Abstract
Zinc (Zn) is essentially required by plants for their growth and development. It plays very important role in various physiological procedures of plants such as photosynthesis, membrane integrity, protein synthesis, pollen formation, and immunity system. Although Zn is required by the plant in microconcentration, its bioavailable fraction in the soil is very low due to various soil factors. From soil solution it is absorbed by plants by root membrane transport mechanisms. After entering into plant system, it is neither oxidized nor reduced; but remains as divalent cation which has a great tendency to form tetrahedral complexes. From soil solution Zn reaches the plant root surface by three mechanisms, i.e., mass flow, diffusion, and root interception. Once it is absorbed, its transportation from roots to shoots occurs through the xylem and then easily retranslocated by phloem. This transport of ions and molecules from epidermal and cortical cell to xylem occurs through the symplastic or apoplastic route. The uptake of zinc into cells and its permeability into and out of intracellular organelles require some of the specific chemicals, generally known as transporter proteins. These proteins possess a quality to span the cell membranes which facilitate the movement of zinc. In recent years, a number of metal transporters have been identified in plants, including the P1B-ATPase family, zinc-regulated transporter (ZRT), iron-regulated transporter (IRT)-like protein (ZIP), natural resistance-associated macrophage protein (NRAMP) family, and cation diffusion facilitator (CDF) family. The bioavailable content of Zn in the soil can be increased using both chemical and biological approaches. Mineral fertilizers are considered a good source of Zn, but it gets fixed quickly on soil matrix, resulting in poor availability to plants. It is crucial to increase bioavailability of Zn to plants by solubilizing fixed Zn and/or by reducing fixation of the applied Zn fertilizers. This can be achieved either by using organic amendments or potential Zn solubilizing bioinoculants. Organic amendments improve bioavailability of Zn by increasing microbial biomass, which not only enhance the rate of decomposition of organic matter (source of Zn) but also enhance the bioavailability of indigenous Zn by lowering the soil pH and by releasing chelating agents. Similarly, exogenous application of some potential Zn solubilizing microflora has shown huge capability to improve bioavailable Zn content in the soil and its uptake by plant roots. This manuscript critically reviews about the Zn transporters and the role of rhizosphere microflora as a potential tool in enhancing its bioavailability to higher plants.
Similar content being viewed by others
Keywords
1 Introduction
Zinc (Zn), a transition metal, is essentially required by plants for their growth and development (McCall et al. 2000). It plays an important role in photosynthesis, membrane integrity, protein synthesis, pollen formation, and immunity system (Alloway 2008; Hajiboland and Amirazad 2010; Gurmani et al. 2012). It is also an important constituent of nucleic acids and Zn-binding proteins. It has been documented that higher plants possess about 3,000 proteins that contain Zn prosthetic groups (Tapiero and Tew 2003). Moreover, zinc is required as a cofactor for the activity of various enzymes (McCall et al. 2000) and enhances the level of antioxidants within plant tissues (Luo et al. 2010). Furthermore, it acts as an important hormonal regulating agent in plants (Marschner 1997). Apart from this, Zn finger proteins are also known for their involvement in the regulation of signal transduction events. These proteins affect transcription by binding to DNA/RNA or other proteins, leading to cell death (Englbrecht et al. 2004; Ciftci-Yilmaz and Mittler 2008).
Besides, Zn is also observed as a crucial substance for the synthesis of phytohormones such as auxin, abscisic acid, gibberellins, and cytokinins. Its deficiency reduces the level of these phytohormones in plant tissues, resulting in an impairment of cell growth. Thus, its deficiency in plant tissues adversely affects various vital processes occurring within the plant body. Though Zn is required by the plant in microconcentration, its bioavailable fraction in the soil is very low due to various soil factors (Alloway 2009). Some of the soils, in spite of having appreciable quantity of Zn, cannot support plant growth because of its poor bioavailability. It is further documented that around one third of the world’s soils are Zn deficient (Kochian 2000). In this respect, scientifically, it has been estimated that about 90 % of the total available soil Zn has no relevance to bioavailability (Mandal et al. 1988). Even if Zn is supplemented though mineral fertilizers which are considered a good source of it, that to also gets fixed quickly on soil matrix, resulting in poor availability to plants (Zia et al. 1999). Therefore, it is crucial to increase bioavailability of Zn to plants either by solubilizing fixed Zn and/or by reducing fixation of the applied Zn fertilizers. The Zn bioavailable content in soil solution can be increased by using both chemical and biological approaches, and this can be achieved either by using organic amendments or potential Zn solubilizing bioinoculants. Organic amendments improve bioavailability of Zn by increasing microbial biomass, which does not only enhance the rate of decomposition of organic matter (source of Zn) but also enhance the bioavailability of indigenous Zn by lowering the soil pH and by releasing chelating agents after their decomposition. Similarly, exogenous application of some potential Zn solubilizing microflora has also shown huge capability to improve bioavailable Zn content in the soil and its uptake by plant roots (Tariq et al. 2007).
2 Functions of Zn in Plants
Zinc takes part in various vital biochemical reactions within the plants. Under Zn-deficient situations, some of the plants such as maize, sorghum, and sugarcane showed reduced photosynthetic carbon metabolism. Scientific studies conducted on these plants revealed that Zn modifies and/or regulates the activity of carbonic anhydrase enzyme which is known to regulate the conversion of carbon dioxide to reactive bicarbonate species required for fixation of carbohydrates. Zinc is also a part of several other enzymes such as superoxide dismutase and catalase, which is known to prevent oxidative stress in plant cells (Hacisalihoglu et al. 2003). Some of the important roles of zinc in plants are as follows:
-
(i)
It helps in production of auxin which is known as an important essential growth hormone.
-
(ii)
It regulates starch formation in plants and, hence, is responsible for proper root development.
-
(iii)
It plays key role in formation of chlorophyll and carbohydrates
-
(iv)
It provides strength to plants to withstand lower air temperatures.
-
(v)
It also helps in the biosynthesis of cytochrome, a pigment, and maintains plasma membrane integrity and synthesis of leaf cuticle.
3 Zn Uptakes and Transport
3.1 Mechanism of Uptake and Factors Affecting Its Availability
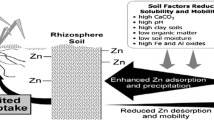
Zinc is a transition metal essential for terrestrial life, as already described. However, it is only required at low concentrations, therefore making its function as a micronutrient. Some of the scientific reports indicate that concentrations between 30 and 100 μg zinc g−1 DW are sufficient to support adequate plant growth, whereas if concentrations exist above 300 μg g−1 DW, then zinc toxicity symptoms appear especially on those plant species that are not adapted to high-zinc exposure (Marschner 1995; Van de Mortel et al. 2006). Therefore, it is proven that for optimal growth, plants need to keep tight control over zinc homeostasis. Zinc homeostasis requires a complex network of cellular or tissue-specific functions to regulate metal uptake, accumulation, trafficking, and detoxification. The ability to take up Zn of higher plants depends much more on its bioavailability from the soil rather than the absolute soil concentrations. Zinc bioavailability is modulated by various physical and chemical soil factors as it is well known that zinc solubility in the soil decreases due to high levels of calcium carbonate, metal oxides, and pH and low levels of organic matter and soil moisture as well as high amounts of phosphate (Robson 1994; Cakmak 2011; Cakmak et al. 2010). When available in the soil solution, zinc is absorbed and transported in the divalent ion form (Zn+2) from roots to shoots through the xylem, being easily retranslocated by phloem (Clemens 2001).
This transport of ions and molecules from epidermal and cortical cells to xylem can occur via symplastic or apoplastic route. Since zinc plays multiple roles in a number of biochemical and physiological processes of plants, hence, slight deficiencies can cause a decrease in growth, yield, by including the zinc content of edible parts. Therefore, it has become imperative to know about the molecular details of the element in relation to its uptake, translocation, and storage in plants. Some of the key factors which affect the Zn bioavailability are as follows:
-
(i)
Though it is well known that Zn availability generally decreases as pH increases, this effect is not universal and can temporarily be overcome by using the band applications of acid-type fertilizers.
