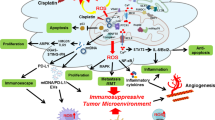
Abstract
Oxidative stress is one of defining features of the tumor microenvironment, which is closely related to the interactions between tumor cells and stromal cells. It affects tumor progression in many aspects. In the tumor microenvironment, primary immune cells have different functions, which together make up the immune defense line of the tumor. This review focuses on the relationship between oxidative stress and tumor-related immune system, specifically the effects and mechanisms of oxidative stress on different cell processes of immune cells in the tumor microenvironment. Then, we discuss the main overall effect of oxidative stress, immunosuppression, and its inspiration for tumor immunotherapy, which provides a theoretical basis for the feasibility of oxidative stress as a new target of tumor immunotherapy.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Fiaschi T, Chiarugi P. Oxidative stress, tumor microenvironment, and metabolic reprogramming: a diabolic liaison. Int J Cell Biol. 2012;2012:762825. https://doi.org/10.1155/2012/762825.
Bhattacharyya S. Tumour, oxidative stress and host T cell response: cementing the dominance. Scand J Immunol. 2015;6:82.
Whiteside TL. Immune suppression in cancer: effects on immune cells, mechanisms and future therapeutic intervention. Semin Cancer Biol. 2006;16(1):3–15. https://doi.org/10.1016/j.semcancer.2005.07.008.
Cheng YT, Yang CC, Shyur LF. Phytomedicine-modulating oxidative stress and the tumor microenvironment for cancer therapy. Pharmacol Res. 2016;114:128–43. https://doi.org/10.1016/j.phrs.2016.10.022.
Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012;30:1–22. https://doi.org/10.1146/annurev-immunol-100311-102839.
Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. https://doi.org/10.1146/annurev.immunol.23.021704.115633.
Binnewies M, Mujal AM, Pollack JL, Combes AJ, Hardison EA, Barry KC, Tsui J, Ruhland MK, Kersten K, Abushawish MA, Spasic M, Giurintano JP, Chan V, Daud AI, Ha P, Ye CJ, Roberts EW, Krummel MF. Unleashing type-2 dendritic cells to drive protective antitumor CD4(+) T cell immunity. Cell. 2019;177(3):556–71. https://doi.org/10.1016/j.cell.2019.02.005.
Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, Barczak A, Rosenblum MD, Daud A, Barber DL, Amigorena S, Veer LJ, Sperling AI, Wolf DM, Krummel MF. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell. 2014;26(5):638–52. https://doi.org/10.1016/j.ccell.2014.09.007.
Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY, Duggan R, Wang Y, Barber GN, Fitzgerald KA, Alegre ML, Gajewski TF. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41(5):830–42. https://doi.org/10.1016/j.immuni.2014.10.017.
Dustin ML, Bromley SK, Kan Z, Peterson DA, Unanue ER. Antigen receptor engagement delivers a stop signal to migrating T lymphocytes. Proc Natl Acad Sci U S A. 1997;94(8):3909–13. https://doi.org/10.1073/pnas.94.8.3909.
Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15(5):751–61. https://doi.org/10.1016/s1074-7613(01)00234-5.
Boissonnas A, Fetler L, Zeelenberg IS, Hugues S, Amigorena S. In vivo imaging of cytotoxic T cell infiltration and elimination of a solid tumor. J Exp Med. 2007;204(2):345–56. https://doi.org/10.1084/jem.20061890.
Breart B, Lemaitre F, Celli S, Bousso P. Two-photon imaging of intratumoral CD8+ T cell cytotoxic activity during adoptive T cell therapy in mice. J Clin Invest. 2008;118(4):1390–7. https://doi.org/10.1172/JCI34388.
Feig C, Jones JO, Kraman M, Wells RJB, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL, Teichmann SA, Janowitz T, Jodrell DI, Tuveson DA, Fearon DT. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A. 2013;110(50):20212–7. https://doi.org/10.1073/pnas.1320318110.
