Abstract
In mammals, two integral membrane proteins, sperm IZUMO1 and egg CD9, regulate sperm–egg fusion, and their roles are critical but as yet unknown. In such situation, a recent study has shown that CD9-containing exosome-like vesicles, which are released from wild-type eggs, can induce the fusion between sperm and Cd9-deficient egg, even though Cd9-deficient eggs are highly refractory to the fusion with sperm. This result provides compelling evidence for the crucial involvement of CD9-containing, fusion-facilitating vesicles in sperm–egg fusion. On the other hand, similarities have been observed between the generation of retroviruses in host cells and the formation of small cellular vesicles, termed exosomes, in mammalian cells. The exosomes are thought to regulate intercellular communication through transfer of proteins and RNAs. These collective studies provide an insight into the molecular mechanisms of gamete fusion and other membrane fusion events.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Fertilization is an event that consists of cell–cell adhesion, cell–cell fusion, and activation of cell signaling to allow the resumption of the egg cell cycle (Fig. 31.1). In mammals, two membrane protein families, a cell adhesion molecule “integrin” (Almeida et al. 1995) and a membrane-anchored protease “ADAM (a disintegrin and metalloprotease)” (Blobel et al. 1992), were biochemically identified and immunocytochemically confirmed to localize on the outer cell membranes of egg and sperm, respectively; furthermore, antibodies against these protein families were shown to significantly reduce the rate of sperm–egg binding and fusion in mice (Almeida et al. 1995). Integrins, which are expressed in many types of cells in animals, mediate cell–cell and cell-matrix interaction and intercellular communication, including cell adhesion and cell–cell fusion (Almeida et al. 1995). On the other hand, the ADAM family has a characteristic domain that is homologous to an extracellular region of integrin family (Evans 2001). Thus, the presence of the domain conserved between integrins and ADAMs indicates that these two protein families play a role in sperm–egg adhesion and/or fusion (Evans 2001). Unexpectedly, when their genetically manipulated mice were produced, both male and female mice displayed no overt anomalies in either sperm–egg fusion or adhesion (Miller et al. 2000; He et al. 2003). In the past, many factors predicted to participate in sperm–egg fusion have emerged in mice, but despite expectations, most were found to be unnecessary (Okabe and Cummins 2007). From these studies, to ensure the continuous success of the reproduction cycle in mice, compatible pathways tuned by overlapping functions of multiple proteins seem to regulate the mechanism of fertilization. In other words, there will be more than one way for a sperm and egg to fuse and the network of multiple pathways may minimize the severity of the malfunction that may occur in sperm or eggs lacking a single gene. Concerning sperm–egg fusion, CD9 on the egg membrane (Miyado et al. 2000; Le Naour et al. 2000; Kaji et al. 2000) and IZUMO1 on the sperm membrane (Inoue et al. 2005) are exceptionally essential factors in gene disruption experiments (Fig. 31.2).
Series of steps from sperm–egg interaction to fusion during mammalian fertilization: an overview of mammalian fertilization. Fertilization is divided into multiple steps: interaction of sperm-somatic cells (termed cumulus cells), binding of sperm to the extracellular matrix (termed zona pellucida), and penetration of the egg. After the sperm penetrates the zona pellucida, it can bind and fuse to the egg cell membrane. Successful fertilization requires not only that a sperm and egg fuse, but also that polyspermy block occurs
Players identified in sperm–egg fusion. IZUMO1 is expressed on the sperm membrane, and Izumo1-deficient sperm show a defect in fusion with the egg cell membrane. CD9 is expressed on the egg cell membrane and functions in fusion with the sperm. Two membrane proteins, IZUMO1 and CD9, are essential for sperm–egg fusion in mice. Direct interaction between CD9 and IZUMO1 has not been identified, and the unidentified sperm and egg factors may be involved in sperm–egg fusion. After sperm–egg membrane fusion occurs, a sperm factor triggers Ca2+ oscillations and initiates egg activation in mammals
2 CD9 and Its Role in Cellular Function
The CD9 gene encoding a 24-kDa protein is transcribed in all types of mammalian cells (Hemler 2008). This protein is localized on the cell membranes and partially on endosomes, and it is expected to be involved in cell–cell adhesion, because CD9 associates with the integrin family (Hemler 2008). CD9 is known as a motility-related protein 1 (MRP-1), which plays a role in suppressing tumor metastasis (Miyake et al. 1991). CD9 has two extracellular loops, four transmembrane domains, and two short cytoplasmic domains. Its functional domain is expected to be included in a large extracellular loop (LEL) (Fig. 31.3), because CD9 associates with other membrane proteins via LEL in vitro (Hemler 2008). In addition, because the amount of CD9 in mesenchymal and embryonic stem cells is significantly higher than in fibroblastic cells, CD9 is useful as a cell-surface marker for isolating undifferentiated cells from cell pools in mice and humans (Akutsu et al. 2009).