-
(ii)
Zn availability is also affected by Zn:P balance. It is well documented that high levels of soil P adversely affect the Zn availability.
-
(iii)
Presence of good amount of organic matter is reported to increase Zn availability because the organic compounds produced by decomposition of soil organic matter chelate the inorganic sources of Zn and enhance its availability.
-
(iv)
Under N stress situations, generally, plants fail to take adequate amount of Zn because of the decreases in vigor of plants.
-
(v)
Soil saturation situation is also reported to cause Zn deficiency because Zn does not undergo the valence changes as Mn does. Therefore, various research reports indicated that rice cannot take up Zn effectively under flooded conditions. The causes of this condition appear to be applicable to other crops too for some extent.
-
(vi)
Zn:Cu balance is also reported to cause Zn deficiency because the absorption mechanism of both elements by plant roots is the same. Therefore, this causes interference in the uptake of one when the other is in excess in the root zone.
-
(vii)
Contrary scientific reports are available for Zn:Mn balance Some results indicate an antagonistic relationship, while others indicate a sympathetic relationship.
-
(viii)
Zn:Mg balance reported for their sympathetic relationship; various reports indicate that additions of Mg can increase the uptake of zinc.
-
(ix)
As far as Zn:As balance is concern, it was observed that high levels of arsenic in the soil inhibit seriously both phosphorus and zinc uptake.
3.2 Zn Uptake from Soil
Generally, from soil solution and at neutral pH, zinc is mostly absorbed by most of the plants as a divalent cation (Zn++), and at high pH, it can also be taken up as a monovalent (ZnOH+) cation (Marschner 1995). Once taken up, it is neither oxidized nor reduced; therefore, the role of Zn in cells sticks to its behavior as a divalent cation, and in this valence form it is known for their strong tendency to form tetrahedral complexes. The ability to take up Zn of higher plants depends much more on its bioavailability from soil rather than the absolute soil concentrations. As we know the bioavailability of zinc is modulated by various physical and chemical properties of the soil. In most of the soil, zinc solubility is reported to decrease by high levels of calcium carbonate, metal oxides, and pH and low levels of organic matter and soil moisture as well as high amounts of phosphate (Robson 1994; Cakmak 2011).
From soil solution, uptake of Zn mainly depends on ion concentrations at the root surface, plant demand, and root absorption capacity. Mass flow, diffusion, and root interception mechanisms were reported by which Zn reaches to the plant root surface. Mass flow is considered as a passive nutrient transport from the soil to the roots and is driven by transpiration. Mass flow becomes the predominant mechanism when the soil solution contains a relatively large concentration of Zn, whereas diffusion operates when the Zn concentration is low, particularly in soils with low plant-available Zn. Contrary to mass flow, diffusion operates only in the immediate volume of soil surrounding by a root. Thus, root interception which includes root growth and root surface area is also an important factor in determining plant availability of Zn. It is seen if root interception is poor, it can limit Zn uptake even if granules of ZnSO4 are banded in the soil; particularly, it has more relevance to the fact at a low rate of ZnSO4 application.
3.2.1 Zn Movement in Plants
3.2.1.1 Transportation from Root Surface to Xylem and Phloem
When available in the soil solution, zinc is absorbed and transported in the divalent ion (Zn+2) form from roots to shoots via the xylem where it is easily retranslocated by phloem (Clemens 2001). This kind of transportation of ions and molecules from epidermal and cortical cells to xylem can occur through the symplastic or apoplastic route. It is observed that accumulation of Zn in plant roots exhibits biphasic kinetics, with initial rapid entry and then binding within the root cell wall followed by a slower linear transport phase across the plasma membrane. Movement of Zn2+ from the external solution to the root cell wall-free space occurs via diffusion and then is subsequently transported across the plasma membrane by the influence of ion transport proteins. Since the cytoplasm of a plant cell is negatively charged, therefore, it favors entry of Zn2+ and other cations into the cell. Root Zn uptake is mediated by different transport systems which include a high-velocity, low-affinity membrane transporter system (Km = 2–5 μM) and a low-velocity, high-affinity system (Km = 0.6–2 nM). Most likely the second transport system dominantly operates under low soil Zn conditions (Hacisalihoglu et al. 2001). It is generally observed that Zn-inefficient plants have lower rates of uptake as well as the comparable affinity values compared with Zn-efficient plants (Rengel and Wheal 1997; Hacisalihoglu et al. 2001). Most of the nutrients are known to transport into the root xylem via the epidermis, cortex, and endodermis (Marschner 1995). Zn may pass through the root to the xylem either through the extracellular spaces between root cells (apoplast) (White et al. 2002) or the cytoplasmic continuum of root cells linked by plasmodesmata (symplast). The apoplastic cation flux is largely dependent on the cation exchange capacity of the cell wall, water flows, and the existence of Casparian band; whereas the symplastic pathway involves specific transporters in the plasma membrane, particular cations can be selected for transport (Sattelmacher 2001). For example, in Thlaspi caerulescens and T. arvense, Zn reaches the xylem via the symplastic pathway, and the entry point for Zn accumulation is across the plasma membrane of root cells (Lasat et al. 1996; Lasat and Kochian 2000). With an increasing external Zn concentration, the apoplastic pathway plays a greater role in Zn uptake and influx to the xylem (White et al. 2002; Rengel and Wheal 1997).
3.2.1.2 Transportation from Xylem/Phloem to Shoot
Nutrients in the xylem move toward shoots in the transpiration stream. This driving force for transpiration arises from the gradient in water potential between stomatal cells and the atmosphere. An analysis of xylem fluid content showed that Zn moves as a complexed form (e.g., anionic) in the xylem. Zn in the xylem has been measured in both soluble and insoluble forms. In a soluble form it bound to small proteinous molecules, whereas Zn-phytate complexes exist as insoluble form of Zn. Metal complexes and ionic activities of micronutrients differ in the xylem and phloem saps (Welch 1995). The activity of cationic micronutrients, such as Mn, Zn, Fe, Cu, and Ni, is low in the phloem sap due to a high phosphate level and high pH. Hence, these micronutrients are likely to form metal complexes to move easily in the phloem stream (Welch 1995). Regardless of the chosen path, the solutes that reach the xylem parenchyma cells are transferred to the xylem elements in a tightly controlled process mediating membrane transport. Via the xylem sap, the minerals are released in the apoplast of the leaves and are subsequently distributed intracellularly (Clemens et al. 2002).
3.2.2 Zinc Transporters (Transportation to Cytoplasm)
Since Zn cannot diffuse across cell membrane, therefore for these purposes, some of the specific chemicals generally known as transporters are required to transport Zn into cytoplasm. Zn transporters are proteinous molecules and known to span cell membranes to facilitate the movement of zinc into cells and its transport into and out of intracellular organelles. Similarly, few P-type ATPases have also been demonstrated to play roles in eukaryotic zinc transport. In recent years, a number of metal transporter families such as P1B-ATPase, zinc-regulated transporter (ZRT), iron-regulated transporter (IRT)-like protein (ZIP), natural resistance-associated macrophage protein (NRAMP), and cation diffusion facilitator (CDF) have not only been identified in plants, archaea, bacteria, fungi, and mammals, but also their involvement in relation to metal uptake and transport was demonstrated thoroughly. ZIP proteins are generally known to contribute toward metal ion homeostasis by means of transporting cations into the cytoplasm. Functional complementation in yeast indicated that ZIP proteins are able to transport various divalent cations, including Fe2+, Zn2+, Mn2+, and Cd2+. The ZIP proteins are reported to consist about 309–476 amino acid residues which consist eight potential transmembrane domains and a similar membrane topology. Known eukaryotic zinc transporters come largely from two families, the ZIP (SLC39) and CDF/ZnT (SLC30) proteins.
