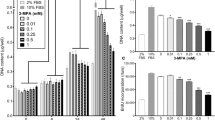
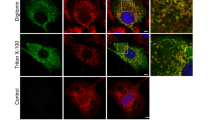
Abstract
Creatine Kinase (CK) is a key enzyme of eukaryotic energy metabolism. In chicken, the two cytosolic isoforms, brain- (B-CK) and muscle-type (M-CK), are expressed in a tissue specific manner. During muscle contraction, CK catalyzes the regeneration of ATP from phosphorylcreatine (PCr) and ADP (reviewed in Wallimann et al., 1992). In differentiating chicken embryonic skeletal muscle cells, during myotube formation, a CK isoenzyme transition from B-CK to M-CK takes place, which was also observed in cultured embryonic skeletal muscle cells (Perriard et al., 1978). B-CK is expressed at a high level until shortly after M-CK expression has started. This isoenzyme switch is part of the biochemical differentiation, which accompanies the morphological development of myoblasts. After leaving the cell cycle, postmitotic myoblasts align themselves into arrays and subsequently fuse to form multinucleated myotubes (see references in David et al., 1990). The myotubes show sarcomeric striations and are able to contract in culture, suggesting that their cellular metabolism resembles that of the in vivo situation. Essential steps in the process of myoblast fusion depend on the action of various protein kinases, such as the phospholipid/Ca2+-dependent protein kinase (PKC) and the Ca2+/calmodulin-dependent protein (CaM-) kinase (David et al., 1990). Cyclic AMP-dependent protein kinase (PKA) activity is also present during the time of fusion (Rogers et al., 1985).
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Chida, K., K. Kasahara, M. Tsunenaga, Y. Kohno, S. Yamada, S. Ohmi and T. Kuroki. 1990a. Purification and identification of creatine phosphokinase B as a substrate of protein kinase C in mouse skin in vivo. Biochem. Biophys. Res. Commun. 173:351–357.
Chida, K., M. Tsunenaga, K. Kasahara, Y. Kohno and T. Kuroki. 1990b. Regulation of creatine phosphokinase B activity by protein kinase C. Biochem. Biophys. Res. Commun. 173:346–350.
Cohen, P. 1989. The structure and regulation of protein phosphatases. Ann. Rev. Biochem. 58:453–508.
David, J. D., C. R. Faser and G. P. Perrot. 1990. Role of protein kinase C in chick embryo skeletal myoblast fusion. Dev. Biol. 139:89–99.
Entwistle, A., D. H. Curtis and R. J. Zalin. 1986. Myoblast fusion is regulated by a prostanoid of the one series independently of a rise in cyclic AMP. J. Cell Biol. 103:857–866.
Friedman, D. L., P. R. Puelo and M. B. Perryman. 1990. Phosphorylation of MM creatine kinase in striated muscle. FASEB J. 4:A2232.
Hemmer, W., S. J. Glaser, G. R. Hartmann, H. M. Eppenberger and T. Wallimann. 1991. Covalent modification of creatine kianse by ATP: evidence for autophosphorylation. In NATO ASI Series: “Cellular Regulation by Protein Phosphorylation.” L. M. G. Heilmeyer, eds., Springer-Verlag, Berlin. H 56:143–147.
Kemp, B. E. and R. B. Pearson. 1990. Protein kinase recognition sequence motifs. Trends Biochem. Sci. 15:342–346.
Kim, H. S., C. H. Chung, M.-S. Kang and D. B. Ha. 1991. Okadaic acid blocks membrane fusion of chick embryonic myoblasts in culture. Biochem. Biophys. Res. Commun. 176:1044–1050.
Mahadevan, L. C., S. A. Whatley, T. K. C. Leung and L. Lim. 1984. The brain form of a key ATP-regulating enzyme, creatine kinase, is a phosphoprotein. Biochem. J. 222:139–144.
Perriard, J.-C., M. Caravatti, E. R. Perriard and H. M. Eppenberger. 1978. Quantitation of creatine kinase isoenzyme transitions in differentiating chicken embryonic breast muscle and myogenic cell cultures by immunoadsorption. Arch. Biochem. Biophys. 191:90–100.
Quest, A. F. G., T. Soldati, W. Hemmer, J.-C. Perriard, H. M. Eppenberger and T. Wallimann. 1990. Phosphorylation of chicken brain-type creatine kinase affects a physiologically important kinetic parameter and gives rise to protein microheterogeneity in vivo. FEBS Lett. 269:457–464.
Rogers, J. E., S. Narindrasorasak, G. A. Cates and B. D. Sanwal. 1985. Regulation of protein kinase A and its regulatory subunits during skeletal myogenesis. J. Biol. Chem. 260:8002–8007.
Rosenberg, U. B., H. M. Eppenberger and J.-C. Perriard. 1981. Occurence of heterogenous forms of the subunits of creatine kinase in various muscle and nonmuscle tissues and their behaviour during myogenesis. Eur. J. Biochem. 116:87–92.
Schäfer, B. W. and J.-C. Perriard. 1988. Intracellular targeting of isoproteins in muscle cytoarchitecture. J. Cell Biol 106:1161–1170.
Soldati, T. and J.-C. Perriard. 1991. Intracompartmental sorting of essential myosin light chains: molecular dissection and in vivo monitoring by epitope tagging. Cell. 66:277–289.
Soldati, T., B. W. Schäfer and J.-C. Perriard. 1990. Alternative ribosomal initiation gives rise to chicken brain-type creatine kinase isoproteins with heterogeneous amino termini. J. Biol. Chem. 265:4498–4506.
Wallimann, T., M. Wyss, D. Brdiczka, K. Nicolay and H. M. Eppenberger. 1992. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem. J. 281:21–40.
Wirz, T., U. Brändie, T. Soldati, J. P. Hossle and J.-C. Perriard. 1990. A unique chicken B-creatine kinase gene gives rise to two B-creatine kinase isoproteins with distinct N termini by alternative splicing. J. Biol. Chem. 265:11656–11666.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1993 Springer-Verlag Berlin Heidelberg
About this paper
Cite this paper
Hemmer, W., Skarli, M., Eppenberger, H.M., Wallimann, T. (1993). Phosphorylation of Creatine Kinase in Myogenic Cells: Effects of Okadaic Acid and other Agents Affecting Cellular Protein Phosphorylation. In: Heilmeyer, L.M.G. (eds) Tyrosine Phosphorylation/Dephosphorylation and Downstream Signalling. NATO ASI Series, vol 76. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-78247-3_30
Download citation
DOI: https://doi.org/10.1007/978-3-642-78247-3_30
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-78249-7
Online ISBN: 978-3-642-78247-3
eBook Packages: Springer Book Archive