Structural features of tetraspanin CD9, a member of the tetraspan-membrane protein family, termed tetraspanin, and its molecular mass is 24 kDa. The structural features of CD9 include four transmembrane domains, two extracellular loops, short and large extracellular loops (SEL and LEL), and two short cytoplasmic tails. CD9 has cysteine-cysteine-glycine (CCG) residues (amino acids 152–154) as a tetraspanin-specific motif and two other cysteines within LEL
Despite two decades of effort, however, the in vivo role of CD9 was unclear, and therefore three laboratories have independently generated Cd9 −/− mice (Miyado et al. 2000; Le Naour et al. 2000; Kaji et al. 2000). Consequently, all strains of the Cd9 −/− mice have shown severe female subfertility whereas the Cd9 −/− male mice were fertile. Moreover, the Cd9 −/− female mice exhibited severely reduced sperm fusion ability. Since these findings, CD9 has been studied as one of the crucial factors in sperm–egg fusion in mammals. A functionally essential domain of CD9 is predicted to be located within the LEL (Zhu et al. 2002; Kaji et al. 2002); however, even though CD9-binding proteins have been identified in non-gamete cells, LEL-binding, potentially fusion-related proteins have not been found yet.
3 Tetraspanin
CD9 belongs to a membrane protein superfamily collectively termed “tetraspanin” that encompasses 35 members in mammals (including CD9, CD37, CD53, CD63, CD81, CD82, and CD151) (Hemler 2008), 30 in nematodes (Moribe et al. 2004), and 30 in flies (Todres et al. 2000). In mammals, tetraspanin is related to infectious diseases; in particular, these proteins are involved in cell–cell transmission of human immunodeficiency virus (HIV-1) (Garcia et al. 2005; Wiley and Gummuluru 2006). After its primary infection into host cells, CD9, CD63, CD81, and CD82 are enriched at HIV-1 budding sites of HIV-1 virions. When tetraspanin-containing HIV-1 particles are secondarily formed and released from host cells, they become tenfold more infectious than the cell-free virus particles. In mice, CD81 is linked to infection of hepatocytes with the malaria parasite (Silvie et al. 2003). Malaria sporozoites, a cell form that infects new hosts, are transmitted into the liver of the mammalian host through bites from infected mosquitoes, but the sporozoites fail to infect Cd81 −/− mouse hepatocytes, suggesting that CD81 is involved in sporozoite entry into hepatocytes as a host factor. Otherwise, Cd81 −/− female mice are subfertile, because Cd81-deficient eggs exhibit impaired sperm fusion ability (Rubinstein et al. 2006; Tanigawa et al. 2008). CD81 is expressed on Cd9-deficient eggs, and CD9 is also expressed on Cd81-deficient eggs at an expression rate comparable with that of wild-type eggs, indicating that CD9 and CD81 work independently in sperm–egg fusion (Ohnami et al. 2012).
More than 60 members of tetraspanin are known in plants (Huang et al. 2005; Chiu et al. 2007). Tetraspanin-like proteins have also been identified in fungi, and their molecular mass (more than 200 kDa) is greater than those of tetraspanin identified in animals and plants (20–30 kDa) (Lambou et al. 2008). An appressorium is a specialized cell typical of many fungal plant pathogens that is used to infect host plants. By analyzing a nonpathogenic mutant, punchless, isolated from the rice blast fungus Magnaporthe grisea, tetraspanin-like PLS1 (MgPLS1) has been shown to control the appressorrial function, which is essential for fungal penetration into host leaves (Clergeot et al. 2001). Similarly, Colletotrichum lindemuthianum PLS1 (ClPLS1) is a functional homologue of MgPLS1. The nonpathogenic ClPLS1-deficient mutant to bean leaves exhibits a defect in the formation and positioning of the penetration pore (Veneault-Fourrey et al. 2005). As the invasion of pathogenic fungi into leaves is an event closely related to membrane fusion events, these studies indicate that tetraspanin-like PLS1s are involved in the membrane fusion-related event between fungus and plant.