3.2.2.1 The ZIP Family
Plants, bacteria, fungi, and humans are reported to contain the ZIPs (ZRT, IRT-like proteins). These proteins are said to be involved in the transport of Fe, Zn, Mn, and Cd with a number of family members differing in their substrate range and specificity (Guerinot 2000). Structurally, the ZIP proteins are said to contain eight transmembrane domains with the amino- and carboxyl-terminal ends situated on the outer surface of the plasma membrane between transmembrane domains III and IV (Guerinot 2000; Colangelo and Guerinot 2004). In most of the cases, the expression of genes encoding the plant ZIP transporters appears to be induced by zinc or iron deprivation (Eide et al. 1996; Eide and Guarente 1992; Grotz et al. 1998). Some of the ZIP family members, such as irt genes, are also constitutively expressed, but in this case also expression levels increase with iron deprivation (Eckhardt et al. 2001; Vert et al. 2002). Presently about 85 ZIP family members have been identified from bacteria, archaea, and all types of eukaryotes, including 15 genes in Arabidopsis. The function of several members of the ZIP family in Escherichia coli (Grass et al. 2002), yeast (Saccharomyces cerevisiae; Zhao and Eide 1996a, b), plants (Eide et al. 1996; Grotz et al. 1998; Guerinot 2000; Pence et al. 2000; Eckhardt et al. 2001; Vert et al. 2002; Moreau et al. 2002), and humans (Gaither and Eide 2000, 2001) has been studied. In plant community, the dicots, i.e., pea (Pisum sativum), Arabidopsis, and Thlaspi caerulescens, were mainly reported to have ZIP transporters. Many members of ZIP family like the zinc-regulated transporter (ZRT) and iron-regulated transporter (IRT)-like protein were thought to be involved in transporting zinc into the cytosol across the plasma membrane, which is an important process for plant zinc uptake (Palmer and Guerinot 2009; Song et al. 2010).
As per the alignment of the predicted amino acid sequences, the ZIPs can be grouped into four subfamilies, although all of the higher plant genes appear to fall into a single group. They range widely in overall length, this being due to a variable region between TM-3 and TM-4. This region is predicted to be on the cytoplasmic side and is a potential metal-binding domain rich in histidine residues. ZIPs 1, 3, and 4 are expressed in the roots of Zn-deficient plants, while ZIP4 is also found in the shoots and is predicted to have a chloroplast targeting sequence (Grotz et al. 1998; Guerinot 2000). The proposed role of ZIP transporters in Zn nutrition is supported by the characterization of homologues from other species. ZRT3 is proposed to function in the mobilization of stored Zn from the vacuole (MacDiarmid et al. 2000).
A ZIP gene homologue, TcZNT1, from the Zn/Cd-hyperaccumulating plant, Thlaspi caerulescens, was shown to mediate high-affinity Zn2+ uptake and low-affinity Cd2+ uptake following expression in yeast (Pence et al. 2000; Zhao et al. 2002). Assuncao et al. (2010) reported that TcZNT1 and TcZNT2 were predominantly expressed in roots although their expression was seldomly Zn responsive. In contrary to that in case of the non-hyperaccumulator, i.e., T. arvense, these genes were exclusively expressed under conditions of Zn deficiency (Assuncao et al. 2010). Recently, a member of the ZIP family, GmZIP1, has been identified in soybean (Moreau et al. 2002). By functional complementation of the ZRT1 and ZRT2 yeast cells, GmZIP1 was observed to be highly selective for Zn, while yeast Zn uptake was inhibited by Cd. It was found that GmZIP1 was specifically expressed in the nodules and not in roots, stems, or leaves, and the protein was localized to the peribacteroid membrane, which indicates the possibility of its role in the symbiosis (Moreau et al. 2002) (Table 11.1).
3.2.2.2 Cation Diffusion Facilitator (CDF) Proteins
Members of the cation diffusion facilitator (CDF) family are known to control cation concentrations in cells through sequestration into internal compartments and efflux from cell and hence play an important role in living organisms (Gustin et al. 2011). These proteins belong to a family of heavy metal efflux transporters that presumably play an essential role in homeostasis and tolerance to metal ions. Some of the members of the cation diffusion facilitator (CDF) family such as MTP1 (metal tolerance protein, previously ZAT) are thought to transport heavy metals into the vacuoles of leaf epidermal cells in the metal ion-hyperaccumulating plants (hyperaccumulators) (Kupper et al. 1999; Persans et al. 2001; Delhaize et al. 2003). The Zn tolerance of A. halleri has been found to be due to an increased copy number of the MTP1 gene and an elevated level of transcription (Becher et al. 2004; Drager et al. 2004).
Zn transport activity of MTP1 in Arabidopsis thaliana (AtMTP1) was demonstrated by using reconstituted proteoliposomes of the protein expressed in Escherichia coli (Bloß et al. 2002) and by a yeast complementation assay with a Zn-hypersensitive double mutant of ZRC1 and COT1 (zrc1/cot1) (Drager et al. 2004; Kim et al. 2009). AtMTP1 has been found to retain about 92 % identity with A. halleri AhMTP1-3 (Becher et al. 2004; Drager et al. 2004). Ectopic expression of AtMTP1 in A. thaliana caused in enhancement of Zn resistance (van der Zaal et al. 1999). The increased tolerance to Zn is thought to be due to sequestration of Zn into vacuoles through the ectopically expressed AtMTP1. However, neither the subcellular location of AtMTP1 nor the Zn sequestration organelle has been reported.
3.2.2.3 P1B-ATPases Family
Another family of transporters involved in zinc efflux is the P1B-ATPase. Arabidopsis has been demonstrated with eight genes encoding P1B-ATPases with difference in their structure, function, and regulation (Eren and Arguello 2004). Among these, HMA1, HMA2, HMA3, and HMA4 were observed to involve in zinc transport (Hussain et al. 2004). AtHMA1 is present in the chloroplast envelop and can contribute to Zn detoxification under excess zinc conditions (Kim et al. 2009). The AtHMA2 gene encodes a Zn+2-ATPase located in the plasma membrane. The gene expression is induced by cadmium and zinc (Eren and Arguello 2004).
Since zinc hyperaccumulator species were observed to show higher expression of HMA3 than the non-hyperaccumulator ones, such as Arabidopsis, therefore, the AtHMA3 protein possibly mediates zinc hyperaccumulation (Becher et al. 2004; van de Mortel et al. 2006; Hassan and Aarts 2011). Recently, HMA3 has been cloned from Thlaspi caerulescens Alpine Penny-cress (Ueno et al. 2011) and rice (Ueno et al. 2010); therefore, it is a vacuolar influx transporter, important for cadmium tolerance in both species. This suggests that different homologues of HMA3 may have different metal-substrate specificity. AtHMA4, similar to AtHMA2 and acting alike, plays an important role in translocation of zinc, specifically in loading of zinc into the xylem (van de Mortel et al. 2006; Waters and Sankaran 2011). Hussain et al. (2004) reported that both HMA2 and HMA4 are essential to zinc homeostasis and they show a functional redundancy.
4 Phytosiderophores and Metal Chelators
Roots are well known to change the rhizosphere chemistry by altering the rhizosphere pH (Wang et al. 2006) and/or releasing PS that can chelate soil Zn. Therefore, by both of the ways, it increases Zn availability (Cakmak et al. 1994). This root-mediated decrease in pH caused increases in Zn availability by solubilizing the Zn present in inorganic and organic soil complexes (Hacisalihoglu and Kochian 2003).The role of root exudates in relation to Zn efficiency has been demonstrated by a number of scientific evidences. Generally, PS are chemically identified as non-proteinogenic amino acids released from roots of various plants especially of graminaceous species under Fe- (Marschner 1995) and Zn-deficiency (Zhang et al. 1991; Kochian 1993) situations. These release compounds were found highly efficacious in mobilizing and complexing Zn in calcareous soils. Zhang et al. 1991 reported that PS release from the root apoplast of wheat plants caused mobilization of Zn and may also be involved in the translocation and solubility of Zn within plants (Welch 1995). It was also observed that under Zn-deficiency situations, release of PS in Zn-inefficient durum wheat is approximately 6–8 times lower than that in Zn-efficient bread wheat (Cakmak et al. 1996, 1998).
