Abstract
PET radiopharmaceuticals are currently produced using a centralized approach, which makes sustainable the distribution to few imaging centers of an only small set of tracers (virtually only [18F]FDG). However, a wider set of structures have demonstrated a potential applicability for imaging in a specific manner several disease condition. In order to allow this wider and more personalized use of PET imaging, the production paradigms need to be changed. In this contribution we will explain how Dose-On-Demand systems can be conceptualized and what are the challenges that are still to be overcome in order for such approach to be of widespread utility.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The clinical production of radiopharmaceuticals or radiotracers for positron-emitting tomography (PET) is currently performed in centralized locations such as commercial radiopharmacies or some dedicated radiochemistry facilities. Generally, these facilities contain a cyclotron to produce the PET radioisotope and laboratories furnished with lead-shielded hot cells containing automated radiosynthesis modules to produce the radiotracer. Quality control equipment is also required to validate and confirm the purity of the radiotracer prior to its dispatch to the imaging centers.
The majority of radiotracer production facilities synthesize [18F]FDG, the gold standard for detecting a variety of cancers. Nowadays, [18F]FDG can be produced in a large batch, making it relatively affordable. Portions can then be dispatched and transported by road or air to the relevant hospital owing to the half-life of fluorine-18 (110 min).
A major challenge in clinical PET radiochemistry is that there are a greater number of hospitals or PET clinics than there are PET radiotracer production facilities. Furthermore, the demand for new clinical PET radiotracers is low due to the cost of production in a centralized location. New PET radiotracers are overwhelmingly used for research purposes only.
To overcome this obstacle, a decentralized approach has been envisaged [1]. Here, scientists could produce their radiotracer of interest in-house, economically and on demand, leading to a concept that we have defined as Dose On Demand (DOD). This short review will cover the important aspects of DOD and detail the journey toward the DOD of short-lived radiopharmaceuticals.
2 DOD Features
The current production approach of PET radiotracers imposes several limitations and challenges for guaranteeing the most efficient organization of imaging studies [2]. A possible way to improve this situation would require a system for which the type and the quantity of the produced tracer is defined and directly handled by an as final as possible user (e.g., hospital pharmacy, imaging laboratory). This system should implement the reduction to the minimum possible of the amounts of radioactivity and chemicals needed in the preparation, added to an overall simplification of the production process. Such conceptual process can be defined as “Dose On Demand” (DOD) [3, 4, 5]: the operation of producing a radiopharmaceutical in the shortest time possible, using the minimum amount of chemicals and radioactivity strictly needed for the production of the single (or few) imaging dose(s) required.
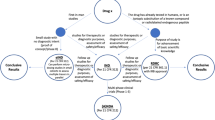
This approach, exemplified in Fig. 6.1, would provide several benefits to the overall PET community. Firstly, it will hand over flexibility in the application directly to the hospital/imaging center, which can decide on a patient basis which tracer to produce and when; this could also happen in small regional centers, thus not forcing anymore interested patients to commute long distances to the few useful imaging hospitals. This flexibility will also allow the utilization of rare or research tracers to be facilitated, as in this system the small amounts of chemicals and radioactivity needed for a DOD production would be economically sustainable for a single imaging center. Secondly, while a fault in production from a centralized approach will have impact on a large number of patients, a fault in one DOD system will have an impact limited to the patients utilizing those doses only. Lastly, due to the reduction in raw materials needed (as well as related topics, e.g., safety, storage) and the redistribution of running costs over more institutes, the imaging doses will result in a reduced cost and in tracers’ availability to a wider population.
In order to realize a DOD process, few requirements can be envisaged.
Firstly, the production needs to be implemented on an automated instrument that can implement preset operations, as well as allowing remote interaction of the operator for minor modifications (i.e., “Automation”). In addition, it has to have real-time monitoring and audit trail capabilities for monitoring and trending purposes. Secondly, the instrument used needs to be able to handle small aliquots of reagents, from fractions to hundreds of μL (i.e., “Discretization”) with accuracy and repeatability. The handling operations comprise moving, merging, mixing, heating, and similar processes. In other words, the instrument has to be capable to give a defined “chemical history” to any given aliquot of reagents used (that can be different among several aliquots). Thirdly, the processes implemented need to be serially repeatable with minimum operator intervention (“Restarting”). This can be achieved by substituting single-use parts in the system or by cleaning it using validated procedures. A final peculiarity that contributes to achieving DOD is the need to use the minimum amount of chemicals (“Reduction”), which would maximize the atom efficiency of the process, as well as allow an acceptable sacrifice in employing single-use parts or realize a faster cleaning of the system.
