Abstract
Mammalian sperm acquire fertilizing capacity during epididymal transit. During this process, the expression of protein disulfide isomerase-P5 (PDI-P5, P5) decreases. Almost all members of the PDI family accelerate the formation, reduction, and isomerization of disulfide bonds, whereas boar P5, which is composed of two active thioredoxin domains, a and a′, and an inactive b domain, inhibits the oxidative refolding of reduced and denatured lysozyme due to nonproductively folded lysozyme resulting from intermolecular isomerization. We investigated the reductive activities of cysteine mutants of boar P5 and of the a and a′b domains, using an insulin turbidity assay, and their ability to inhibit the oxidative refolding of reduced and denatured lysozyme. We also analyzed refolded products by Western blotting. The reductive activities of the C-terminal variants, C171A, C174A, and C171/174A, were lower than those of the N-terminal variants C36A, C39A, C36/39A, and a′b. The inhibitory activities of N- and C-terminal cysteine mutants were ~60 % and ~75 %, respectively, whereas the inhibitory activities of C36/39A and of a′b were ~60 % and 30 %, respectively. The properties of a mixture of a and a′b were similar to those of a′b alone. These results suggested that two active domains of P5 cooperatively inhibited refolding by intermolecular isomerization and that this effect was increased when the a and a′b domains were linked. The findings were supported by Western blotting analysis of the refolded products. These results provide new insight into the molecular mechanism by which P5 inhibits protein refolding.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Caput epididymal sperm released from the testes are biologically incompetent, acquiring functionality during transit from the epididymal caput to the cauda via the corpus. Ejaculated sperm are able to move vigorously and participate in the complex cascades of interactions that culminate in fertilization of the oocyte (Yanagimachi et al. 1994). Various kinds of studies on epididymal sperm maturation have been reported (McLaughlin et al. 1997; Frayne et al. 1998; Zhu et al. 2001; Saxena et al. 2002; Baker et al. 2005, 2012; Ellerman et al. 2006; Toshimori et al. 2006; Inoue et al. 2008; Ijiri et al. 2011). Protein disulfide isomerase-P5 (PDI-P5, P5) has been reported present in mouse sperm membrane fraction (Stein et al. 2006) and to be involved in asymmetrical organogenesis in zebrafish (Hoshijima et al. 2002), in regulating human platelet function (Jordan et al. 2005), and in promoting the ability of human tumors to evade the immune system (Kaiser et al. 2007; Gumireddy et al. 2007). We have reported that boar P5 is downregulated during transit from the epididymal corpus to the cauda and inhibits oxidative refolding of reduced and denatured lysozyme (Akama et al. 2010). To date, however, the mechanism by which P5 inhibits protein refolding has not been elucidated.
P5 is composed of two active thioredoxin domains, a and a′, and an inactive b domain, with each active thioredoxin domain containing an active CGHC site. We investigated the mechanism by which P5 inhibits protein refolding by assaying the inhibitory activities of highly purified domain-deleted or cysteine variants of P5. Our results suggest that the a domain inhibits refolding by promoting the intermolecular isomerization of lysozyme with the a′ domain, with the a-a′b attachment essential for complete inhibition.
2 Materials and Methods
2.1 Expression and Purification of PDI-P5 Variants
The cDNAs corresponding to P5 deletion variants, the a and a′b fragments, were amplified by polymerase chain reaction (PCR) and inserted into the expression vector pET30a(+) (Novagen). The cysteine mutants of P5 cDNA were generated by amplification of wild-type P5 cDNA cloned into pET30a(+) vector using pfu polymerase (Promega). The domain structure of P5 and its variants are shown in Fig. 9.1. Expression and purification of pET-PDI-P5 variants were performed as described (Akama et al. 2010), with ammonium sulfate precipitation and hydrophobic chromatography on phenyl-Sepharose (CL-4B) (GE Healthcare Bio-Science) performed before affinity chromatography.
2.2 Insulin Turbidity and Lysozyme Refolding Assays
Insulin turbidity (Holmgren 1979) and lysozme refolding (Puig and Gilbert 1994) assays were performed as described.
2.3 Western Blotting
Reaction products of lysozyme refolding assay were Western blotted as described (Akama et al. 2010).
