic antiaging and anticancer metabolites. Aging (Albany NY) 13(5):6375–6405
Shi L et al (2020) Combining IL-2-based immunotherapy with commensal probiotics produces enhanced antitumor immune response and tumor clearance. J Immunother Cancer 8(2):e000973
Liu MN et al (2020) [Effects of Akkermansia muciniphila on the proliferation, apoptosis and insulin secretion of rat islet cell tumor cells]. Sichuan Da Xue Xue Bao Yi Xue Ban 51(1):13–17
Zheng Y et al (2019) Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J Immunother Cancer 7(1):193
Greer RL et al (2016) Akkermansia muciniphila mediates negative effects of IFNgamma on glucose metabolism. Nat Commun 7:13329
Peng M et al (2016) Aerobic glycolysis promotes T helper 1 cell differentiation through an epigenetic mechanism. Science 354(6311):481–484
Dighe AS et al (1994) Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity 1(6):447–456
Jorgovanovic D et al (2020) Roles of IFN-gamma in tumor progression and regression: a review. Biomark Res 8:49
Hanada T et al (2006) IFNgamma-dependent, spontaneous development of colorectal carcinomas in SOCS1-deficient mice. J Exp Med 203(6):1391–1397
Zou Q et al (2015) T cell intrinsic USP15 deficiency promotes excessive IFN-gamma production and an immunosuppressive tumor microenvironment in MCA-induced Fibrosarcoma. Cell Rep 13(11):2470–2479
Slotkin W, Nishikura K (2013) Adenosine-to-inosine RNA editing and human disease. Genome Med 5(11):105
Mager LF et al (2020) Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 369(6510):1481–1489
Wang T et al (2020) Inosine is an alternative carbon source for CD8(+)-T-cell function under glucose restriction. Nat Metab 2(7):635–647
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Lakshmanan, A.P., Murugesan, S., Bangarusamy, D.K. (2022). Crosstalk of Immuno-Oncology and Metabolism: Influence of Akkermansia muciniphila and Personalized Therapy Approach. In: Macha, M.A., Bhat, A.A., Wani, N.A. (eds) Immuno-Oncology Crosstalk and Metabolism. Springer, Singapore. https://doi.org/10.1007/978-981-16-6226-3_4
Download citation
DOI: https://doi.org/10.1007/978-981-16-6226-3_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-6225-6
Online ISBN: 978-981-16-6226-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)