If all these requirements can be respected in a process system, a DOD production can be implemented and can be used to produce several doses of the same tracer or of different tracers, using the same system and minimal operator interventions.
3 Early Examples Conducible to DOD
The possibility to tailor the production of nuclear medicine (NM) tracers as much as possible to the needs of the final user has always been present, and indeed some example of approaches that can be linked to DOD concept can be found in early and current practices.
Generators of radioactive raw nuclides have been widely used (e.g., 99mTc, 68Ga) [6]; the elution of the desired radionuclide is generally done upon demand and followed by simple chemical reactions (generally performed directly in the same NM department) to obtain the final radiopharmaceutical. This approach respects the Restarting requirement, though limited to the raw nuclide production and not to the pharmaceutical preparation; however, it generally does not respect the Discretization nor the Reduction requirements, as it is difficult to separately handle the amount of chemicals needed for a single patient (i.e., in the μLs range). It sometimes respects the Automation requirement, but in most NM departments, these preparations are performed manually.
The use of very short half-life nuclides can be natively defined as a DOD application, as their handling must be done shortly before their use in imaging. Typical examples are the production of [15O]-H2O [7] and [13N]-NH3 [8]; shortly after production, the systems utilized can be restarted easily due to their simple setup and the fast decay of process wastes. However, also in this case, these processes cannot be properly defined as DOD since they generally respect the Automation and the Restarting requirements, but neither the Discretization nor the Reduction (i.e., no difference if the production is used for one or several contemporary patients). 11C chemistry [8] also falls within the use of very short half-life nuclides as more productions can be run on the same machine on the same day. However, also in this case, the typical systems used can be even less defined as DOD, due to its relatively longer half-life (compared to 15O and 13N).
Currently, the approach that most resembles DOD is represented by the cassette-based systems. In this case, Automation and Restarting requirements can be easily achieved; Discretization and Reduction are generally not pursued, because nowadays these systems are used for single-batch productions, but the principles underlying cassette philosophy could be used to project single-use/single-dose cassettes. In fact, these systems are basically very compact macrofluidic systems; this understanding clarifies how microfluidic concepts can be the ones that should allow a full implementation of DOD in radiopharmaceutical production.
4 DOD Proof-of-Principle Examples
4.1 Minicyclotron/Minichemistry/MiniQC
The Biomarker Generator (BG75), made by ABT Molecular Imaging, is a small (0.37 m × 1.25 m) self-shielded 7.5 MeV cyclotron coupled to an aseptic single-use card-based automated chemical production module and an automated module for quality control. The BG75 was initially used for the DOD production of [18F]fluoride and [18F]FDG [9]. Using the computer’s software, the operator is able to select whether the [18F]fluoride or [18F]FDG is to be produced. For the production of [18F]fluoride (~1 mCi/min at 5 μA), the process is complete once the product is delivered into the specified vial. Alternatively, for the production of [18F]FDG, the software prompts the operator to prepare the tracer-specific Dose Synthesis Card (DSC) and the chemistry and quality control modules while the cyclotron is preparing the [18F]fluoride. The radiosynthesis is then completed on the DSC, including relevant purification, and dispensed into a shielded, sterile syringe or vial. An aliquot of the product is removed for quality control (pH determination, acetonitrile and ethanol residual solvent determination, radiochemical identity and purity, Kryptofix 2.2.2. determination, and a filter integrity test), which is automatically performed by the system, without any operator input.
The BG75 has been able to consistently produce a 10–13 mCi dose of [18F]FDG at 40 min intervals up to six times per day, with products meeting the required USP limits for release [9]. To date, other DOD radiotracers synthesized using the BG75 include Na[18F]F [10] and [18F]FMISO [11, 12].
4.2 Continuous Flow Microfluidics
Interestingly, proof-of-concept studies have been recently conducted into the production of [18F]FLT using the cyclotron component of the BG75 system and the Advion NanoTek microfluidic system [13]. Between 70 and 80 mCi of [18F]fluoride were produced by the minicyclotron and the radiosynthesis was subsequently performed under continuous-flow microfluidic conditions to yield [18F]FLT in sufficient quantity and purity for clinical trials. The number of radiochemists using a microfluidic approach has been steadily accumulating in recent years. This may be related to the advantages of microfluidic systems over traditional automated radiochemistry modules, which include a decrease in the amount chemical reagents used, shorter reaction times, greater radiochemical yields, the ability to use solvents under supercritical conditions, and reduced radiation exposure to the operator due to the lower amounts of radioactivity used. The NanoTek Microfluidic Synthesis System by Advion was the first commercially available continuous-flow microfluidic system. The system comprises a concentrator module to azeotropically evaporate the [18F]fluoride from the cyclotron and subsequently reconstitute the isotope into the appropriate solvent; a pump module containing two syringe pumps and loops to store chemical precursors and a reactor module, which contains a syringe pump and loop to house the isotope along with thermostatted slots to store up to four microreactors, where the radiochemical reactions occur.