Taken together, these results suggest that tetraspanin is related to membrane fusion-related events in multicellular organisms. On the other hand, the physiological activities of tetraspanin are still unknown, and its fusogenic activity corresponding to fusogenic transmembrane proteins, such as syncytin identified in human placenta (Mi et al. 2000), and virus envelope proteins (Hernandez et al. 1996), has not been identified.
4 Tetraspanin as an Exosome Component
In mammals, cell-cultured media contain nanosized membrane vesicles, but they were not attractive to researchers because they could not be structurally distinguished from the debris of dead cells (Couzin 2005). Recent studies have shown that the vesicles, termed exosomes, are derived from living cells, but not dead cells; furthermore, they have been proven to play a significant role in the mediation of adaptive immune reactions to pathogens and tumors through the enhancement of antigen-specific T-cell responses (Couzin 2005; Simons and Raposo 2009). Besides immune cells, the exosomes are released from a wide range of normal and malignant mammalian cell types, and their diameter is estimated to range from 50 to 90 nm (Simons and Raposo 2009). The protein composition of exosomes varies with the origin of cells, yet the exosomes commonly contain a ganglioside GM3, two kinds of heat shock proteins (HSP70 and HSP90), and tetraspanin (Simons and Raposo 2009). The exosomes also contain transcripts, mRNA and microRNA, which are thought to be shuttled from one cell to another, thereby influencing protein synthesis in recipient cells (Valadi et al. 2007).
5 Exosome-Like Vesicles Released from Eggs
In eggs, two reports have demonstrated the contribution of CD9 in the organization of the egg cell membrane. First, CD9 is transferred from the egg to the fertilizing sperm present in the perivitelline space, implying the involvement of a process similar to trogocytosis, which is a mechanism for the cell-to-cell contact-dependent transfer of membrane fragments from antigen-presenting cells to lymphocytes in immune responses for pathogens (Barraud-Lange et al. 2007). Second, CD9 deficiency alters the length and density of microvilli on the egg cell membrane (Runge et al. 2007).
On the other hand, a recent study has reported the potential of enhanced green fluorescent protein-tagged CD9 (CD9-EGFP) as a reporter protein for studying sperm-egg fusion in living mouse eggs (Miyado et al. 2008). Interestingly, in eggs just before fertilization, CD9-EGFP is significantly accumulated within the perivitelline space that completely surrounds the eggs and lies between the egg cell membrane and the zona pellucida. Consistent with the images from CD9-EGFP, immunoelectron microscopic analysis of wild-type eggs has revealed that CD9 is not only present in the perivitelline space but is also incorporated into vesicles of varying size (50–200 nm in diameter) without a sectional profile of a typical lipid bilayer. Furthermore, in opossums (Talbot and DiCarlantonio 1984) and humans (Dandekar et al. 1992), as well as in mice, membrane vesicles have been detected by electron microscopy within the perivitelline space of their eggs. Moreover, recent study has demonstrated that the vesicles identified in mouse eggs share CD9, GM3, and HSP90 with exosomes, and these components are absent in eggs lacking CD9 and are reproduced by CD9-EGFP expression restricted to the eggs (Miyado et al. 2008). These results provide two types of evidence for the nature of CD9 in mouse eggs. First, CD9-incroporated exosome-like vesicles are produced in mouse eggs and are released outside the egg cell membrane just before fertilization. Second, CD9 is essential for the formation and release of the exosome-like vesicles (hereafter referred to as egg exosomes) in mouse eggs.
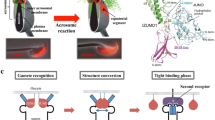
CD9-containing egg exosomes render sperms capable of fusing with Cd9-deficient eggs (Miyado et al. 2008) (Fig. 31.4). Cd9-deficient eggs cannot fuse with eggs, but the coexistence of wild-type eggs results in 60–70 % of the Cd9-deficient eggs fusing with at least one sperm. Thus, sperm can fuse with Cd9-deficient eggs with impaired microvilli via the egg exosomes of wild-type eggs, which means that the egg exosomes, but not the microvilli, are essential for sperm-egg fusion.
