Abstract
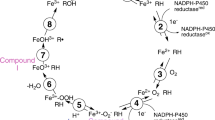
Cytochrome P-450-dependent monooxygenases are widely distributed in living organisms like mammals, fish, birds, yeasts and plants. In man, they exist in several organs and tissues with a maximum concentration in the liver. Some of them are in charge of the elimination of foreign compounds (xenobiotics) from the body and have to hydroxylate a broad spectrum of lipophilic compounds making them more hydrophilic and more easily excreted (Ullrich 1979). These cytochromes P-450 play a key role not only in pharmacology since they control the elimination rate of drugs, but also in toxicology since they seem to be the privileged sites of formation of reactive intermediates inside the cell.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Ahr HJ, King LJ, Nastainczyk W, Ullrich V (1982) The mechanism of reductive dehalogenation of halothane by liver cytochrome P-450. Biochem Pharmacol 13: 383
Augusto O, Ortiz de Montellano PR, Quintanilha A (1981) Spin-trapping of free radicals formed during microsomal metabolism of ethylhydrazine and acetylhydrazine. Biochem Biophys Res Comm 101: 1324
Battioni P, Mahy JP, Gillet G, Mansuy D (1983) Ironporphyrin dependent oxidation of methyl- and phenylhydrazine: isolation of iron(II)-diazene and a-alkyl-iron (III) complexes. Relevance to the reactions of hemoproteins with hydrazines. J Amer Chem Soc 105: 1399
Battioni P, Mahy JP, Delaforge M, Mansuy D (1983) Reaction of monosubstituted hydrazines with rat liver cytochrome P-450. Eur J Biochem 134: 241
Franklin MR (1977) Inhibition of mixed-function oxidations by substrates forming reduced cytochrome P-450 metabolic intermediate complexes. Pharmacol Ther A2: 227
Hines EN, Prough RA (1980) The characterization of an inhibitory complex formed with cytochrome P-450 and a metabolite of 1,1-disubstituted hydrazines. J Pharm Exp Therap 214: 80
Jonen HG, Werringloer J, Prough RA, Estabrook RW (1982) The reaction of phenylhy¬drazine with microsomal cytochrome P-450. J Biol Chem 257: 4404
Mahy JP, Battioni P, Mansuy D, Fischer M, Weiss R, Mispelter J, Morgernstern I, Gans P (in press) Iron-porphyrin-nitrene complexes. J Am Chem Soc
Mansuy D, Battioni JP, Chottard JC, Ullrich V (1979) Preparation of a porphyrin- iron-carbene model for the cytochrome P-450 complexes obtained upon metabolic oxidation of 1,3-benzodioxoles. J Am Chem Soc 101: 3971
Mansuy D (1980) New iron-porphyrin complexes with metalcarbon bonds. Biological implications, Pure and Applied Chem 52: 681
Mansuy D (1981) Use of model systems in biochemical toxicology: heme models. Rev Biochem Toxicol 3: 283
Mansuy D, Fontecave M, Battioni JP (1982) Intermediate formation of a a-alkyl-iron (III) complex in the reduction of 4-nitro-benzylchloride by iron(II)-porphyrins. JCS Chem Comm 317
Mansuy D, Battioni JP (1982) Isolation of a-alkyl-iron(III) or carbene-iron(II) complexes from reduction of polyhalogenated compounds by iron(II)-porphyrins: the particular case of halothane, CF3CHClBr. JCS Chem Comm 638
Mansuy D, Battioni P, Mahy JP (1982) Isolation of an iron-nitrene complex from the dioxygen and iron-porphyrin-dependent oxidation of a hydrazine. J Amer Chem Soc 104: 4487
Mansuy D, Fontecave M (1983) Reduction of benzyl halides by liver microsomes: formation of 478 run absorbing a-alkyl ferric cytochrome P-450 complexes. Biochem Pharmacol 32: 1871
Mason RP (1979) Free radical metabolites of foreign compounds and their toxicological significance. Rev Biochem Toxicol 1: 151
Ullrich V (1979) Cytochrome P-450 and biological hydroxylation reactions. Topics Curr Chem 83: 68
Wolff CR, Mansuy D, Nastainczyk W, Deutschmann G, Ullrich V (1977) The reduction of polyhalogenated methanes by liver microsomal cytochrome P-450. Molec Pharmacol 13: 698
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1983 Springer-Verlag Berlin Heidelberg
About this paper
Cite this paper
Mansuy, D. (1983). Reactive Intermediates Derived from Cytochrome P-450 Monooxygenases. In: Sund, H., Ullrich, V. (eds) Biological Oxidations. Colloquium der Gesellschaft für Biologische Chemie 14.–16. April 1983 in Mosbach/Baden, vol 34. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-69467-7_14
Download citation
DOI: https://doi.org/10.1007/978-3-642-69467-7_14
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-69469-1
Online ISBN: 978-3-642-69467-7
eBook Packages: Springer Book Archive