The previous example of the production of [18F]FLT is the latest in a growing list of radiotracers prepared using the NanoTek system. The first instance was the production of [18F]fallypride for use in micro-PET studies [14]. Initially, the radiochemical optimization of [18F]fallypride was conducted by dispensing 10 μL solutions of the tosyl-fallypride precursor and [18F]fluoride complex into the microreactor at 10 μL/min to obtain 1–1.5 mCi doses of [18F]fallypride. These optimization reactions were performed sequentially and could be considered an early form of DOD. Once the optimal radiochemical conditions were determined, the authors were able to prepare a dose of [18F]fallypride sufficient for human injection (15 mCi) by increasing the volume of the two solutions from 10 μL to 200 μL. The authors also alluded to the fact that multiple high doses of [18F]fallypride could be produced using the same microreactor. Soon after, Pascali et al. [15] described the sequential radiolabeling of ethyl-ditosylate and propyl-ditosylate in the NanoTek system using the same solution of [18F]fluoride complex and swapping the precursor between productions by emptying and refilling the precursor loop with a different substrate. These examples of DOD demonstrated the economical use of the [18F]fluoride solution to yield two radiotracers on the same day. The authors also sequentially prepared several injectable doses of [18F]CB102, a cannabinoid type 2 receptor agonist, for small animal PET imaging, suggesting that freshly prepared doses using a DOD approach were superior to a batch solution to be used over a certain shelf life.
To further evaluate the robustness and reliability of a DOD approach, Pascali et al. were able to produce three sequential doses of three different [18F]fluorocholines with a total processing time of 13–15 min for each dose, including SPE purification [5]. While this example includes a modification to the NanoTek system to incorporate SPE purification, typically, the radiochemical outputs are purified externally to the NanoTek system, particularly HPLC purification. Recent examples include the preparation of [18F]FPEB [16], whereby the radiosynthesis occurred in the NanoTek and the reaction output was sent to a vial preloaded with water and pre-concentrated onto an Oasis HLB Light SPE cartridge to remove DMSO present in the reaction mixture. The cartridge was eluted with acetonitrile and water before being transferred to a GE TRACERlab FXFN synthesis unit to conduct semi-preparative HPLC purification and formulation. Additionally, the Tau imaging agent, [18F]T807, was produced with the same modifications [17]. Three consecutive >100 mCi productions of [18F]T807 were performed for validation purposes, and [18F]T807 became the first example of human use of a radiopharmaceutical prepared by continuous-flow microfluidics.
The NanoTek system has been modified recently to include HPLC and SPE purification [18]. By utilizing the cable harnessing of the system, a custom-made electrical board was engineered whereby additional switches and analog signals could be added and be controlled by the NanoTek software to activate externally powered devices and record external signals (e.g., detectors), if applicable. This customized system was able to produce 1- or 2-step radiotracers such as [18F]CB102, [18F]fluoroethylcholine [18], [18F]MEL050 (melanin targeting) [19], [18F]fallypride, and [18F]PBR111 (TSPO receptor) [20], in a DOD manner. Similarly, [18F]FMISO has been produced by integrating a HPLC system to the NanoTek through a six-port valve [21]. By fine-tuning the HPLC conditions for [18F]FMISO, the authors were able to eliminate the requirement for SPE.