Overview of studies of Cd9-deficient eggs. In wild-type eggs, CD9-containing egg exosomes are released from wild-type eggs before any interaction with the sperm (upper right). Shortly after the sperm penetrates the perivitelline space, the egg exosomes are transferred on the acrosome-reacted sperm head. Then, a sperm fuses with the egg cell membrane. Interaction between sperm and exosomes is an essential step for sperm-fusing ability. In contrast, Cd9-deficient eggs cannot release egg exosomes, which are correlated with the formation of microvilli on the egg cell membrane (upper left). Sperm cannot fuse to the cell membrane of the Cd9-deficient egg. However, when the zona pellucida is removed from the eggs, the sperm is able to interact with the egg exosomes released from wild-type eggs and can fuse with the Cd9-deficient egg (lower left). By coincubation with wild-type eggs, the sperm can fuse with a similar number of Cd9-deficient and wild-type eggs. Intracytoplasmic sperm injection (ICSI) is an in vitro fertilization procedure in which a single sperm head is injected directly into an egg (lower right diagram). This procedure is most commonly used to overcome male infertility and fusion defects in Cd9-deficient eggs
6 Membrane Fusion and Exosomes
The close relationship between egg exosomes and sperm–egg fusion raises the question of how egg exosomes facilitate fusion. According to a previous report, exosomes contain both functional mRNA and microRNA, which are shuttled from one cell to another, affecting recipient cell ability to produce protein (Valadi et al. 2007). Moreover, HIV-1 utilizes the exosome biogenesis pathway for the formation of infectious particles, and in macrophages, HIV-1 assembles into an intracellular plasma membrane domain-containing tetraspanin (i.e., CD9, CD81, CD53, or CD63) (Wiley and Gummuluru 2006). Thus, the exosomes may play at least two roles in regulating cell function: first, shuttling proteins and RNAs (mRNAs and microRNAs) from one cell to another, and second, forming infectious particles. In fertilization, these two actions may be required for sperm–egg fusion in mammals. The studies of exosomes underscore the relevance of CD9 for healthy and pathogenic cell–cell fusion processes and may present a useful strategy for regulating the cell-to-cell spread of specific viruses and fertilization ability.
References
Akutsu H, Miura T, Machida M et al (2009) Maintenance of pluripotency and self-renewal ability of mouse embryonic stem cells in the absence of tetraspanin CD9. Differentiation 78:137–142
Almeida EA, Huovila AP, Sutherland AE et al (1995) Mouse egg integrin alpha-6-beta-1 functions as a sperm receptor. Cell 81:1095–1104
Barraud-Lange V, Naud-Barriant N, Bomsel M et al (2007) Transfer of oocyte membrane fragments to fertilizing spermatozoa. FASEB J 21:3446–3449
Blobel CP, Wolfsberg TG, Turck CW et al (1992) A potential fusion peptide and an integrin ligand domain in a protein active in sperm-egg fusion. Nature (Lond) 356:248–252
Chiu WH, Chandler J, Cnops G et al (2007) Mutations in the TORNADO2 gene affect cellular decisions in the peripheral zone of the shoot apical meristem of Arabidopsis thaliana. Plant Mol Biol 63:731–744
Clergeot PH, Gourgues M, Cots J et al (2001) PLS1, a gene encoding a tetraspanin-like protein, is required for penetration of rice leaf by the fungal pathogen Magnaporthe grisea. Proc Natl Acad Sci USA 98:6963–6968
Couzin J (2005) Cell biology: the ins and outs of exosomes. Science 308:1862–1863
Dandekar P, Aggeler J, Talbot P (1992) Structure, distribution and composition of the extracellular matrix of human oocytes and cumulus masses. Hum Reprod 7:391–398
Evans JP (2001) Fertilin beta and other ADAMs as integrin ligands: insights into cell adhesion and fertilization. Bioessays 23:628–639
Garcia E, Pion M, Pelchen-Matthews A et al (2005) HIV-1 trafficking to the dendritic cell–T-cell infectious synapse uses a pathway of tetraspanin sorting to the immunological synapse. Traffic 6:488–501
He ZY, Brakebusch C, Fassler R et al (2003) None of the integrins known to be present on the mouse egg or to be ADAM receptors are essential for sperm–egg binding and fusion. Dev Biol 254:226–237
Hemler ME (2008) Targeting of tetraspanin proteins: potential benefits and strategies. Nat Rev Drug Discov 7:747–758
Hernandez LD, Hoffman LR, Wolfsberg TG et al (1996) Virus–cell and cell–cell fusion. Annu Rev Cell Dev Biol 12:627–661