In the case of rice when grown in nutrient solution, the efficiency of Zn uptake was found highly to be correlated with the exudation rates of low molecular weight organic anions (Hoffland et al. 2006). Some of the root-exuded metal chelators were observed to play important role in metal homeostasis. One of the most important examples of this kind of compound is nicotianamine (NA) made by the action of NA synthase; this is known as a strong metal-chelating compound for binding of zinc, iron, copper, as well as nickel (Curie et al. 2009; Delhaize et al. 2003). NA is thought to be involved in long-distance transport, perhaps also playing a role in the entry of metals into the phloem or xylem through metal-NA chelate transporters of the yellow stripe-like (YSL) family (Gendre et al. 2007). NA synthase is encoded by four genes in Arabidopsis, AtNAS1–AtNAS4, which act functionally redundant, although they show different expression patterns, suggesting that each NAS gene may have a specialized function (Klatte et al. 2009). As an example, only AtNAS2 and AtNAS4 are highly expressed in roots under zinc deficiency (Van de Mortel et al. 2006).
5 Mechanism of Improving Zn Availability
Zn is an essential micronutrient for all living organisms. Approximately 40 % of the world’s human population is zinc deficient; moreover this problem is worse in developing countries where people depend on cereal-rich-based diets for their sustenance. The improvement of plants for their ability to mitigate zinc deficiency and in turn to improve crop yield under zinc-limiting conditions (Reid et al. 1996) has thus far been hampered by lack of knowledge of the mechanisms and regulation of the zinc homeostasis network in plants. Therefore, by properly understanding the zinc homeostasis network in plants, bio-fortification of crops with zinc by using effective application of fertilizers, plant breeding, and other genetic approaches can offer a sustainable solution to this global problem. Various strategies such as application of chemical fertilizers, effectively chelated Zn, organic amendments, and bioinoculants are advocated to increase Zn concentration in the rhizosphere.
5.1 Effective Application of Zinc
Except some of the very insoluble materials, all zinc carriers are effective sources of zinc for crops, provided they are properly applied (Hodgson 1963). Zinc-containing materials may either be broadcast on the soil or thoroughly incorporated or used in a band near the seed at planting time. These materials are generally advocated to apply at a rate enough to grow the current crop; however, with this element it may be more feasible to increase the soil zinc content in order to ensure a multiyear supply. Granular zinc sulfate or finely divided zinc oxide or carbonates are cost-effective carriers of Zn and can easily be broadcast. These materials can also be applied as band application beside the seed at planting time. It was always found profitable to include a dose of zinc if we are going to apply approximately 10 lb of nitrogen per acre. Phosphorus is also an essential nutrient needed for good crop, and Zn can be applied effectively with liquid phosphatic fertilizers. For band application of Zn, carrier selection is more crucial; moreover, selection of suitable carriers plays more role under severe zinc-deficient situations, because the material is not well distributed in the soil. Under severe Zn-deficient conditions, if dry, granular fertilizer is chosen as a source of Zn as band application, then the organic zinc chelates should be preferred because chelated materials were found more effective than the other zinc carriers. To give satisfactory results with liquid polyphosphate fertilizers, finely divided zinc carriers should be used; however, under very extreme zinc-deficiency conditions, the role of zinc carrier does not show any difference when applied in a band with liquid polyphosphate fertilizers. Under these extreme deficiency conditions, it is difficult to supply enough zinc in a band; thus, broadcast treatment with thoroughly incorporation is likely to be more effective.
Zn deficiency can be mitigated by applying Zn-containing materials properly. Generally, three basic types of compounds are available for Zn fertilization; these include inorganic mineral compounds, synthetic chelates, and natural organic materials; however, their performance greatly depends on their water-solubility properties. Some of the common sources of Zn fertilizers are listed below in Table 11.2.
5.2 Organic Amendments
Soil organic matter is considered a very important factor in nutrient mobility in soil. Various organic amendments, such as compost, farmyard manure (FYM), poultry manure, olive husk, etc., are applied to soil for improving its health, fertility, as well as crop yields. The organic material can improve the availability of Zn by releasing Zn with time and by altering the physicochemical properties of soil. These properties may increase soluble/available fraction of Zn in soil for plant uptake. Moreover, application of organic amendments also improves biological properties of the soil (Tejada et al. 2006). For instance, microbial biomass and soil enzyme activities are substantially increased with application of organic amendments (Blagodatsky and Richter 1998; Liang et al. 2003). This increase in microbial population and activities is an important indicator of soil health and soil productivity. Soils having more microbial biomass and microbial activities are supposed to be productive soils as they have good nutrient mobility and availability to plants. Organic amendments improve soil microbial biomass carbon (Cmic) and the Zn content in soil and plant tissues.
5.3 Rhizosphere Microflora
Rhizosphere comprises the narrow zone of the soil around roots that is directly influenced by root secretions and is considered a hot spot of microflora, having manifold increase in microbial population than bulk soil. All the microbial communities residing in this region constitute rhizosphere microflora. The rhizosphere microflora may benefit plants through multifarious mechanisms including fixation of atmospheric nitrogen; mobilization of nutrients; production of phytohormones; altering indigenous level of phytohormones; improving plant stress tolerance to salinity, toxicity, drought, metal, and pesticide load; and also acting as a biocontrol agent (Glick and Bashan 1997; Lucy et al. 2004; Khalid et al. 2009).
Although, each and every mechanism has its own significance, but mobilization of nutrients by microflora has considered the most crucial function they perform in order to improve nutrient content in plant tissues. There is a good deal of research on mobilization of phosphorus in the rhizosphere through these tiny creatures, but increase in Zn bioavailable fraction in the rhizosphere due to the activities of rhizosphere microbes has not been well explored yet. However, there are sufficient reports indicating substantial potential of these microbes in improving Zn bioavailable fraction in the rhizosphere of plants and Zn content in plant tissues. Among microbes, both bacteria and fungi have shown tremendous ability to improve plant-available Zn in the rhizosphere and also increase Zn in plant parts (Whiting et al. 2001; Fasim et al. 2002; Biari et al. 2008; Subramanian et al. 2009). The ways through which rhizosphere microflora may cause mobilization/solubilization of Zn include reduction in soil pH (Koide and Kabir 2000; Subramanian et al. 2009), chelation (Whiting et al. 2001), or through improving root growth and root absorptive area (Burkert and Robson 1994). These mechanisms vary from one microorganism to other acquisition/uptake in plant tissues. Importance and examples of these mechanisms have been discussed in detail here.
5.3.1 Reduction in pH
Availability of micronutrients in the soil is very much sensitive to soil. A little change in soil pH may have a great impact on micronutrient mobility/solubility in soil. It has been reported that availability of Zn decreases 100 times with one unit increase in pH (Havlin et al. 2014). Thus by decreasing the pH of alkaline soil, bioavailable fraction of Zn can be enhanced to an appreciable level. Rhizosphere microflora has been reported to lower the soil pH to a good extent (Wu et al. 2006), which may occur due to secretion of some organic acids and protons extrusion (Fasim et al. 2002). For instance, Pseudomonas fluorescens secreted gluconic acid and 2-ketogluconic acid in the culture during solubilization of Zn phosphate. In addition, concentration of protons was also found higher in the culture after incubation period (Simine et al. 1998). Likewise, Fasim et al. (2002) observed that solubilization of Zn oxide and phosphate was accompanied by proton extrusion and production of 2-ketogluconic acid. Martino et al. (2003) documented that ericoid mycorrhizal fungi secreted organic acid to solubilize Zn from insoluble ZnO and Zn3 (PO4)2. A change in pH was observed when Pseudomonas and Bacillus spp. were used to solubilize ZnS, ZnO, and ZnCO3 in broth culture (Saravanan et al. 2004). Koide and Kabir (2000) proposed that mycorrhizal plants facilitate Zn availability by lowering the pH of the soil by the release of some organic acids.