3 Results
3.1 Reductive Activity of a′ Domain
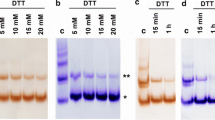
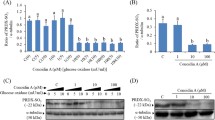
Purified recombinant mature P5 showed thiol-dependent reductase activity, catalyzing the reduction of insulin disulfides with DTT as previously reported (Akama et al. 2010). All cysteine-to-alanine mutations decreased the reductive activity of P5. C-terminal mutants (C171A, C174A, and C171/174A) had about ~50 % of the activity of wild type, whereas N-terminal mutants (C36A, C39A, C36/39A and a′b) had about ~75 % of the activity, suggesting that the disulfide reducing activity of the a′ domain was greater than that of the other domains. The quadruple mutant C36/39/171/174A, in which all four active cysteines were mutated to alanine, had no reducing activity, indicating that cysteines located in the inactive thioredoxin domain, b, were not involved in disulfide reduction.
3.2 Chaperone Activities of the P5 Mutants
Reduced and denatured lysozyme is commonly used as a substrate to determine whether PDIs have chaperone activity and promote oxidative refolding in vitro. Most PDI family members possess chaperone activity, catalyzing the formation and rearrangement of disulfide bonds during the correct folding of nascent proteins. We have found, however, that P5 possesses anti-chaperone activity, inhibiting the oxidative refolding of the denatured lysozyme (Akama et al. 2010). Western blot analysis has suggested that the anti-chaperone activity of P5 is the result of its intermolecular isomerization with lysozyme, yielding lysozyme aggregates (Akama et al. 2010).
To clarify this mechanism, we compared the ability of P5 and P5 mutants to inhibit oxidative refolding. The a domain enhanced lysozyme refolding, behaving like thioredoxin alone, whereas the a′b fragment without the N-terminal a domain inhibited folding ~30 % compared with the wild type (100 %). The inhibitory activity of equal concentrations of the a and a′b fragments was similar to that of a′b alone, suggesting that the enhancement of refolding by the a domain did not occur when a′b was present. The double N-terminal mutant C36/39A had greater inhibitory activity (60 %) than a′b. These results suggested that the presence of the a domain enhanced the intermolecular isomerization of P5 with denatured lysozyme, and that the covalent bonding of a to a′b was essential for complete inhibition. The other N-terminal active domain mutants (C36A, C39A) had ~60 % and the C-terminal active domain mutants (C171A, C174A and C171/174A) had ~75 % of the activity of the wild type, indicating that the a domain inhibited refolding when combined with the a′ domain.
3.3 Detection of Lysozyme Aggregates by Western Blotting
Our previous study has indicated that the P5 inhibition of lysozyme refolding is caused by lysozyme aggregates resulting from intermolecular isomerization. Using Western blotting, we found that the quantity of lysozyme aggregates was reduced when refolding was catalyzed by C36A and C171A, compared with native P5 (Fig. 9.2). Spontaneous crosslinking of reduced and denatured lysozyme alone was observed in refolding buffer (Fig. 9.2), explaining why its refolding rate was no higher than 40 %. In the presence of native P5, lysozyme aggregates were detected in the high molecular region, while lysozyme intermediates were not, similar to our earlier findings (Akama et al. 2010). In the presence of C36A or C171A, lysozyme intermediates had higher molecular weights than those of lysozyme alone, with the amounts of aggregates being smaller than for P5. No aggregates were observed in the presence of a′b, but the sizes of the intermediates were smaller than those observed with the cysteine mutants. These results further indicated that the a and a′ domains cooperatively contributed to the production of lysozyme aggregates and that the a domain accelerated the aggregate production.
