Abstract
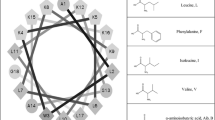
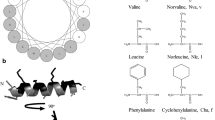
Cationic peptides are a common component of many host defense systems [1]. Upon binding to the plasma membrane of target bacteria, many of these peptides can adopt a conformation that exhibits amphipathic character. The accumulation of peptides at the membrane surface eventually results in a breakdown of the permeability barrier in the target organism, resulting in cell death. Naturally occurring cationic antimicrobial peptides usually fall into one of two classes. Linear peptides such as magainins, PGLa and cecropins, adopt α-helical structure, while cysteine-containing peptides such as defensins (constrained by intramolecular disulfide bonds) form β-sheet conformation in order to maximize amphipathicity. Recently we designed an 18-residue linear peptide, (KIGAKI)3-NH2, with no amphipathic propensity as an α-helix, but a highly amphipathic β-sheet structure [2]. Although this peptide possesses similar antimicrobial activity to that of linear a-helical peptides of comparable size, charge and hydrophobicity, it appears to be significantly more selective for bacterial membranes as compared to mammalian membranes. In this study, we examined the relationship between amphipathicity, secondary structure, antimicrobial activity, and lipid selectivity among a representative group of model peptides.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Maloy, W.L., Kari, U.P. Biopolymers 37, 105–122 (1995).
Blazyk, J., Wiegand, R., Klein, J., Hammer, J., Epand, R.M., Epand, R.F., Maloy, W.L. Kari, U.P. J. Biol. Chem. 276, 27899–27906 (2001).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2001 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Blazyk, J., Hammer, J., Jin, Y., Zhang, Y., Zhu, F. (2001). Relationship Between Amphipathic Secondary Structure and Activity in Model Linear Cationic Antimicrobial Peptides. In: Lebl, M., Houghten, R.A. (eds) Peptides: The Wave of the Future. American Peptide Symposia, vol 7. Springer, Dordrecht. https://doi.org/10.1007/978-94-010-0464-0_221
Download citation
DOI: https://doi.org/10.1007/978-94-010-0464-0_221
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-010-3905-5
Online ISBN: 978-94-010-0464-0
eBook Packages: Springer Book Archive