5.3.2 Zn Chelation
Zinc ions have high interaction with the soil constituent due to which its persistency in the soil solution is very low (Alloway 2009). Due to low persistency/high reactivity of Zn in soil solution, plant-available fraction of Zn in the soil is poor. However, bioavailability of Zn could be increased by means of Zn-chelating compounds (Obrador et al. 2003). These compounds are either synthetic or synthesized and released by the plant roots and potential rhizosphere microflora into the rhizosphere to chelate the Zn and improve its bioavailability. The chelates of microflora are the metabolites, which form complexes with metal cations like Zn2+ (Tarkalson et al. 1998), which reduces their reaction with the soil. These Zn chelates subsequently move toward the roots and release chelating ligand (Zn2+) at the root surface, making them free to chelate another Zn ion from the soil solution.
In some microorganisms, chelation has been observed as dominant phenomena to improve bioavailability and uptake by plant roots. For instance, Whiting et al. (2001) suggested that possible mechanism used by bacteria (Microbacterium saperdae, Pseudomonas monteilii, and Enterobacter cancerogenes) for increasing water soluble Zn (bioavailable) in soil was the production of Zn-chelating metallophores. In another report, Tariq et al. (2007) found that Azospirillum lipoferum (JCM-1270, ER-20), Pseudomonas sp. (96-51), and Agrobacterium sp. (Ca-18) mobilized Zn and made it bioavailable for longer period of time when they were applied as a biofertilizer to rice by producing chelating agent like ethylenediaminetetraacetate (EDTA).
5.3.3 Changes in Root Architecture
Zinc is immobile in soil and is taken up by plant mainly by diffusion (Imran et al. 2014). Due to poor native bioavailable Zn and low exogenous supply, depletion zones are formed around roots. Therefore, to improve Zn uptake, it should be in close proximity to roots. This can be achieved either by application of more Zn or improving root growth and surface area so that roots can take nutrients beyond the depletion zone. A rhizosphere microflora especially mycorrhizal fungus is widely known for its impact on root architecture. Mycorrhizal plants uptake Zn over more distances, crossing the depletion zone. According to Burkert and Robson (1994), arbuscular mycorrhizae can acquire Zn from a distance of 40 mm from the root surface. Jansa et al. (2003) noted that Glomus intraradices can take up Zn from a distance of 50 mm from the roots of maize. In the absence of Zn fertilization, Subramanian et al. (2009) observed that mycorrhizal fungus significantly increased root length, spread, and volume compared to the plants without fungal inoculation, and this increased the Zn concentration in the grain up to 4 %.
6 Bioinoculants
Several studies have revealed that bioinoculants help in mitigation of Zn deficiency in plants through improving mobilization of Zn in the soil. Many bacterial and fungal strains have been found capable of solubilizing fixed Zn and consequently increasing its uptake by plants. The role of fungal and bacterial inoculants in improving availability of Zn to plants is comprehensively discussed below.
6.1 Fungal Inoculants
Among the fungal inoculants, AM fungus is considered highly effective in improving the availability and absorption of immobile nutrients by higher plants (Ikram et al. 1992; Tarafdar and Marschner 1994; Liu et al. 2000). AM fungi are well known in improving the availability of phosphorus to plant roots. It has also been reported that mycorrhizal symbiosis is also very effective in improving availability of Zn to plants (Ortas et al. 2002; Gao et al. 2007; Ryan et al. 2007; Subramanian et al. 2009). There are good reports about increase in Zn uptake by the application of bioinoculants (Smith and Read 1997; Liu et al. 2000; Ryan and Angus 2003; Subramanian et al. 2009), which might have occurred through the increase in bioavailable Zn in soil. For instance, Swaminathan and Verma (1979) observed a great improvement in bioavailable Zn fraction in the soil through fungal (Glomus macrocarpum) treatment which subsequently increased the Zn concentration in the leaves of wheat, maize, and potato grown on Zn-deficient soils. Likewise, Subramanian et al. (2009) found that inoculation of Glomus intraradices caused an overall increase of 43 % in bioavailable Zn in the soil after 75 days. Response of AM fungus inoculation has also been found very promising in terms of Zn accumulation in shoot and leaves. Giri et al. (2005) found about 15 times more Zn in the shoots of + AM compared to -AM plants. Chen et al. (2003) also recorded substantial increase in shoot Zn concentration through fungal inoculation. Similarly, mycorrhizal infection also improved concentration of Zn in the leaves of AM plants compared to uninoculated plants (Swaminathan and Verma 1979; Wu et al. 2011).
6.2 Bacterial Inoculants
Like fungi, bacterial bioinoculants are also helpful in increasing solubilization/availability of Zn in the soil and its further uptake by plants to improve plant Zn content. Several bacterial species have been reported that are able to solubilize insoluble Zn compounds in liquid medium (Simine et al. 1998; Fasim et al. 2002; Saravanan et al. 2007) and in the soil (Whiting et al. 2001; Tariq et al. 2007). For instance, in an in vitro study, Saravanan et al. (2004) found that Pseudomonas and Bacillus can solubilize various Zn compounds like ZnS, ZnO, and ZnCO3 to a good extent in liquid medium. Likewise, in another study, Saravanan et al. (2007) demonstrated Zn solubilizing potential of Glomus diazotrophicus PA15. Inoculation with G. diazotrophicus PA15 resulted in 41, 15.7, and 60 times increase in soluble Zn content in the cases of ZnO, ZnCO3, and Zn3(PO4)2, respectively, after 48 h of incubation compared to uninoculated control. Similarly, Fasim et al. (2002) found a high potential of Pseudomonas aeruginosa to solubilize ZnO in liquid medium. Bacteria have also shown high mobilization of soil Zn as Tariq et al. (2007) observed almost 5.6 time higher bioavailable Zn in inoculated soil compared to uninoculated soil. Whiting et al. (2001) have also documented about 0.45-fold increase in bioavailable Zn in rhizosphere soil through bacterial inoculation. It has also been widely reported that bacterial inoculation improves plant Zn content (Whiting et al. 2001; Sadaghiani et al. 2008; Biari et al. 2008).
7 Improved Genotypes for Zn Use Efficiency
Plant genotypes vary widely in their tolerance to soils with low plant-available Zn with respect to both Zn uptake and utilization. Tolerance of plant genotypes to Zn deficiency, as a genetic trait, is usually referred to as Zn efficiency and defined as the ability of a cultivar to grow and yield well in soils that are too deficient in Zn to support a standard cultivar. The physiological and molecular mechanisms of Zn-deficiency tolerance are just beginning to be understood, and these mechanisms can be exploited in crop breeding programs (Hacisalihoglu and Kochian 2003) For example, Zn-efficient genotypes with better Zn utilization may contain higher amounts of chelators that bind Zn and increase its physiological availability at the cellular level. A better understanding of the physiological, morphological, and genetic bases of Zn efficiency is needed for the development of fast, simple, and reliable screening procedures for identifying and breeding genotypes for Zn efficiency. The first step in breeding for Zn efficiency is the assessment of a large number of segregating populations from crosses of Zn-efficient and Zn-inefficient parents.