4.3 Peptide Labeling
While microfluidic systems have mainly been utilized to radiolabel small molecules, reports of peptide or protein radiolabeling using microfluidics are limited. Early work in this area featured the direct [18F]radiolabeling of bombesin derivatives (with 7–8 amino acid residues) that had been modified to incorporate trimethylammonium or triarylsulfonium leaving groups [22]. The peptides could be radiolabeled reproducibly, suggesting a possible DOD approach; however, due to the harsh temperature conditions required for radiolabeling, this method would be unsuitable for protein radiolabeling. An alternative route to radiolabel a peptide is through the use of a prosthetic group as an indirect radiolabeling method. [18F]SFB [23] and even, the most abundantly used PET tracer, [18F]FDG [24] have been utilized as prosthetic groups for the radiolabeling of peptides. Although both prosthetic groups were synthesized on macroscale equipment, the subsequent peptide radiolabeling was performed under microfluidic conditions. In each case, the peptide was radiolabeled in a shorter period of time, in higher radiochemical yield (RCY), and using a smaller quantity of the peptide compared to conventional radiolabeling techniques. Only recently has the first microfluidic radiosynthesis of a prosthetic group and the ensuing peptide radiolabeling been reported [25]. Here, the [18F]F-Py-TEP prosthetic group was prepared in the first microreactor of an Advion NanoTek system from [18F]fluoride and the corresponding precursor. After exiting the microreactor, the [18F]F-Py-TEP was transferred to a second microreactor, where it reacted with a model peptide containing free amines. Once again, the peptide coupling was faster than conventional methods and obtained in higher RCY. These accounts all imply that the DOD of radiolabeled peptides for molecular imaging is currently being explored and may be employed in the future.
4.4 Solid-Phase Approaches
Although the use of microfluidic conditions is leading to radiochemical reactions being completed in less time than traditional approaches, to further decrease the overall radiochemical processing times, new methods are required to decrease or eliminate the time taken to process and activate the starting [18F]fluoride. One option is to trap the [18F]fluoride onto a resin and subsequently perform on-resin radiofluorinations, thus eliminating the need for azeotropic evaporations and re-solubilization of the [18F]fluoride complex. Reusable polymer-supported phosphazenes have been investigated as suitable resins to perform the [18F]fluoride trapping and radiofluorination [26]. The PS-P2 tBu resin was able to trap >99% of [18F]fluoride, with no leaching of activity was observed when the column was subsequently dried with helium gas. It was found that substrates with sulfonate leaving groups resulted in the highest RCY when subjected to on-column radiofluorination. The same phosphazene resin could be recycled at least three times using the same substrate, or at least two times using a different substrate, which implies that the DOD production of radiotracers is possible through solid-phase radiofluorination.
Other work in this area includes a continuous-flow system comprising a polystyrene-imidazolium-chloride (PS-Im+Cl−) monolith which traps [18F]fluoride [27]. A solution of base and the relevant precursor could then be flowed through the PS-Im+[18F]F− monolith into a preheated microfluidic chip where the radiochemical reaction takes place. The advantage of this method is that the entire process is performed in continuous flow and the microfluidic platform has a very small footprint compared to current processes.
4.5 Droplet Systems
An interesting extension in the field of microfluidic radiochemistry is through the use of droplets. Also sometimes referred to as segmented flow chemistry, it features droplets (nL- μL) which are separated by an immiscible carrier fluid, similar to oil droplets in water. Droplets can be thought of as individual nano- or microreactors and can be used to aid radiolabeling optimization, whereby each droplet is the result of a predetermined set of reaction parameters. Droplets consisting of approximately 120 nL were formed during the coupling of [18F]FSB with an anti-prostate stem cell antigen diabody [28]. Using a 5 μL sample of the diabody solution was sufficient to screen over 100 different reaction conditions using the droplets, and hence, the optimal reaction conditions were determined rapidly with minimal use of the precious diabody solution. Droplet systems have also been utilized in electrowetting-on-dielectric (EWOD) devices. In EWOD systems, droplets are sandwiched between two plates; the bottom plate consists of electrode pads to manipulate the movement of the droplets throughout the microchip, while the top plate electrically grounds the droplets. The EWOD chip was first used in the synthesis of [18F]FDG [29], but its use has more recently expanded to include [18F]FLT [30], [18F]fallypride [31], and [18F]SFB [32]. While the EWOD chip produces these radiolabeled molecules in comparable, if not greater, RCY than previously, drawbacks of the system include off-chip purification and the potential for radioactivity and volatile side products to escape since the chip is exposed to air. It is envisaged that with advances in technology, the EWOD chip could be further automated, be disposable, and lead to scientists producing their desired radiotracer on demand.
5 Challenges and Future of DOD
As it can be seen, a perfect DOD system is still not existent, but several data are available demonstrating that such approach should represent the reality in the next future. However, to witness this paradigmatic change, several challenges need to be fronted, and they will represent the future of DOD research in radiopharmaceutical production.
