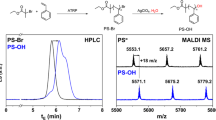
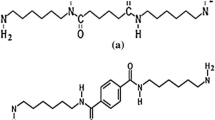
Abstract
High or good yield is essential for peptide production. The side chain protecting groups are cleaved usually in solution, after peptide detachment from the solid carrier. This can, however, be accompanied by the loss of material and the decrease in yield. Such problems can be circumvented when the side chain deprotection steps are carried out on the peptide still bound to resin. Just before purification, the final product can be detached from resin. Analogues of Vasopressin and LH-RH were synthesized on hydroxyalkylated aminopolystyrene. Stability of polymeric like alkyl ester towards hard acid (TFMSA, HF) makes possible the assembling of peptides protected with Benzyl and Tosyl groups. All cleavage steps were carried out on the peptide bound to resin. In this way salts and scavengers can also be washed away prior to detachment of peptide from the resin. Ammonolysis in gas [1] or ethylaminolysis was employed as the detachment step. The crude peptide amides were obtained in the high yield and purity. Conditions for the complete cleavage of Tos group were developed. Moreover, the resin could be used again for the new peptide synthesis.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Flegel, M., et al., In Kaumaya, P.T.P. and Hodges, R.S. (Eds.) Peptides: Chemistry, Structure and Biology (Proc. 14th Am. Pept. Symp.), Mayflower, 1996, p. 119.
Pánek, Z., Pavlik, M., Flegel, M., In Epton, R. (Ed.) Solid phase synthesis & combinatorial libraries, Mayflower Scientific Ltd, 1998, p. 365.
Buserelin, In European Pharmacopoeia 1997, p. 500
Bergot, J., Noble, R.F., Geiser, T., In Theodoropoulos, D. (Ed.) Peptides 1986 (Proceeding of the 19thEuropean Peptide Symposium), Walter de Gruyter, 1987, p. 97.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2001 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Lepša, L., Ammerová, A., Flegel, M. (2001). Acidic Cleavage of the Side Chain Protecting Groups of Peptides Attached to a Modified Polystyrene Carrier: Synthesis of Peptide Generics. In: Lebl, M., Houghten, R.A. (eds) Peptides: The Wave of the Future. American Peptide Symposia, vol 7. Springer, Dordrecht. https://doi.org/10.1007/978-94-010-0464-0_37
Download citation
DOI: https://doi.org/10.1007/978-94-010-0464-0_37
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-010-3905-5
Online ISBN: 978-94-010-0464-0
eBook Packages: Springer Book Archive