8 Conclusion
Since Zn is involved in various plant physiological processes, therefore, it is a very important micronutrient for plant health and yield, and to get maximum yield, it should be available to plants in appropriate quantity. Although most of the soils have a fair quantity of Zn, unfortunately most of the cases posses extremely low plant-available fraction due to various soil factors. Therefore, recommendations were always made to apply Zn from exogenous sources in order to maintain the appropriate concentration of it in soil solution. Various chemical fertilizers, chelated Zn, and organic fertilizers are available for this purpose. Potential of microbial biotechnology has also been tested to improve the indigenous Zn availability and reduce the fixation of applied Zn. Application of various fungal and bacterial bioinoculants to the soil had shown very promising results in terms of improving Zn content in soil and plant tissues and improving yield. Apart from all these factors, high Zn efficiency in crops also appears to be related to various morphological and physiological traits, such as root surface area, Zn-mobilizing root exudates, and better utilization of Zn at the cellular level. In terms of understanding the roles of genes involved in uptake and translocation of zinc in plants, a lot has been achieved, but information on where in the plant each transporter functions and how each one is controlled in response to nutrient availability remains still unclear. The identification of transcription factors involved in the control of zinc-deficiency response offers interesting opportunities to modulate zinc-deficiency responsive gene expression to make plants less sensitive to zinc deficiency or to confer a constitutive zinc-deficiency response, which can induce plants to overaccumulate metals. Therefore, understanding the regulator identity of the zinc homeostasis network in plants should provide new insights for the development of crops in areas suffering from low zinc bioavailability and for biofortification strategies. Furthermore, understanding how zinc interacts with other metals, such as cadmium and lead, during its absorption is important to be known, avoiding undesirable accumulation of such heavy metals in plants.
References
Alloway BJ (2008) Zinc in soils and crop nutrition. International zinc Association and International Fertilizer Industry Association, Brussels/Paris, p 130
Alloway BJ (2009) Soil factors associated with zinc deficiency in crops and humans. Environ Geochem Health 31:537–548
Assuncao AGL, Herrero E, Lin YF, Huettel B, Talukdar S, Smaczniak C, Immink RGH, Eldik MV, Fiers M, Schat H, Aarts MGM (2010) Arabidopsis thaliana transcription factors bZIP19 and bZIP23 regulate the adaptation to zinc deficiency. Proc Natl Acad Sci U S A 107:10296–10301
Becher M, Talke IN, Krall L, Kramer U (2004) Cross-species microarray transcript profiling reveals high constitutive expression of metal homeostasis genes in shoots of the zinc hyperaccumulator Arabidopsis halleri. Plant J 37(2):251–268
Biari A, Gholami A, Rahmani HA (2008) Growth promotion and enhanced nutrient uptake of maize (Zea mays L.) by application of plant growth promoting rhizobacteria in arid region of Iran. J Biol Sci 8:1015–1020
Blagodatsky SA, Richter O (1998) Microbial growth in soil and nitrogen turnover: a theoretical model considering the activity state of microorganisms. Soil Biol Biochem 30:1743–1755
Bloß B, Clemens S, Nies DH (2002) Characterization of the ZAT1p zinc transporter from Arabidopsis thaliana in microbial model organisms and reconstituted proteoliposomes. Planta 214:783–791
Burkert B, Robson A (1994) 65Zn uptake in subterranean clover (Trifolium subterraneum L.) by three vesicular – arbuscular mycorrhizal fungi in a root – free sandy soil. Soil Biol Biochem 26:1117–1124
Cakmak I (2011) Breeding, transformation and physiological strategies for the development of wheat with high zinc and iron grain concentration. In: Bonjean A (ed) The world wheat book. Lavoisier, Paris, New York
Cakmak I, Gulut KY, Marschner H, Graham RD (1994) Effect of zinc and iron deficiency on phytosiderophore release in wheat genotypes differing in zinc efficiency. J Plant Nutr 17:1–17
Cakmak I, Sary N, Marschner H, Ekiz H, Kalaycy M, Yilmaz A, Braun HJ (1996) Phytosiderophore release in bread and durum wheat genotypes differing in zinc efficiency. Plant Soil 180:183–189
Cakmak I, Torun B, Erenoglu B, Ozturk L, Marschner H, Kalayci M, Ekiz H, Yilmaz A (1998) Morphological and physiological differences in the response of cereals to zinc deficiency. Euphytica 100:349–357
Cakmak I, Pfeiffer W, Mcclafferty B (2010) Biofortification of durum wheat with zinc and iron. Cereal Chem 87:10–20
Chen BD, Li XL, Tao HQ, Christie P, Wong MH (2003) The role of arbuscular mycorrhiza in zinc uptake by red clover growing in a calcareous soil spiked with various quantities of zinc. Chemosphere 50:839–846
Ciftci-Yilmaz S, Mittler R (2008) The zinc finger network of plants. Cell Mol Life Sci 65:1150–1160
Clemens S (2001) Molecular mechanisms of plant metal tolerance and homeostasis. Planta 212:475–486
Clemens S, Palmgren M, Kramer U (2002) A long way ahead: understanding and engineering plant metal accumulation. Trends Plant Sci 7:309–315
Colangelo E, Guerinot M (2004) Put the metal to the petal: metal uptake and transport throughout plants. Curr Opin Plant Biol 9:322–330
Curie C, Cassin G, Couch D, Divol F, Higuchi K, Le Jean M (2009) Metal movement within the plant: contribution of nicotianamine and yellow stripe 1-like transporters. Ann Bot 103:1–11
Delhaize E, Kataoka T, Hebb DM, White RG, Ryan PR (2003) Genes encoding proteins of the cation diffusion facilitator family that confer manganese tolerance. Plant Cell 15:1131–1142
Drager DB, Desbrosses-Fonrouge AG, Krach C, Chardonnens AN, Meyer RC, Saumitou-Laprade P, Kramer U (2004) Two genes encoding Arabidopsis halleri MTP1 metal transport proteins co-segregate with zinc tolerance and account for high MTP1 transcript levels. Plant J 39:425–439
Eckhardt U, Mas Marques A, Buckhout TJ (2001) Two iron-regulated cation transporters from tomato complement metal uptake-deficient yeast mutants. Plant Mol Biol 45:437–448
Eide D, Guarente L (1992) Increased dosage of a transcriptional activator gene enhances iron-limited growth of Saccharomyces cerevisiae. J Gen Microbiol 138:347–354
Eide D, Broderius M, Fett J, Guerinot M (1996) A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc Natl Acad Sci U S A 93:5624–5628
Englbrecht CC, Schoof H, Bohm S (2004) Conservation, diversification and expansion of C2H2 zinc finger proteins in the Arabidopsis thaliana genome. BMC Genomics 5:39–55
Eren E, Arguello J (2004) Arabidopsis HMA2, a divalent heavy metal-transporting PIB-Type ATPase, is involved in cytoplasmic Zn2+ homeostasis. Plant Physiol 136:3712–3723
Fasim FN, Ahmed R, Parsons GM (2002) Solubilization of zinc salts by bacterium isolated by the air environment of tannery. FEMS Microbiol Lett 213:1–6
Gaither LA, Eide DJ (2000) Functional expression of the human hZIP2 zinc transporter. J Biol Chem 275(8):5560–5564
Gaither LA, Eide DJ (2001) Eukaryotic zinc transporters and their regulation. Biometals 14:251–270