One of the biggest challenges is to understand whether one only system could achieve the desired spread of operations that a DOD process should implement. This should cover not only the production steps but also the switching of chemicals, cleaning, and priming steps. It is very likely that these systems will be based on micronized approaches (e.g., microfluidics, nanodroplets), but understanding which philosophy they should implement is still under discussion. For example, a system can be projected to implement several different preexisting routes, which would lead to different products in different quantities, or, on the contrary, be represented by a fixed framing to which flexibly interface single-use components/modules (i.e., similar to microcassettes) to build up the desired process. Another possibility might also be represented by the possibility to use the exact same system into which different chemicals are delivered, depending on the production needs. All these options are amenable to deliver a DOD system, but the choice of one or the other will drive the final performance and actual ease/flexibility of use.
Even once the underlying philosophy is clarified, some technical problems are still unsolved or partially addressed. Purification of the finished product represents probably the most important issue, and while there are several excellent systems to perform chemical reactions, there is a notable lack in miniaturization of purification methods or their interfacing with micronized chemistry systems. Some research is now available on micro-chromatographic systems [33], mainly facilitated by the advancements of monolith polymers [34] that can be easily integrated with micron-sized channels/reservoirs [35]. These solutions allow the reduction of inherent void volumes, therefore improving the atom efficiency in the purification process. Also, similar solutions may be useful for the cases in which a simple solid-phase extraction (SPE) would be sufficient to purify the relevant molecule [36, 37]. Polymer chemistry advancement possibly represents the field where useful innovations can have a relevant impact on miniaturized purifications. As an example, molecularly imprinted polymers (MIP) represent a promising approach that would allow to streamline the selective separation of the molecule of interest and its efficient elution [38]. MIP structures are prepared by building the polymer pores around a desired template molecule; once the template is removed, the material acquires selectivity of shape and electronic interaction (i.e., with functional groups of polymer) for the desired molecule [39]. MIP systems are in fact also referred as “synthetic receptors.”
Another innovation that would be generally useful in radiochemistry but particularly applicable to DOD systems (due to their preferred micronized nature) is the use of supported precursors. These systems should be projected in such a way that the labeling reaction is the only event that would make the molecular structure to become free from the solid support bond. In this way, no other complex organic species will be present in the resulting mixture, and only simple filtration and reformulation steps would be required in order to retrieve the radiopharmaceutical. Some systems based on supported sulfonates [40] or triazene [41] have been reported up to date, and patent literature also refers to examples of supported ionic precursors (e.g., iodonium compounds) [42]. However, none of these systems have demonstrated a preferential use compared to traditional methods, probably because of the mismatch between the support active surface and reagent accessibility to it. The use of micronized systems could be beneficial in solving this mismatch, and indeed the use of sulfonate precursors supported on a monolith structure grown directly in a microfluidic chip gave satisfying yields of radiofluorination [43].
A further modification to this approach, which would facilitate the respect of DOD requirements, could be the use of reversibly linked precursors. In this concept, the precursor should form a bond (e.g., covalent coordination) with the support material, and as usual, the labeling reaction should be able to selectively cleave the structure from the support; however, in a second “recycling” step, a change in conditions will allow the recovery of the precursor out of the system and offer a support free to be reversibly functionalized again with a different precursor. A recent paper reported the catalysis by TiO2 nanoparticles in radiofluorinating a tosylate precursor [44]; interestingly enough, the authors suspect that the process is catalyzed due to selective coordination of the tosylate moiety with the titania surface, therefore opening to the idea of a reversible functionalization of metal nanoparticles with several different precursors prior to radiolabeling. Another possibility, drawn from the field of self-healing materials, might be the use of reversible click reactions, a nice example of which is represented by the 1,2,4-triazoline-3,5-dione chemistry (TAD) moieties. This structure reacts in a reversible way (using different temperatures, Fig. 6.2) with indoles through an ene click reaction [45]; however, it undergoes fast Diels-Alder reactions with dienes and is widely used in biology for its capacity to bind irreversibly tyrosine residues [46]. Such an approach could be very useful in DOD processes aimed at protein labeling, for which radiolabeled prosthetic groups enabling click chemistry are now widely employed [47].
TAD residue in reversible click-chemistry transformations (Taken from [45])
Another important point to clarify is whether DOD systems should produce product vials (as in the traditional approach), a syringe/cartridge dose, or even directly deliver the radiopharmaceutical preparation into the vein of the subject (see Fig. 6.3). Though currently unlikely, the possibility to overcome the concept of product vial is very appealing on the base of flexibility, atom efficiency, and procedure streamlining. Therefore, an outstanding challenge is represented by modifying the regulators’ view [48, 49] on the requirements needed to prepare injectable radiopharmaceuticals for human use, in order to allow easier and more personalized modalities of dose delivery. One of the ways to achieve such result is represented by the change in quality control (QC) paradigms; in fact, the traditional way to produce a separate vial for QC [50] should be overtaken by the possibility of realizing a DOD process whose precise control and monitoring would represent itself a guarantee of good-quality end product.