Markiewicz MA, Wise EL, Buchwald ZS, Cheney EE, Hansen TH, Suri A, Cemerski S, Allen PM, Shaw AS. IL-12 enhances CTL synapse formation and induces self-reactivity. J Immunol. 2009;182(3):1351–61. https://doi.org/10.4049/jimmunol.182.3.1351.
Melssen M, Slingluff CL Jr. Vaccines targeting helper T cells for cancer immunotherapy. Curr Opin Immunol. 2017;47:85–92. https://doi.org/10.1016/j.coi.2017.07.004.
Kennedy R, Celis E. Multiple roles for CD4+ T cells in anti-tumor immune responses. Immunol Rev. 2008;222:129–44. https://doi.org/10.1111/j.1600-065X.2008.00616.x.
Tateyama M, Oyaizu N, McCloskey TW, Than S, Pahwa S. CD4 T lymphocytes are primed to express Fas ligand by CD4 cross-linking and to contribute to CD8 T-cell apoptosis via Fas/FasL death signaling pathway. Blood. 2000;96(1):195–202.
Kumar P, Bhattacharya P, Prabhakar BS. A comprehensive review on the role of co-signaling receptors and Treg homeostasis in autoimmunity and tumor immunity. J Autoimmun. 2018;95:77–99. https://doi.org/10.1016/j.jaut.2018.08.007.
Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6(4):295–307. https://doi.org/10.1038/nri1806.
Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21(4):589–601. https://doi.org/10.1016/j.immuni.2004.09.002.
Sarhan D, Hippen KL, Lemire A, Hying S, Luo X, Lenvik T, Curtsinger J, Davis Z, Zhang B, Cooley S, Cichocki F, Blazar BR, Miller JS. Adaptive NK cells resist regulatory T-cell suppression driven by IL37. Cancer Immunol Res. 2018;6(7):766–75. https://doi.org/10.1158/2326-6066.cir-17-0498.
Shevach EM, Thornton AM. tTregs, pTregs, and iTregs: similarities and differences. Immunol Rev. 2014;259(1):88–102. https://doi.org/10.1111/imr.12160.
Wei T, Zhong W, Li Q. Role of heterogeneous regulatory T cells in the tumor microenvironment. Pharmacol Res. 2020;153:104659. https://doi.org/10.1016/j.phrs.2020.104659.
Kamada T, Togashi Y, Tay C, Ha D, Sasaki A, Nakamura Y, Sato E, Fukuoka S, Tada Y, Tanaka A, Morikawa H, Kawazoe A, Kinoshita T, Shitara K, Sakaguchi S, Nishikawa H. PD-1(+) regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc Natl Acad Sci U S A. 2019;116(20):9999–10008. https://doi.org/10.1073/pnas.1822001116.
Tokunaga R, Naseem M, Lo JH, Battaglin F, Soni S, Puccini A, Berger MD, Zhang W, Baba H, Lenz HJ. B cell and B cell-related pathways for novel cancer treatments. Cancer Treat Rev. 2019;73:10–9. https://doi.org/10.1016/j.ctrv.2018.12.001.
Yuen GJ, Demissie E, Pillai S. B lymphocytes and cancer: a love-hate relationship. Trends Cancer. 2016;2(12):747–57. https://doi.org/10.1016/j.trecan.2016.10.010.
Zhang CY, Xin H, Zhang W, Yazaki PJ, Zhang ZF, Le K, Li WZ, Lee H, Kwak L, Forman S, Jove R, Yu H. CD5 binds to interleukin-6 and induces a feed-forward loop with the transcription factor STAT3 in B cells to promote cancer. Immunity. 2016;44(4):913–23. https://doi.org/10.1016/j.immuni.2016.04.003.
Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, Malchinkhuu E, Wersto RP, Biragyn A. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4(+) T cells to T-regulatory cells. Cancer Res. 2011;71(10):3505–15. https://doi.org/10.1158/0008-5472.CAN-10-4316.
Wang SS, Liu W, Ly D, Xu H, Qu LM, Zhang L. Tumor-infiltrating B cells: their role and application in anti-tumor immunity in lung cancer. Cell Mol Immunol. 2019;16(1):6–18. https://doi.org/10.1038/s41423-018-0027-x.