Western blotting of refolded products of lysozyme. *1, intermolecular crosslinked aggregates. *2, crosslinked intermediates (highly crosslinked, lanes 7 and 8). *3, intermediates of lysozyme produced during spontaneous refolding. *4l, lysozyme monomers. P5s, P5 and its variants; nP5, native P5; R, reduced; NR, nonreduced. P5 concentration, 0.6 μM
4 Discussion
4.1 Collaborative Isomerization by Two Active Domains
The results of the lysozyme refolding assay showed that N-terminal active cysteines contribute more to the inhibition of refolding than C-terminal active cysteines, similar to findings with rat and human P5 (Kramer et al. 2001; Kikuchi et al. 2002). Using human P5 variants, a single active domain, particularly the a domain, has been shown sufficient for isomerase activity (Kikuchi et al. 2002). When both cysteines in either the a or a′ domain are replaced with serine, the isomerase activities of C36/39S and C171/174S, using insulin as a substrate, are 50 % and 75 %, respectively, relative to the wild type, suggesting that the a domain can compensate for the replacement of cysteines in the a′ domain. However, a study of yeast PDI has shown that the isomerase activity of mutants, in which cysteines residues were replaced with alanine, is less than that of the serine mutants, a finding thought to be the result of the reducing activity remaining in the hydroxyl group of serine (Tian et al. 2006).
Using a lysozyme refolding assay, we found that the inhibitory activity of the N-terminal mutants (C36A, C39A, and C36/39A) was ~60 % of that of the wild type, whereas the inhibitory activity of the C-terminal mutants (C171A, C174A, and C171/174A) was ~75 %, indicating that both active domains collaborate in intermolecular isomerization. Western blotting showed that comparable amounts of lysozyme aggregates were present in the presence of C36A or C171A, indicating that the a and a′ domains cooperatively inhibited refolding. The relatively stronger reducing activity of the a′ relative to the a domain observed in the insulin turbidity assay also suggested collaborative isomerization. The refolding inhibitory activity of both a′b and the mixture of a and a′b was 30 % of that of the wild type, suggesting that a covalent bond between the two was essential for complete inhibition. This finding was supported by our Western blotting results, with a′b alone showing lysozyme intermediates but no aggregates. The collaborative inhibition by the a and a′ domains suggests that they have comparable access to substrates, although these domains are adjacent to each other, possibly because the amino acid chain is relatively long (36 residues) between these domains, enabling the a domain to move widely. The more efficient inhibition by C36/39A, in which both cysteines in the a domain were mutated to inactive alanine, than by a′b, which lacked an a domain, suggests that the mobile a domain holds and stabilizes large lysozyme aggregates.
4.2 Importance of Thioredoxin Domain Order
Evolution has given rise to the PDI family of proteins, with 1–6 thioredoxin domain(s). The ancestral PDIs may have been characterized by active domains packed together, with these domains structurally separated in descendant PDIs (Pedone et al. 2010). Protein disulfide oxidoreductases (PDOs), composed of only two thioredoxin domains, can be classified as ancestral types. P5 may also be classified as an ancestral PDI because it contains three thioredoxin domains, in the order a, a′, and b, similar to PDOs. The domain structure of P5, consisting of two contiguous active domains and a third inactive domain, may be responsible for its potent isomerization activity and unique function; this in turn may be the result of the nonspecialized roles of both the a and a′ domains. Rather, they cooperatively isomerize disulfides. The downregulation of P5 from the epididymal corpus to the cauda suggests that its potent isomerization activity may be involved in sperm maturation.
5 Conclusion
We investigated the properties of domain-deleted and cysteine variants of P5 having an active a and a′ domain and an inactive b domain. The reductive activities of C171A, C174A, and C171/174A (a′ domain variants) were ~50 % of the wild type, whereas those of C36A, C39A, C36/39A (a domain variants), and a′b were ~75 %. The inhibitory activities of lysozyme refolding of a and a′ domain variants were ~60 % and ~75 % of wild type, respectively, whereas the inhibitory activity of a mixture of a and a′b was 30 % as well as that of a′b. Together with the results of Western blotting, these findings suggested that two active domains of P5 cooperatively inhibited lysozyme refolding by intermolecular isomerization, and that the isomerase activity was enhanced by a domain and the covalent bond between a and a′b was essential for the “potent” isomerase activity. Our finding on its isomerization mechanism may contribute for further understanding of molecular basis of sperm maturation, asymmetrical organogenesis, and tumor immune evasion.