6 Conclusions
Miniaturization and optimization of the biochemical hardware involved have created a substantial personalization of several medical practices. A typical example of this trend that has improved the treatment of diabetic subjects is the current possibility for any person to check their glucose levels using a straightforward handheld system, instead of reaching the nearest hospital and performing a proper blood examination. This level of simplicity, flexibility, and personalization is currently lacking in the important field of radiopharmaceutical production. However, several studies are starting to demonstrate that new chemical technologies (e.g., microfluidics, high-tech polymers) can represent useful tools to achieve what we can define Dose-On-Demand systems. Several challenges are still to be faced before reaching such a useful target in an efficient and affordable way; we however think that the realization of this capability will be the main way to allow the use of rare and disease dedicated tracers whose widespread utilization is currently hindered [51] by the existing radiopharmaceutical production paradigms.
References
Keng PY, Esterby M, van Dam RM. Emerging technologies for decentralized production of PET tracers. In: Positron emission tomography – current clinical and research aspects. 2012. doi:10.5772/1280
Satyamurthy N. Electronic generators for the production of positron-emitter labeled radiopharmaceuticals where would PET be without them? Clin Positron Imaging. 1999;2:233–53.
Wang M-W, Lin W-Y, Liu K, Masterman-Smith M, Kwang-Fu Shen C. Microfluidics for positron emission tomography probe development. Mol Imaging. 2010;9:175–91.
Arima V, Pascali G, Lade O, et al. Radiochemistry on chip: towards dose-on-demand synthesis of PET radiopharmaceuticals. Lab Chip. 2013;13:2328–36.
Pascali G, Nannavecchia G, Pitzianti S, Salvadori PA. Dose-on-demand of diverse 18F-fluorocholine derivatives through a two-step microfluidic approach. Nucl Med Biol. 2011;38:637–44.
Knapp FF (Russ., Mirzadeh S. The continuing important role of radionuclide generator systems for nuclear medicine. Eur J Nucl Med. 1994;21:1151–65.
Welch MJ, Kilbourn MR. A remote system for the routine production of oxygen-15 radiopharmaceuticals. J Label Compd Radiopharm. 1985;22:1193–200.
Miller PW, Long NJ, Vilar R, Gee AD. Synthesis of 11C, 18F, 15O, and 13N radiolabels for positron emission tomography. Angew Chem Int Ed Engl. 2008;47:8998–9033.
Awasthi V, Watson J, Gali H, Matlock G, McFarland A, Bailey J, Anzellotti A. A “dose on demand” biomarker generator for automated production of [(18)F]F(-) and [(18)F]FDG. Appl Radiat Isot. 2014;89:167–75.
Awasthi V, Gali H, McFarland A, Anzellotti A. Automated manufacture of Na[18F]F at the University of Oklahoma Health Sciences Center (OUHSC). Dose on-demand and imaging studies. J Nucl Med. 2014;55:1243.
Anzellotti A, Bailey J, Ferguson D, McFarland A, Bochev P, Andreev G, Awasthi V, Brown-Proctor C. Automated production and quality testing of [18F]labeled radiotracers using the BG75 system. J Radioanal Nucl Chem. 2015;305:387–401.
Anzellotti A, Yuan H. Automated manufacture of [18F]FMISO in the BG 75 system. Synthesis and purification using solid phase extraction. J Nucl Med. 2015;56:1001.
Gali H, Nkepang G, Galbraith W, Hammond K, Awasthi V, Collier TL. Preparation of [F-18]FLT using an ABT compact cyclotron and continuous flow microfluidics. J Label Compd Radiopharm. 2015;58 Suppl 1:S362.
Lu S, Giamis AM, Pike VW. Synthesis of [F]fallypride in a micro-reactor: rapid optimization and multiple-production in small doses for micro-PET studies. Curr Radiopharm. 2009;2:49–55.
Pascali G, Mazzone G, Saccomanni G, Manera C, Salvadori PA. Microfluidic approach for fast labeling optimization and dose-on-demand implementation. Nucl Med Biol. 2010;37:547–55.