Sharonov GV, Serebrovskaya EO, Yuzhakova DV, Britanova OV, Chudakov DM. B cells, plasma cells and antibody repertoires in the tumour microenvironment. Nat Rev Immunol. 2020; https://doi.org/10.1038/s41577-019-0257-x.
Di Vito C, Mikulak J, Zaghi E, Pesce S, Marcenaro E, Mavilio D. NK cells to cure cancer. Semin Immunol. 2019;41:101272. https://doi.org/10.1016/j.smim.2019.03.004.
Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9(5):495–502. https://doi.org/10.1038/ni1581.
Close HJ, Wurdak H, Short SC, Melcher AA, Stead LF, Wilson EB, Cook GP. Natural killer cell recognition of glioblastoma. Immunology. 2014;143:138.
Giorda R, Rudert WA, Vavassori C, Chambers WH, Hiserodt JC, Trucco M. NKR-P1, a signal transduction molecule on natural killer cells. Science. 1990;249(4974):1298–300.
Zhu YT, Huang B, Shi J. Fas ligand and lytic granule differentially control cytotoxic dynamics of natural killer cell against cancer target. Oncotarget. 2016;7(30):47163–72. https://doi.org/10.18632/oncotarget.9980.
Fehniger TA, Cooper MA. Harnessing NK cell memory for cancer immunotherapy. Trends Immunol. 2016;37(12):877–88. https://doi.org/10.1016/j.it.2016.09.005.
Porta C, Riboldi E, Ippolito A, Sica A. Molecular and epigenetic basis of macrophage polarized activation. Semin Immunol. 2015;27(4):237–48. https://doi.org/10.1016/j.smim.2015.10.003.
Belgiovine C, D'Incalci M, Allavena P, Frapolli R. Tumor-associated macrophages and anti-tumor therapies: complex links. Cell Mol Life Sci. 2016;73(13):2411–24. https://doi.org/10.1007/s00018-016-2166-5.
Chittezhath M, Dhillon MK, Lim JY, Laoui D, Shalova IN, Teo YL, Chen J, Kamaraj R, Raman L, Lum J, Thamboo TP, Chiong E, Zolezzi F, Yang H, Van Ginderachter JA, Poidinger M, Wong AS, Biswas SK. Molecular profiling reveals a tumor-promoting phenotype of monocytes and macrophages in human cancer progression. Immunity. 2014;41(5):815–29. https://doi.org/10.1016/j.immuni.2014.09.014.
Dimitrios M. Naturally occurring regulatory T cells show reduced sensitivity toward oxidative stress-induced cell death. Blood. 2009;15:113.
King MR, Ismail AS, Davis LS, Karp DR. Oxidative stress promotes polarization of human T cell differentiation toward a T helper 2 phenotype. J Immunol. 2006;176(5):2765–72. https://doi.org/10.4049/jimmunol.176.5.2765.
Chen X, Song M, Zhang B, Zhang Y. Reactive oxygen species regulate T cell immune response in the tumor microenvironment. Oxidative Med Cell Longev. 2016;2016:1580967. https://doi.org/10.1155/2016/1580967.
Cemerski S, van Meerwijk JPM, Romagnoli P. Oxidative-stress-induced T lymphocyte hyporesponsiveness is caused by structural modification rather than proteasomal degradation of crucial TCR signaling molecules. Eur J Immunol. 2003;33(8):2178–85. https://doi.org/10.1002/eji.200323898.
Mak TW, Saunders ME. 14 - T cell activation. In: Mak TW, Saunders ME, editors. The immune response. Burlington: Academic; 2006. p. 373–401. https://doi.org/10.1016/B978-012088451-3.50016-8.
Cemerski S, Cantagrel A, van Meerwijk JPM, Romagnoli P. Reactive oxygen species differentially affect T cell receptor-signaling pathways. J Biol Chem. 2002;277(22):19585–93. https://doi.org/10.1074/jbc.M111451200.
