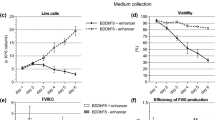
Abstract
Increasing demands for quality, identity and high expression rates have been the driving forces for the development of new expression systems over the last 10 years. In the future, human cell systems will range among leading technologies for expression and production of human therapeutic proteins. Primary human amniocytes obtained by routine amniocentesis have been immortalized by adenoviral E1/pIX functions. CEVEC’s Amniocyte Production (CAP) cells are of non-tumor origin, they are immortalized by a function not oncogenic in human, and they are from ethically accepted source of origin. Moreover, the development of CAP cells follows relevant guidelines and is completely documented. Starting from several hundred transformed cell lines, two CAP cell lines have been selected based on growth stability, performance in permanent and transient protein expression and capacity of growth in suspension. CAP cells grow as single cell suspension in chemically defined serum-free medium. CAP cells have been tested for permanent expression of reference proteins like human alpha-1 antitrypsin (hAAT), erythropoietin and human IgG1, very high protein expressing cell lines have been obtained. The hAAT expressed in CAP cell lines was fully glycosylated and sialylated. In addition, CAP cells show very high transfection efficiency, a major requirement for efficient transient expression of protein. Thus, we have developed a new expression system based on human amniocytes which offers significant advantages over existing production technologies, like a human glycosylation pattern or other post-translational modifications and high permanent and transient protein expression.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Andus, T., Gross, V., Tran-Thi, T.A., and Heinrich, P.C. (1983) Interaction of mature, unglycosylated and cell-free synthesized rat alpha 1-proteinase inhibitor with elastase. Eur. J. Biochem. 136, 253–257.
Cho, M.S., Yee, H., and Chan, S. (2002) Establishment of a human somatic hybrid cell line for recombinant protein production. J. Biomed. Sci. 9, 631–638.
Fallaux, F.J., and Hoeben, R.C. (1996) Gene therapy for the hemophilias. Curr. Opin. Hematol. 3(5), 385–389.
Fallaux, F.J., Bout, A., van der Velde, I., van den Wollenberg, D.J., Hehir, K.M., Keegan, J., Auger, C., Cramer, S.J., van Ormondt, H., van der Eb, A.J., Valerio, D., and Hoeben, R.C. (1998) New helper cells and matched early region 1-deleted adenovirus vectors prevent generation of replication-competend adenoviruses. Hum. Gene Ther. 9, 1909–1917.
Gallimore, P.H., Grand, R.J., and Byrd, P.J. (1986) Transformation of human embryo retinoblasts with simian virus 40, adneovirus and ras oncogenes. Anticancer Res. 6, 499–508.
Girod, P.A. and Mermod, N. (2003) Use of scaffold/matrix-attachment regions for protein production. In: Makrides, S.C. (eds.), Gene Transfer and Expression in Mammalian Cells. Elsevier Science BV, Amsterdam, pp. 359–379.
Graham, F.L., Smiley, J., Russell, W.C., and Nairn, R. (1977). Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 36, 59–74.
Gross, V., Tran-Thi, T.A., Schwarz, R.T., Elbein, A.D., Decker, K., and Heinrich, P.C. (1986) Different effects of the glucosidase inhibitors 1-deoxynojirimycin, N-methy1-1-deoxynojirimycin and castanospermine on the glycosylation of rat alpha 1-proteinase inhibitor and alpha 1-acid glycoprotein. Biochem. J. 236(3), 853–860.
Guzdek, A., Potempa, J., Dubin, A., and Travis, J. (1990) Comparative properties of human alpha-1-proteinase inhibitor glycosylation variants. FEBS Lett. 272, 125–127.
Hodges, L.C., Laine, R., and Chan, S.K. (1979) Structure of the oligosaccharide chains in human α1-protease inhibitor. J. Biol. Chem. 254, 8208–8212.
Hoehn, H., Bryant, E.M., Karp, L.E., and Martin, G.M. (1974) Cultivated cells from diagnostic amniocentesis in second trimester pregnancies. 1. Clonal morphology and growth potential. Pediatr. Res. 8, 756–754.
McLean, G.R., Nakouzi, A., Casadevall, A., and Green, N.S. (2000) Human and murine immunoglobulin expression vector cassettes. Mol. Immunol. 37, 5837–845.
Mega, T., Lujan, E., and Yoshida, A. (1980) Studies on the oligosaccharide chains of human α1-protease inhibitor. II. Structure of oligosaccharides. J. Biol. Chem. 255, 4057–4061.
Schiedner, G., Hertel, S., Johnston, M., Biermann, V., Dries, V., and Kochanek, S. (2002) Variables affecting in vivo performance of high-capacity adenoviral vectors. J. Virol. 76, 1600–1609.
Schiedner, G., Hertel, S., and Kochanek, S. (2000) Efficient transformation of primary human amniocytes by E1 functions of Ad5: generation of new cell lines for adenoviral vector production. Hum. Gene Ther. 11, 2105–2116.
van den Heuvel, S.J., The, S.I., Klein, B., Jochemsen, A.G., Zentema, A., and van der Eb, A.J. (1992) p53 share an antigenic determinant with proteins of 92 and 150 kilodaltons that may be involved in senescene of human cells. J. Virol. 66, 591–595.
Weber, W., Steube, K., Gross, V., Tran-Thi, T.A., Decker, K., Gerok, W., and Heinrich, P.C. (1985) Unglycosylated rat alpha 1-proteinase inhibitor has a six-fold shorter plasma half-life than the mature glycoprotein. Biochem. Biophys. Res. Commun. 126, 630–635.
Wong, C.H. (2005) Protein glycosylation: new challenges and opportunities. J. Org. Chem. 70, 4219–4225.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media B.V.
About this paper
Cite this paper
Schiedner, G. et al. (2010). Human Cell Lines for Production of Biopharmaceuticals. In: Noll, T. (eds) Cells and Culture. ESACT Proceedings, vol 4. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-3419-9_89
Download citation
DOI: https://doi.org/10.1007/978-90-481-3419-9_89
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-90-481-3418-2
Online ISBN: 978-90-481-3419-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)