Gao X, Kuyper TW, Zou CF, Zhang HE (2007) Mycorrhizal responsiveness of aerobic rice genotypes is negatively correlated with their zinc uptake when nonmycorrhizal. Plant Soil 290:283–291
Gendre D, Czernic P, Conejero G, Pianelli K, Briat JF, Lebrun M, Mari S (2007) TcYSL3, a member of the YSL gene family from the hyperaccumulator Thlaspi caerulescens, encodes a nicotianamine-Ni/Fe transporter. Plant J 49:1–15
Giri B, Giang PH, Kumari R, Prasad R, Varma A (2005) Microbial diversity in soils. In: Buscot F, Varma S (eds) Micro-organisms in soils: roles in genesis and functions. Springer, Heidelberg, pp 195–212
Glick BR, Bashan Y (1997) Genetic manipulation of plant growth-promoting bacteria to enhance biocontrol of phytopathogens. Biotechnol Adv 15:353–378
Grass G, Wong MD, Rosen BP, Smith RL, Rensing C (2002) ZupT is a Zn(II) uptake system in Escherichia coli. J Bacteriol 184:864–866
Grotz N, Fox T, Connolly E, Park W, Guerinot ML, Eide D (1998) Identification of a family of zinc transporter genes from Arabidopsis that respond to zinc deficiency. Proc Natl Acad Sci U S A 95:7220–7224
Guerinot ML (2000) The ZIP family of metal transporters. Biochim Biophys Acta 1465:190–198
Gurmani AR, Khan SU, Andaleep R, Waseem K, Khan A (2012) Soil application of zinc improves growth and yield of tomato. Int J Agric Biol 14:91–96
Gustin JL, Zanis MJ, Salt DE (2011) Structure and evolution of the plant cation diffusion facilitator family of ion transporters. BMC Evol Biol 11:76
Hacisalihoglu G, Kochian LV (2003) How do some plants tolerate low levels of soil zinc? Mechanisms of zinc efficiency in crop plants. New Phytol 159(2):341–350
Hacisalihoglu G, Hart JJ, Kochian LV (2001) High-and low-affinity zinc transport systems and their possible role in zinc efficiency in bread wheat. Plant Physiol 125:456–463
Hacisalihoglu G, Hart JJ, Wang Y, Cakmak I, Kochian LV (2003) Zinc efficiency is correlated with enhanced expression and activity of Cu/Zn superoxide dismutase and carbonic anhydrase in wheat. Plant Physiol 131:595–602
Hajiboland R, Amirazad F (2010) Growth, photosynthesis and antioxidant defense system in Zn-deficient red cabbage plants. Plant Soil Environ 56:209–217
Hassan Z, Aarts M (2011) Opportunities and feasibilities for biotechnological improvement of Zn, Cd or Ni tolerance and accumulation in plants. Environ Exp Bot 72:53–63
Havlin JL, Tisdale SL, Nelson WL, Beaton JD (2014) Soil fertility and nutrient management: an introduction to nutrient management, 8th edn. Pearson, Upper Saddle River, p 505
Hodgson JF (1963) Chemistry of the micronutrient elements in soils. Adv Agron 15:119–159
Hoffland E, Wei C, Wissuwa M (2006) Organic anion exudation by lowland rice (Oryza sativa L.) at zinc and phosphorus deficiency. Plant Soil 283(1):155–162
Hussain D, Haydon MJ, Wang Y, Wong E, Sherson SM, Young J, Camakaris J, Harper JF, Cobbett CS (2004) P-type ATPase heavy metal transporters with roles in essential zinc homeostasis in Arabidopsis. Plant Cell 16:1327–1339
Ikram A, Mahmud AW, Ghani MN, Ibrahim MT, Zainal AB (1992) Field nursery inoculation of Hevea brasiliensis Muell. Arg. seedling rootstock with vesicular–arbuscular mycorrhizal (VAM) fungi. Plant Soil 145:231–236
Imran M, Arshad M, Khalid A, Kanwal S, Crowley DE (2014) Perspectives of rhizosphere microflora for improving Zn bioavailability and acquisition by higher plants. Int J Agric Biol 16:653–662
Jansa J, Mozafar A, Frossard E (2003) Long–distance transport of P and Zn through the hyphae of an arbuscular mycorrhizal fungus in symbiosis with maize. Agron 23:481–488
Khalid A, Arshad M, Shaharoona B, Mahmood T (2009) Plant growth promoting rhizobacteria and sustainable agriculture. In: Khan MS, Zaidi A, Musarrat J (eds) Microbial strategies for crop improvement. Springer, Berlin/Heidelberg, pp 133–160
Kim YY, Choi H, Segami S, Cho HT, Martinoia E, Maeshima M, Lee Y (2009) AtHMA1 contributes to detoxification cation of excess Zn(II) in Arabidopsis. Plant J 58:737–753
Klatte M, Schuler M, Wirtz M, Fink-Straube C, Hell R, Bauer P (2009) The analysis of Arabidopsis nicotianamine synthase mutants reveals functions for nicotianamine in seed iron loading and iron deficiency responses. Plant Physiol 150:257–271
Kochian LV (1993) Zinc absorption from hydroponic solutions by plant roots. In: Robson AD (ed) Zinc in soils and plants. Kluwer, Dordrecht, pp 45–57
Kochian LV (2000) Molecular physiology of mineral nutrient acquisition, transport, and utilization. Biochem Mol Biol Plants 20:1204–1249
Koide RT, Kabir Z (2000) Extra radical hyphae of the mycorrhizal fungus Glomus intraradices can hydrolyse organic phosphate. New Phytol 148:511–517
Kupper H, Zhao FJ, McGrath SP (1999) Cellular compartmentation of zinc in leaves of the hyperaccumulator Thlaspi caerulescens. Plant Physiol 119:305–311
Lasat MM, Kochian LV (2000) Physiology of Zn hyperaccumulation in Thlaspi caerulescens. In: Terry N, Banuelos G (eds) Phytoremediation of contaminated soil and water. CRC Press/LLC, Boca Raton, pp 159–169
Lasat MM, Baker AJ, Kochian LV (1996) Physiological characterization of root Zn2+ absorption and translocation to shoots in Zn hyperaccumulator and nonaccumulator species of Thlaspi. Plant Physiol 112:1715–1722
Liang YC, Chen Q, Liu Q, Zhang WH, Ding RX (2003) Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt – stressed barley (Hordeum vulgare L.). J Plant Physiol 160:1157–1164
Liu A, Hamel C, Hamilton RI, Ma BL (2000) Acquisition of Cu, Zn, Mn and Fe by mycorrhizal maize (Zea mays L.) grown in soil at different P and micronutrient levels. Mycorrhiza 9:331–336
Lucy M, Reed E, Glick BR (2004) Applications of free living plant growth – promoting rhizobacteria. Anton Leeuw 86:1–25
Luo ZB, He XJ, Chen L, Tang L, Gao S, Chen F (2010) Effects of zinc on growth and antioxidant responses in Jatropha curcas seedlings. Int J Agric Biol 12:119–124
MacDiarmid CW, Gaither LA, Eide D (2000) Zinc transporter that regulate vacuolar Zinc storage in Saccharomyces cerevisiae. EMBO J 19(12):2845–2855
Mandal B, Hazra GC, Pal AK (1988) Transformation of zinc in soils under submerged condition and its relation with zinc nutrition of rice. Plant Soil 106:121–126
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic, London
Marschner H (1997) Mineral nutrition of higher plants. Academic, San Diego, p 889
Martino E, Perotto S, Parsons R, Gadd GM (2003) Solubilization of insoluble inorganic zinc compounds by ericoid mycorrhizal fungi derived from heavy metal polluted sites. Soil Biol Biochem 35:133–141
McCall KA, Huang C, Carol AF (2000) Function and mechanism of zinc metalloenzymes. J Nutr 130:1437–1446
Moreau S, Thomson RM, Kaiser BN, Trevaskis B, Guerinot ML, Udvardi MK, Puppo A, Day DA (2002) GmZIP1 encodes a symbiosis-specific zinc transporter in soybean. J Biol Chem 277:4738–4746
Obrador A, Novillo J, Alvarez JM (2003) Mobility and availability to plants of two zinc sources applied to a calcareous soil. Soil Sci Soc Am J 67:564–572
Ortas I, Ortakçi D, Kaya Z, Cinar A, Onelge N (2002) Mycorrhizal dependency of sour orange in relation to phosphorus and zinc nutrition. J Plant Nutr 25:1263–1279
Palmer C, Guerinot M (2009) Facing the challenges of Cu, Fe and Zn homeostasis in plants. Nat Chem Biol 5:333–340