Mizoguchi H. Alterations in signal transduction molecules in T lymphocytes from tumor-bearing mice. Science. 1992;5089:258.
Otsuji M. Oxidative stress by tumor-derived macrophages suppresses the expression of CD3 zeta chain of T-cell receptor complex and antigen-specific T-cell responses. Proc Natl Acad Sci U S A. 1996;23:93.
Sukumar M, Liu J, Mehta GU, Patel SJ, Roychoudhuri R, Crompton JG, Klebanoff CA, Ji Y, Li P, Yu Z, Whitehill GD, Clever D, Eil RL, Palmer DC, Mitra S, Rao M, Keyvanfar K, Schrump DS, Wang E, Marincola FM, Gattinoni L, Leonard WJ, Muranski P, Finkel T, Restifo NP. Mitochondrial membrane potential identifies cells with enhanced stemness for cellular therapy. Cell Metab. 2016;23(1):63–76. https://doi.org/10.1016/j.cmet.2015.11.002.
Olmos Y, Valle I, Borniquel S, Tierrez A, Soria E, Lamas S, Monsalve M. Mutual dependence of Foxo3a and PGC-1alpha in the induction of oxidative stress genes. J Biol Chem. 2009;284(21):14476–84. https://doi.org/10.1074/jbc.M807397200.
Scharping NE, Menk AV, Moreci RS, Whetstone RD, Dadey RE, Watkins SC, Ferris RL, Delgoffe GM. The tumor microenvironment represses T cell mitochondrial biogenesis to drive intratumoral T cell metabolic insufficiency and dysfunction. Immunity. 2016;45(2):374–88. https://doi.org/10.1016/j.immuni.2016.07.009.
Shen BC, Huang SC. Predominant Th2/Tc2 polarity of tumor-infiltrating lymphocytes in cervical cancer. Int J Gynecol Obstet. 2000;70:A62. https://doi.org/10.1016/S0020-7292(00)82673-9.
Liang Z. Enhanced Th17 differentiation and aggravated arthritis in IEX-1-deficient mice by mitochondrial reactive oxygen species-mediated signaling. J Immunol. 2012;4:189.
Jeremias I. Inhibition of nuclear factor kappaB activation attenuates apoptosis resistance in lymphoid cells. Blood. 1998;12:91.
Malmberg KJ. Inhibition of activated/memory (CD45RO(+)) T cells by oxidative stress associated with block of NF-kappaB activation. J Immunol. 2001;5:167.
Tang G, Minemoto Y, Dibling B, Purcell NH, Li Z, Karin M, Lin A. Inhibition of JNK activation through NF-κB target genes. Nature. 2001;414(6861):313–7. https://doi.org/10.1038/35104568.
Sankar B. Tumor-induced oxidative stress perturbs nuclear factor-kappaB activity-augmenting tumor necrosis factor-alpha-mediated T-cell death: protection by curcumin. Cancer Res. 2007;1:67.
Stuelten CH, Byfield SD, Arany PR, Karpova TS, Stetler-Stevenson WG, Roberts AB. Breast cancer cells induce stromal fibroblasts to express MMP-9 via secretion of TNF-α and TGF-β. J Cell Sci. 2005;118(10):2143. https://doi.org/10.1242/jcs.02334.
Bowie A. Oxidative stress and nuclear factor-kappaB activation: a reassessment of the evidence in the light of recent discoveries. Biochem Pharmacol. 2000;1:59.
Belikov AV, Schraven B, Simeoni L. T cells and reactive oxygen species. J Biomed Sci. 2015;22:85. https://doi.org/10.1186/s12929-015-0194-3.
Min L-W. Vitamin E inhibits CD95 ligand expression and protects T cells from activation-induced cell death. J Clin Invest. 2002;5:110.
Kiessling R, Wasserman K, Horiguchi S, Kono K, Sjöberg J, Pisa P, Petersson M. Tumor-induced immune dysfunction. Cancer Immun. 1999;48(7):353–62. https://doi.org/10.1007/s002620050586.