Liang SH, Yokell DL, Jackson RN, Rice PA, Callahan R, Johnson KA, Alagille D, Tamagnan G, Collier TL, Vasdev N. Microfluidic continuous-flow radiosynthesis of [(18)F]FPEB suitable for human PET imaging. Medchemcomm. 2014;5:432–5.
Collier T, Yokell D, Rice P, Jackson R, Shoup T, Normandin M, Brady T, El Fakhri G, Liang S, Vasdev N. First human use of the tau radiopharmaceutical [18F]T807 by microfluidic flow chemistry. J Nucl Med. 2014;55:1246.
Pascali G, Berton A, DeSimone M, Wyatt N, Matesic L, Greguric I, Salvadori PA. Hardware and software modifications on the Advion NanoTek microfluidic platform to extend flexibility for radiochemical synthesis. Appl Radiat Isot. 2014;84:40–7.
Matesic L, Kallinen A, Wyatt NA, Pham TQ, Greguric I, Pascali G. [18F]Fluorination optimisation and the fully automated production of [18F]MEL050 using a microfluidic system. Aust J Chem. 2015;68:69–71.
Matesic L, Kallinen A, Greguric I, Pascali G. Dose-on-demand production of multiple 18F-radiotracers on a microfluidic system. J Label Compd Radiopharm. 2015;58 Suppl 1:S22.
Zheng M-Q, Collier L, Bois F, Kelada OJ, Hammond K, Ropchan J, Akula MR, Carlson DJ, Kabalka GW, Huang Y. Synthesis of [(18)F]FMISO in a flow-through microfluidic reactor: development and clinical application. Nucl Med Biol. 2015;42:578–84.
Selivanova SV, Mu L, Ungersboeck J, Stellfeld T, Ametamey SM, Schibli R, Wadsak W. Single-step radiofluorination of peptides using continuous flow microreactor. Org Biomol Chem. 2012;10:3871.
Richter S, Bouvet V, Wuest M, Bergmann R, Steinbach J, Pietzsch J, Neundorf I, Wuest F. (18)F-Labeled phosphopeptide-cell-penetrating peptide dimers with enhanced cell uptake properties in human cancer cells. Nucl Med Biol. 2012;39:1202–12.
Bouvet VR, Wuest F. Application of [18F]FDG in radiolabeling reactions using microfluidic technology. Lab Chip. 2013;13:4290–4.
Cumming RC, Olberg DE, Sutcliffe JL. Rapid 18 F-radiolabeling of peptides from [18 F]fluoride using a single microfluidics device. RSC Adv. 2014;4:49529–34.
Mathiessen B, Zhuravlev F. Automated solid-phase radiofluorination using polymer-supported phosphazenes. Molecules. 2013;18:10531–47.
Ismail R, Irribaren J, Javed MR, Machness A, Michael van Dam R, Keng PY. Cationic imidazolium polymer monoliths for efficient solvent exchange, activation and fluorination on a continuous flow system. RSC Adv. 2014;4:25348.
Liu K, Lepin EJ, Wang M-W, et al. Microfluidic-based 18F-labeling of biomolecules for immuno-positron emission tomography. Mol Imaging. 2011;10:168–76.
Keng PY, Chen S, Ding H, et al. Micro-chemical synthesis of molecular probes on an electronic microfluidic device. Proc Natl Acad Sci U S A. 2012;109:690–5.
Javed MR, Chen S, Kim H-K, Wei L, Czernin J, Kim C-JCJ, van Dam RM, Keng PY. Efficient radiosynthesis of 3′-deoxy-3′-18F-fluorothymidine using electrowetting-on-dielectric digital microfluidic chip. J Nucl Med. 2014;55:321–8.
Javed MR, Chen S, Lei J, Collins J, Sergeev M, Kim H-K, Kim C-J, van Dam RM, Keng PY. High yield and high specific activity synthesis of [18F]fallypride in a batch microfluidic reactor for micro-PET imaging. Chem Commun (Camb). 2014;50:1192–4.
Chen S, Javed MR, Kim H-K, Lei J, Lazari M, Shah GJ, van Dam RM, Keng P-Y, Kim C-JCJ. Radiolabelling diverse positron emission tomography (PET) tracers using a single digital microfluidic reactor chip. Lab Chip. 2014;14:902–10.
Ali I, Aboul-Enein HY, Gupta VK. Nanochromatography and nanocapillary electrophoresis. 2009. doi:10.1002/9780470434925
Ghanem A, Ikegami T. Recent advances in silica-based monoliths: preparations, characterizations and applications. J Sep Sci. 2011;34:1945–57.