Pence NS, Larsen PB, Ebbs SD, Letham DLD, Lasat MM, Garvin DF, Eide D, Kochian LV (2000) The molecular physiology of heavy metal transport in the Zn/Cd hyperaccumulator Thlaspi caerulescens. Proc Natl Acad Sci U S A 97:4956–4960
Persans MW, Nieman K, Salt DE (2001) Functional activity and role of cation-efflux family members in Ni hyperaccumulation in Thlaspi goesingense. Proc Natl Acad Sci U S A 98:9995–10000
Reid RJ, Brookes JD, Tester MA, Smith FA (1996) The mechanism of zinc uptake in plants. Planta 198:39–45
Rengel Z, Wheal MS (1997) Kinetic parameters of zinc uptake by wheat are affected by the herbicide chlorsulfuron. J Exp Bot 48:935–941
Robson AD (1994) Zinc in soils and plants. Springer, New York
Ryan MH, Angus JF (2003) Arbuscular mycorrhizae in wheat and field pea crops on a low P soil: increased Zn-uptake but no increase in P uptake or yield. Plant Soil 250:225–239
Ryan MH, McCully ME, Huang CX (2007) Relative amounts of soluble and insoluble forms of phosphorus and other elements in intraradical hyphae and arbuscules of arbuscular mycorrhizas. Funct Plant Biol 34:457–464
Sadaghiani MR, Barin M, Jalili F (2008) The effect of PGPR inoculation on the growth of wheat. International meeting on soil fertility land management and agroclimatology, Turkey, pp 891–898
Saravanan VS, Subramoniam SR, Raj SA (2004) Assessing in vitro solubilization potential of different zinc solubilizing bacterial (zsb) isolates. Brazil J Microbiol 35:121–125
Saravanan VS, Madhaiyan M, Thangaraju M (2007) Solubilization of zinc compounds by the diazotrophic, plant growth promoting bacterium Gluconacetobacter diazotrophicus. Chemosphere 66:1794–1798
Sattelmacher B (2001) The apoplast and its significance for plant mineral nutrition. New Phytol 149:167–192
Simine CDD, Sayer JA, Gadd GM (1998) Solubilization of zinc phosphate by a strain of Pseudomonas fluorescens isolated from a forest soil. Biol Fertil Soils 28(1):87–94
Smith S, Read DJ (1997) Mycorrhizal symbiosis. Academic, London, pp 11–33
Song WY, Choi KS, Kim Y, Geisler M, Park J, Vincenzetti V, Schellenberg M, Kim SH, Lim YP, Noh EW, Lee Y, Martinoia E (2010) Arabidopsis PCR2 is a zinc exporter involved in both zinc extrusion and long-distance zinc transport. Plant Cell 22:2237–2252
Subramanian KS, Tenshia V, Jayalakshmi K, Ramachandran V (2009) Role of arbuscular mycorrhizal fungus (Glomus intraradices)–(fungus aided) in zinc nutrition of maize. Agric Biotechnol Sustain Dev 1:29–38
Swaminathan K, Verma BC (1979) Response of three crop species to vesicular arbuscularmycorrhizal infection on Zinc deficient Indian soils. New Phytol 114:1–38
Tapiero H, Tew KD (2003) Trace elements in human physiology and pathology: zinc and metallothioneins. Biomed Pharmacother 57:399–411
Tarafdar JC, Marschner H (1994) Efficiency of VAM hyphae in utilization of organic phosphorus by wheat plants. Soil Sci Plant Nutr 40:593–600
Tariq M, Hameed S, Malik KA, Hafeez FY (2007) Plant root associated bacteria for zinc mobilization in rice. Pak J Bot 39:245–253
Tarkalson DD, Jolley VD, Robbins CW, Terry RE (1998) Mycorrhizal colonization and nutrition of wheat and sweet corn grown in manure‐treated and untreated topsoil and subsoil. J Plant Nutr 21(9):1985–1999. doi:10.1080/01904169809365538
Tejada M, Hernandez MT, Garcia C (2006) Application of two organic amendments on soil restoration: effects on the soil biological properties. J Environ Qual 35:1010–1017
Ueno D, Yamaji N, Kono I, Huang CF, Ando T, Yano M, Feng J (2010) Gene limiting cadmium accumulation in rice. Proc Natl Acad Sci U S A 107:16500–16505
Ueno D, Milner MJ, Yamaji N, Yokosho K, Koyama E, Clemencia Zambrano M, Kaskie M, Ebbs S, Kochian LV, Feng J (2011) Elevated expression of TcHMA3 plays a key role in the extreme Cd tolerance in a Cd-hyperaccumulating ecotype of Thlaspi caerulescens. Plant J 66:852–862
van de Mortel JE, Almar Villanueva L, Schat H, Kwekkeboom J, Coughlan S, Moerland PD, Themaat EVL, Koornneef M, Arts MGM (2006) Large expression differences in genes for iron and zinc homeostasis, stress response, and lignin biosynthesis distinguish roots of Arabidopsis thaliana and the related metal hyperaccumulator Thlaspi caerulescens. Plant Physiol 42:1127–1147
van der Zaal BJ, Neuteboom LW, Pinas JE, Chardonnens AN, Schat H, Verkleij JAC, Hooykaas PJJ (1999) Overexpression of a novel Arabidopsis gene related to putative zinc transporter genes from animals can lead to enhanced zinc resistance and accumulation. Plant Physiol 119:1047–1055
Vert G, Grotz N, Dedaldechamp F, Gaymard F, Guerinot ML, Briat JF, Curie C (2002) IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 14:1223–1233
Wang P, Bi S, Ma L, Han W (2006) Aluminum tolerance of two wheat cultivars (Brevor and Atlas66) in relation to their rhizosphere pH and organic acids exuded from roots. J Agric Food Chem 54:10033–10039
Waters B, Sankaran R (2011) Moving micronutrients from the soil to the seeds: genes and physiological processes from a biofortification perspective. Plant Sci 180:562–574
Welch RM (1995) Micronutrient nutrition of plants. Crit Rev Plant Sci 14:49–82
White PJ, Whiting SN, Baker AJM, Broadley MR (2002) Does zinc move apoplastically to the xylem in roots of Thlaspi caerulescens? New Phytol 153:201–207
Whiting SN, Desouza M, Terry N (2001) Rhizosphere bacteria mobilize Zn for hyperaccumulator by Thlaspi caerulescens. Environ Sci Technol 35:3144–3150
Willams DJ, Hall KB (2000) Experimental and computational studies of the G (UUCG)C RNA tetraloop. J Mol Biol 297(5):1045–1061
Wu SC, Cheung KC, Luo YM (2006) Wong effects of inoculation of plant growth promoting rhizobacteria on metal uptake by Brassica juncea. Environ Pollut 140:124–135
Wu QS, Li GH, Zou YN (2011) Roles of arbuscular mycorrhizal fungi on growth and nutrient acquisition of peach (Prunus persica L. Batsch) seedlings. J Anim Plant Sci 21:746–750
Zhang FS, Romheld V, Marschner H (1991) Diurnal rhythm of release of phytosiderophores and uptake rate of zinc in iron-deficient wheat. J Plant Nutr Soil Sci 37:671–678
Zhao H, Eide D (1996a) The ZRT2 gene encodes the low-affinity zinc transporter in Saccharomyces cerevisiae. J Biol Chem 271:23203–23210
Zhao H, Eide DJ (1996b) The yeast ZRT1 gene encodes the zinc transporter protein of a high-affinity uptake system induced by zinc limitation. Proc Natl Acad Sci U S A 93:2454–2458
Zhao FJ, Hamon RE, Lombi E, McLaughlin MJ, McGrath SP (2002) Characteristics of cadmium uptake in two contrasting ecotypes of the hyper-accumulator Thlaspi caerulescens. J Exp Bot 53:535–543
Zia MS, Aslam M, Baig MB, Ashraf A (1999) Fertility issues and fertilizer management in rice–wheat system: a review. Quart Sci Vis 5:59–73
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer India
About this chapter
Cite this chapter
Kumar, L., Meena, N.L., Singh, U. (2016). Zinc Transporter: Mechanism for Improving Zn Availability. In: Singh, U., Praharaj, C., Singh, S., Singh, N. (eds) Biofortification of Food Crops. Springer, New Delhi. https://doi.org/10.1007/978-81-322-2716-8_11
Download citation
DOI: https://doi.org/10.1007/978-81-322-2716-8_11
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-2714-4
Online ISBN: 978-81-322-2716-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)