Thor FB. Oxygen radicals induce poly(ADP-ribose) polymerase-dependent cell death in cytotoxic lymphocytes. J Immunol. 2006;176(12):7301–7.
Kim EK, Seo HS, Chae MJ, Jeon IS, Song BY, Park YJ, Ahn HM, Yun CO, Kang CY. Enhanced antitumor immunotherapeutic effect of B-cell-based vaccine transduced with modified adenoviral vector containing type 35 fiber structures. Gene Ther. 2014;21(1):106–14. https://doi.org/10.1038/gt.2013.65.
Hamilos DL, Zelarney P, Mascali JJ. Lymphocyte proliferation in glutathione-depleted lymphocytes: direct relationship between glutathione availability and the proliferative response. Immunopharmacology. 1989;18(3):223–35. https://doi.org/10.1016/0162-3109(89)90020-9.
Tanja H. The role of low molecular weight thiols in T lymphocyte proliferation and IL-2 secretion. J Immunol. 2005;1950(12):175.
Yan Z, Garg SK, Banerjee R. Regulatory T cells interfere with glutathione metabolism in dendritic cells and T cells. J Biol Chem. 2010;285(53):41525–32. https://doi.org/10.1074/jbc.M110.189944.
Suthanthiran M. Glutathione regulates activation-dependent DNA synthesis in highly purified normal human T lymphocytes stimulated via the CD2 and CD3 antigens. Proc Natl Acad Sci U S A. 1990;9:87.
Messina JP. Cell cycle progression of glutathione-depleted human peripheral blood mononuclear cells is inhibited at S phase. J Immunol. 1989;6:143.
Debaprasad M. Failure in peripheral immuno-surveillance due to thymic atrophy: importance of thymocyte maturation and apoptosis in adult tumor-bearer. Life Sci. 2005;21:77.
Francisco B. NK cell metabolism and tumor microenvironment. Front Immunol. 2019;10:2278.
Aydin E, Johansson J, Nazir FH, Hellstrand K, Martner A. Role of NOX2-derived reactive oxygen species in NK cell-mediated control of murine melanoma metastasis. Cancer Immunol Res. 2017;5:9.
Mellqvist UH. Natural killer cell dysfunction and apoptosis induced by chronic myelogenous leukemia cells: role of reactive oxygen species and regulation by histamine. Blood. 2000;5:96.
Johan A. Monocytic AML cells inactivate antileukemic lymphocytes: role of NADPH oxidase/gp91(phox) expression and the PARP-1/PAR pathway of apoptosis. Blood. 2012;24:119.
Seong-Woon Y. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;5579:297.
Tentori L. Potential clinical applications of poly(ADP-ribose) polymerase (PARP) inhibitors. Pharmacol Res. 2002;2:45.
Fenerty KE, Padget M, Wolfson B, Gameiro SR, Su Z, Lee JH, Rabizadeh S, Soon-Shiong P, Hodge JW. Immunotherapy utilizing the combination of natural killer– and antibody dependent cellular cytotoxicity (ADCC)–mediating agents with poly (ADP-ribose) polymerase (PARP) inhibition. J Immunother Cancer. 2018;6:1.
Tentori L. Chemopotentiation by PARP inhibitors in cancer therapy. Pharmacol Res. 2005;1:52.
Zhu H, Wang B, Kong L, An T, Li Y. Parvifoline AA promotes susceptibility of hepatocarcinoma to natural killer cell-mediated cytolysis by targeting peroxiredoxin. Cell Chem Biol. 2019;26:8.
Sporn MB, Liby KT. NRF2 and cancer: the good, the bad and the importance of context. Nat Rev Cancer. 2012;12(8):564–71. https://doi.org/10.1038/nrc3278.
Maj T, Wang W, Crespo J, Zhang H, Wang W, Wei S, Zhao L, Vatan L, Shao I, Szeliga W, Lyssiotis C, Liu JR, Kryczek I, Zou W. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat Immunol. 2017;18(12):1332–41. https://doi.org/10.1038/ni.3868.
Silvia D. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;6:204.