Wouters B, De Vos J, Desmet G, Terryn H, Schoenmakers PJ, Eeltink S. Design of a microfluidic device for comprehensive spatial two-dimensional liquid chromatography. J Sep Sci. 2015;38:1123–9.
Zhou D, Chu W, Peng X, McConathy J, Mach RH, Katzenellenbogen JA. Facile purification and click labeling with 2-[18F]fluoroethyl azide using solid phase extraction cartridges. Tetrahedron Lett. 2015;56:952–4.
Fedorova O, Kuznetsova O, Stepanova M, Maleev V, Belokon Y, Wester H-J, Krasikova R. A facile direct nucleophilic synthesis of O-(2-[18F]fluoroethyl)-l-tyrosine ([18F]FET) without HPLC purification. J Radioanal Nucl Chem. 2014;301:505–12.
Turiel E, Martín-Esteban A. Molecularly imprinted polymers for sample preparation: a review. Anal Chim Acta. 2010;668:87–99.
Haupt K, Mosbach K. Molecularly imprinted polymers and their use in biomimetic sensors. Chem Rev. 2000;100:2495–504.
Brown LJ, Bouvet DR, Champion S, et al. A solid-phase route to18F-labeled tracers, exemplified by the synthesis of [18F]2-Fluoro-2-deoxy-D-glucose. Angew Chemie. 2007;119:959–62.
Riss PJ, Kuschel S, Aigbirhio FI. No carrier-added nucleophilic aromatic radiofluorination using solid phase supported arenediazonium sulfonates and 1-(aryldiazenyl)piperazines. Tetrahedron Lett. 2012;53:1717–9.
Brady F, Luthra S, Robins E. Solid-phase fluorination of uracil and cytosine. 2006. US 10/538,904.
Ismail R, Machness A, van Dam RM, Keng P-Y. Solid-phase [18F]fluorination on a flow-through glass microfluidic chip. In: 16th int. conf. miniaturized syst. chem. life sci. 2012. Okinawa, Japan, p. 629–631
Sergeev ME, Morgia F, Lazari M, Wang C, van Dam RM. Titania-catalyzed radiofluorination of tosylated precursors in highly aqueous medium. J Am Chem Soc. 2015;137:5686–94.
Billiet S, De Bruycker K, Driessen F, Goossens H, Van Speybroeck V, Winne JM, Du Prez FE. Triazolinediones enable ultrafast and reversible click chemistry for the design of dynamic polymer systems. Nat Chem. 2014;6:815–21.
Ban H, Nagano M, Gavrilyuk J, Hakamata W, Inokuma T, Barbas CF. Facile and stabile linkages through tyrosine: bioconjugation strategies with the tyrosine-click reaction. Bioconjug Chem. 2013;24:520–32.
Kim DW. Bioorthogonal click chemistry for fluorine-18 labeling protocols under physiologically friendly reaction condition. J Fluor Chem. 2015;174:142–7.
Salvadori AP. Radiopharmaceuticals, drug development and pharmaceutical regulations in Europe. Curr Radiopharm. 2015;1:7–11.
VanBrocklin FH. Radiopharmaceuticals for drug development: United States regulatory perspective. Curr Radiopharm. 2015;1:2–6.
Yu S. Review of 18F-FDG synthesis and quality control. Biomed Imaging Interv J. 2006;2:e57.
Reilly RM, Lam K, Chan C, Levine M. Advancing novel molecular imaging agents from preclinical studies to first-in-humans phase I clinical trials in academia – a roadmap for overcoming perceived barriers. Bioconjug Chem. 2015;26:625–32.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution-Noncommercial 2.5 License (http://creativecommons.org/licenses/by-nc/2.5/) which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
The images or other third party material in this chapter are included in the work’s Creative Commons license, unless indicated otherwise in the credit line; if such material is not included in the work’s Creative Commons license and the respective action is not permitted by statutory regulation, users will need to obtain permission from the license holder to duplicate, adapt or reproduce the material.
Copyright information
© 2016 The Author(s)
About this paper
Cite this paper
Pascali, G., Matesic, L. (2016). How Far Are We from Dose On Demand of Short-Lived Radiopharmaceuticals?. In: Kuge, Y., Shiga, T., Tamaki, N. (eds) Perspectives on Nuclear Medicine for Molecular Diagnosis and Integrated Therapy. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55894-1_6
Download citation
DOI: https://doi.org/10.1007/978-4-431-55894-1_6
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55892-7
Online ISBN: 978-4-431-55894-1
eBook Packages: MedicineMedicine (R0)