References
Akama K et al (2010) Protein disulfide isomerase-P5, downregulated in the final stage of boar epididymal sperm maturation, catalyzes disulfide formation to inhibit protein function in oxidative refolding of reduced denatured lysozyme. Biochem Biophys Acta 1804:1272–1284
Baker MA et al (2005) Identification of post-translational modifications that occur during sperm maturation using difference in two-dimensional gel electrophoresis. Proteomics 5:1003–1012
Baker MA et al (2012) Analysis of phosphopeptide changes as spermatozoa acquire functional competence in the epididymis demonstrates changes in the post-translational modification of Izumo1. J Proteome Res 11:5252–5264
Ellerman DA et al (2006) A role for sperm surface protein disulfide isomerase activity in gamete fusion: evidence for the participation of ERp57. Dev Cell 10:831–837
Frayne J et al (1998) The MDC family of proteins and their processing during epididymal transit. J Reprod Fertil Suppl 53:149–155
Gumireddy K et al (2007) In vivo selection for metastasis promoting genes in the mouse. Proc Natl Acad Sci USA 104:6696–6701
Holmgren A (1979) Thioredoxin catalyzes the reduction of insulin disulfides by dithiothreitol and dihydrolipoamide. J Biol Chem 254:9627–9632
Hoshijima K et al (2002) A protein disulfide isomerase expressed in the embryonic midline is required for left/right asymmetries. Genes Dev 16:2518–2529
Ijiri TW et al (2011) Identification and validation of mouse sperm proteins correlated with epididymal maturation. Proteomics 11:4047–4062
Inoue N et al (2008) Putative sperm fusion protein IZUMO and the role of N-glycosylation. Biochem Biophys Res Commun 377:910–914
Jordan PA et al (2005) A role for the thiol isomerase protein ERP5 in platelet function. Blood 105:1500–1507
Kaiser BK et al (2007) Disulphide-isomerase-enabled shedding of tumour-associated NKG2D ligands. Nature (Lond) 447:482–486
Kikuchi M et al (2002) Functional analysis of human P5, protein disulfide isomerase homologue. J Biochem 132:451–455
Kramer B et al (2001) Functional roles and efficiencies of the thioredoxin boxes of calcium-binding proteins 1 and 2 in protein folding. Biochem J 357:83–95
McLaughlin EA et al (1997) Cloning and sequence analysis of rat fertilin alpha and beta developmental expression, processing and immunolocalization. Mol Hum Reprod 3:801–809
Pedone E et al (2010) Multiple catalytically active thioredoxin folds: a winning strategy for many functions. Cell Mol Life Sci 67:3797–3814
Puig A, Gilbert HF (1994) Protein disulfide isomerase exhibits chaperone and anti-chaperone activity in the oxidative refolding of lysozyme. J Biol Chem 269:7764–7771
Saxena DK et al (2002) Behaviour of a sperm surface transmembrane glycoprotein basigin during epididymal maturation and its role in fertilization in mice. Reproduction 123:435–444
Stein KK et al (2006) Proteomic analysis of sperm regions that mediate sperm–egg interactions. Proteomics 6:3533–3543
Tian G et al (2006) The crystal structure of yeast protein disulfide isomerase suggests cooperativity between its active sites. Cell 124:61–73
Toshimori K et al (2006) The involvement of immunoglobulin superfamily proteins in spermatogenesis and sperm–egg interaction. Reprod Med Biol 5:87–93
Yanagimachi R et al (1994) Mammalian fertilization. In: Knobil K, Neil JD (eds) The physiology of reproduction. Raven, New York, pp 189–317
Zhu GZ et al (2001) Testase 1 (ADAM 24), A plasma membrane-anchored sperm protease implicated in sperm function during epididymal maturation or fertilization. J Cell Sci 114: 1787–1794
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
This chapter is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
Copyright information
© 2014 The Author(s)
About this paper
Cite this paper
Miyakawa, M., Shigihara, S., Zukeran, G., Tomioka, T., Yoshino, T., Akama, K. (2014). Analysis of the Mechanism That Brings Protein Disulfide Isomerase-P5 to Inhibit Oxidative Refolding of Lysozyme. In: Sawada, H., Inoue, N., Iwano, M. (eds) Sexual Reproduction in Animals and Plants. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54589-7_9
Download citation
DOI: https://doi.org/10.1007/978-4-431-54589-7_9
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54588-0
Online ISBN: 978-4-431-54589-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)