Arvey A, van der Veeken J, Samstein RM, Feng Y, Stamatoyannopoulos JA, Rudensky AY. Inflammation-induced repression of chromatin bound by the transcription factor Foxp3 in regulatory T cells. Nat Immunol. 2014;15(6):580–7. https://doi.org/10.1038/ni.2868.
Faraaz BC. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature. 2010;7317:467.
Reth M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat Immunol. 2002;3(12):1129–34. https://doi.org/10.1038/ni1202-1129.
Salimi A, Roudkenar MH, Sadeghi L, Mohseni A, Seydi E, Pirahmadi N, Pourahmad J. Ellagic acid, a polyphenolic compound, selectively induces ROS-mediated apoptosis in cancerous B-lymphocytes of CLL patients by directly targeting mitochondria. Redox Biol. 2015;6:461–71. https://doi.org/10.1016/j.redox.2015.08.021.
Kantner H-P, Warsch W, Delogu A, Bauer E, Esterbauer H, Casanova E, Sexl V, Stoiber D. ETV6/RUNX1 induces reactive oxygen species and drives the accumulation of DNA damage in B cells. Neoplasia. 2013;15(11):1292. https://doi.org/10.1593/neo.131310.
Zhang H, Wang L, Chu Y. Reactive oxygen species: the signal regulator of B cell. Free Radic Biol Med. 2019;142:16–22. https://doi.org/10.1016/j.freeradbiomed.2019.06.004.
Cubillos-Ruiz JR, Silberman PC, Rutkowski MR, Chopra S, Perales-Puchalt A, Song M, Zhang S, Bettigole SE, Gupta D, Holcomb K, Ellenson LH, Caputo T, Lee AH, Conejo-Garcia JR, Glimcher LH. ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell. 2015;161(7):1527–38. https://doi.org/10.1016/j.cell.2015.05.025.
Christina P. Crosstalk between advanced glycation and endoplasmic reticulum stress: emerging therapeutic targeting for metabolic diseases. J Clin Endocrinol Metab. 2012;7:97.
Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol. 2020; https://doi.org/10.1038/s41580-020-0230-3.
Claudio H. Targeting the unfolded protein response in disease. Nat Rev Drug Discov. 2013;9:12.
Ann-Hwee L. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;21:23.
Vladykovskaya E, Sithu SD, Haberzettl P, Wickramasinghe NS, Merchant ML, Hill BG, McCracken J, Agarwal A, Dougherty S, Gordon SA, Schuschke DA, Barski OA, O'Toole T, D’Souza SE, Bhatnagar A, Srivastava S. Lipid peroxidation product 4-hydroxy-trans-2-nonenal causes endothelial activation by inducing endoplasmic reticulum stress. J Biol Chem. 2012;287(14):11398–409. https://doi.org/10.1074/jbc.M111.320416.
Negre-Salvayre A, Coatrieux C, Ingueneau C, Salvayre R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. J Pharmacol. 2008;153(1):6–20. https://doi.org/10.1038/sj.bjp.0707395.
Giannoni E, Parri M, Chiarugi P. EMT and oxidative stress: a bidirectional interplay affecting tumor malignancy. Antioxid Redox Signal. 2012;16(11):1248–63. https://doi.org/10.1089/ars.2011.4280.
Luput L, Licarete E, Sesarman A, Patras L, Costelalupei M, Banciu M. Tumor-associated macrophages favor C26 murine colon carcinoma cell proliferation in an oxidative stress-dependent manner. Oncol Rep. 2017;37(4):2472–80. https://doi.org/10.3892/or.2017.5466.
Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22(2):231–7. https://doi.org/10.1016/j.coi.2010.01.009.
Kuo C-L, Chou H-Y, Chiu Y-C, Cheng AN, Fan C-C, Chang Y-N, Chen C-H, Jiang SS, Chen N-J, Lee AY-L. Mitochondrial oxidative stress by Lon-PYCR1 maintains an immunosuppressive tumor microenvironment that promotes cancer progression and metastasis. Cancer Lett. 2020;474:138–50. https://doi.org/10.1016/j.canlet.2020.01.019.