Huang S, Yuan S, Dong M et al (2005) The phylogenetic analysis of tetraspanins projects the evolution of cell–cell interactions from unicellular to multicellular organisms. Genomics 86:674–684
Inoue N, Ikawa M, Isotani A et al (2005) The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature (Lond) 434:234–238
Kaji K, Oda S, Shikano T et al (2000) The gamete fusion process is defective in eggs of Cd9-deficient mice. Nat Genet 24:279–282
Kaji K, Oda S, Miyazaki S et al (2002) Infertility of CD9-deficient mouse eggs is reversed by mouse CD9, human CD9, or mouse CD81; polyadenylated mRNA injection developed for molecular analysis of sperm-egg fusion. Dev Biol 247:327–334
Lambou K, Tharreau D, Kohler A et al (2008) Fungi have three tetraspanin families with distinct functions. BMC Genomics 9:63
Le Naour F, Rubinstein E, Jasmin C et al (2000) Severely reduced female fertility in CD9-deficient mice. Science 287:319–321
Mi S, Lee X, Li X et al (2000) Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature (Lond) 403:785–789
Miller BJ, Georges-Labouesse E, Primakoff P et al (2000) Normal fertilization occurs with eggs lacking the integrin alpha6beta1 and is CD9-dependent. J Cell Biol 149:1289–1296
Miyado K, Yamada G, Yamada S et al (2000) Requirement of CD9 on the egg plasma membrane for fertilization. Science 287:321–324
Miyado K, Yoshida K, Yamagata K et al (2008) The fusing ability of sperm is bestowed by CD9-containing vesicles released from eggs in mice. Proc Natl Acad Sci USA 105:12921–12926
Miyake M, Koyama M, Seno M et al (1991) Identification of the motility-related protein (MRP-1), recognized by monoclonal antibody M31-15, which inhibits cell motility. J Exp Med 174:1347–1354
Moribe H, Yochem J, Yamada H et al (2004) Tetraspanin protein (TSP-15) is required for epidermal integrity in Caenorhabditis elegans. J Cell Sci 117:5209–5220
Ohnami N, Nakamura A, Miyado M et al (2012) CD81 and CD9 work independently as extracellular components upon fusion of sperm and oocyte. Biology Open 1:640–647
Okabe M, Cummins JM (2007) Mechanisms of sperm–egg interactions emerging from gene-manipulated animals. Cell Mol Life Sci 64:1945–1958
Rubinstein E, Ziyyat A, Prenant M et al (2006) Reduced fertility of female mice lacking CD81. Dev Biol 290:351–358
Runge KE, Evans JE, He ZY et al (2007) Oocyte CD9 is enriched on the microvillar membrane and required for normal microvillar shape and distribution. Dev Biol 304:317–325
Silvie O, Rubinstein E, Franetich JF et al (2003) Hepatocyte CD81 is required for Plasmodium falciparum and Plasmodium yoelii sporozoite infectivity. Nat Med 9:93–96
Simons M, Raposo G (2009) Exosomes: vesicular carriers for intercellular communication. Curr Opin Cell Biol 21:575–581
Talbot P, DiCarlantonio G (1984) Ultrastructure of opossum oocyte investing coats and their sensitivity to trypsin and hyaluronidase. Dev Biol 103:159–167
Tanigawa M, Miyamoto K, Kobayashi S et al (2008) Possible involvement of CD81 in acrosome reaction of sperm in mice. Mol Reprod Dev 75:150–155
Todres E, Nardi JB, Robertson HM (2000) The tetraspanin superfamily in insects. Insect Mol Biol 9:581–590
Valadi H, Ekstrom K, Bossios A et al (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9:654–659
Veneault-Fourrey C, Parisot D, Gourgues M et al (2005) The tetraspanin gene ClPLS1 is essential for appressorium-mediated penetration of the fungal pathogen Colletotrichum lindemuthianum. Fungal Genet Biol 42:306–318
Wiley RD, Gummuluru S (2006) Immature dendritic cell-derived exosomes can mediate HIV-1 transinfection. Proc Natl Acad Sci USA 103:738–743
Zhu GZ, Miller BJ, Boucheix C et al (2002) Residues SFQ (173-175) in the large extracellular loop of CD9 are required for gamete fusion. Development (Camb) 129:1995–2002
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
This chapter is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
Copyright information
© 2014 The Author(s)
About this paper
Cite this paper
Yoshida, K., Kawano, N., Harada, Y., Miyado, K. (2014). Role of CD9 in Sperm–Egg Fusion and Virus-Induced Cell Fusion in Mammals. In: Sawada, H., Inoue, N., Iwano, M. (eds) Sexual Reproduction in Animals and Plants. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54589-7_31
Download citation
DOI: https://doi.org/10.1007/978-4-431-54589-7_31
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54588-0
Online ISBN: 978-4-431-54589-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)