Dai E, Han L, Liu J, Xie Y, Kroemer G, Klionsky DJ, Zeh HJ, Kang R, Wang J, Tang D. Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy. 2020; https://doi.org/10.1080/15548627.2020.1714209.
Zuo L, He F, Sergakis GG, Koozehchian MS, Stimpfl JN, Rong Y, Diaz PT, Best TM. Interrelated role of cigarette smoking, oxidative stress, and immune response in COPD and corresponding treatments. Am J Phys. 2014;307(3):L205–18. https://doi.org/10.1152/ajplung.00330.2013.
Kurze AK, Buhs S, Eggert D, Oliveira-Ferrer L, Muller V, Niendorf A, Wagener C, Nollau P. Immature O-glycans recognized by the macrophage glycoreceptor CLEC10A (MGL) are induced by 4-hydroxy-tamoxifen, oxidative stress and DNA-damage in breast cancer cells. Cell Commun Signal. 2019;17(1):107. https://doi.org/10.1186/s12964-019-0420-9.
Tang J, Ramis-Cabrer D, Wang XJ, Barreiro E. Immunotherapy with monoclonal antibodies in lung cancer of mice: oxidative stress and other biological events. Cancer. 2019;11:9.
Cubillos-Ruiz JR, Bettigole SE, Glimcher LH. Tumorigenic and immunosuppressive effects of endoplasmic reticulum stress in cancer. Cell. 2017;168(4):692–706. https://doi.org/10.1016/j.cell.2016.12.004.
Ohl K, Tenbrock K. Reactive oxygen species as regulators of MDSC-mediated immune suppression. Front Immunol. 2018;9:2499. https://doi.org/10.3389/fimmu.2018.02499.
Sheng KC. Inflammatory mediators hold the key to dendritic cell suppression and tumor progression. Curr Med Chem. 2011;36:18.
Duan H. Novel therapeutic strategies for solid tumor based on body’s intrinsic antitumor immune system. Cell Physiol Biochem. 2018;47(2):441–57. https://doi.org/10.1159/000489979.
Panda AK, Chakraborty D, Sarkar I, Khan T, Sa G. New insights into therapeutic activity and anticancer properties of curcumin. J Exp Pharmacol. 2017;9:31–45. https://doi.org/10.2147/JEP.S70568.
Bhattacharyya S, Md Sakib Hossain D, Mohanty S, Sankar Sen G, Chattopadhyay S, Banerjee S, Chakraborty J, Das K, Sarkar D, Das T, Sa G. Curcumin reverses T cell-mediated adaptive immune dysfunctions in tumor-bearing hosts. Cell Mol Immunol. 2010;7(4):306–15. https://doi.org/10.1038/cmi.2010.11.
Noweeda M. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006;18:66.
Zhang Y, Choksi S, Chen K, Pobezinskaya Y, Linnoila I, Liu Z-G. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Res. 2013;23(7):898–914. https://doi.org/10.1038/cr.2013.75.
Orsolic N, Kunstic M, Kukolj M, Gracan R, Nemrava J. Oxidative stress, polarization of macrophages and tumour angiogenesis: efficacy of caffeic acid. Chem Biol Interact. 2016;256:111–24. https://doi.org/10.1016/j.cbi.2016.06.027.
Netea-Maier RT, Smit JWA, Netea MG. Metabolic changes in tumor cells and tumor-associated macrophages: a mutual relationship. Cancer Lett. 2018;413:102–9. https://doi.org/10.1016/j.canlet.2017.10.037.
Conflict of Interest
Authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Wang, Y., Hu, Y., Jiang, Y., Zhou, S. (2021). Oxidative Stress in the Tumor Immune Microenvironment. In: Huang, C., Zhang, Y. (eds) Oxidative Stress. Springer, Singapore. https://doi.org/10.1007/978-981-16-0522-2_2
Download citation
DOI: https://doi.org/10.1007/978-981-16-0522-2_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-0521-5
Online ISBN: 978-981-16-0522-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)