Abstract
Ecosystem services provided by marine bivalves in relation to nutrient extraction from the coastal environment have gained increased attention to mitigate adverse effects of excess nutrient loading from human activities, such as agriculture and sewage discharge. These activities damage coastal ecosystems and require action from local, regional, and national environmental management. Marine bivalves filter particles like phytoplankton, thereby transforming particulate organic matter into bivalve tissue or larger faecal pellets that are transferred to the benthos. Nutrient extraction from the coastal environment takes place through two different pathways: (i) harvest/removal of the bivalves – thereby returning nutrients back to land; or (ii) through increased denitrification in proximity to dense bivalve aggregations, leading to loss of nitrogen to the atmosphere. Active use of marine bivalves for nutrient extraction may include a number of secondary effects on the ecosystem, such as filtration of particulate material. This leads to partial transformation of particulate-bound nutrients into dissolved nutrients via bivalve excretion or enhanced mineralization of faecal material. In this chapter, concepts in relation to nutrient extraction by bivalves are presented and discussed in relation to nutrient cycling and additional effects of enhancing bivalve communities. In addition, methods to valorise nutrient extraction by bivalves are evaluated. Examples of calculations of the value of nutrient extraction by bivalves are presented.
Abstract in Chinese
摘要:农业和污水排放等人类活动造成的水体富营养化现象已经严重威胁到近海生态系统健康状况,亟需采取有效的环境管理策略进行应对,在这样的背景下,海水双壳类在移除营养物质方面的生态服务功能(受到了越来越多的关注。海水双壳贝类可以通过滤食行为将颗粒有机物转化成软体组织或较易沉淀的较大的粪便颗粒。近岸海域中营养物质的移除包括两种途径:i)收获/移除养殖的双壳贝类,由此将营养盐返回到陆地;或者ii)高密度养殖的双壳贝类能够增强氮的反硝化作用,使水体中的氮释放到大气中。但是,需要指出的是,双壳贝类在发挥净水作用的同时也会产生一系列相关的次生效应,如贝类通过摄食、排泄、粪便的矿化作用等将营养物质由颗粒态转化为溶解态。在本章,我们将介绍并讨论双壳贝类移除营养物质的过程和机制,包括营养盐的循环过程和大量贝类聚集时产生的附加效应。另外,我们评估了双壳贝类净水能力的价值和效率,并给出了贝类净化海域环境的价值计算实例。.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
关键词
1 Introduction
Excess loading of nutrients is one the largest concerns for the marine environment worldwide (Cloern 2001; Duarte et al. 2009). In the coastal zone, nitrogen loading from human activities within contributing watersheds and atmospheric deposition have prompted regulators, managers, and the political system to set standards for water quality and enforce legislation to prevent further deterioration of the marine environment. On a larger scale, examples include the legislative actions enforced by the European Union, e.g. the Nitrate Directive (Anon. 1991), the Water Framework Directive (Anon. 2000) and the Marine Strategy Framework Directive (Anon. 2008) that all aim to reduce nutrient loading – in particular nitrogen – to the marine environment as a means to improve water quality. Traditional measures utilized to reduce nutrient loading to the marine environment are land based. These are directed either towards point sources like sewage treatment plants, or diffuse emissions mainly from cultivated land. Abatement measures for diffuse sources comprise a long list; including restrictions in fertilization, restriction in the periods where fertilization is allowed, requirements for catch crops and winter green fields, wetland restoration and wetland reconstruction, afforestation, and fallowing of intensively cultivated fields. With increasing marginal costs for implementing traditional land-based abatement measures (Hasler et al. 2012), it is appealing to look for alternatives, such as mitigation measures in the recipient water bodies. Strategies less costly than traditional abatement measures are attractive in coastal zones where population densities are low. Finally, internal loading from sediments in areas that have been affected by decades of excess nutrient loading is a problem for water quality that can only be dealt with by marine mitigation measures.
In this context, nutrient extraction services provided by bivalves become interesting. Through their filtering of water, bivalves remove a proportion of the phytoplankton that in large concentrations otherwise is part of the negative effects of excess nutrient loading. By clearing the water column of particles, bivalves contribute to reductions in turbidity and concentrations of particulate organic nutrients, like nitrogen and phosphorous (see e.g. Dame 2012 and references therein). The filtered material is either not ingested and ejected as pseudo faeces or is ingested and digested, then transformed into bivalve tissue or faecal material that settles in proximity of the bivalves. Nutrients in the ingested material that is transformed into bivalve tissue are immobilized, hence temporarily not accessible for primary production. If the bivalves are removed from the water column, e.g. through harvest, the nutrients are permanently made inaccessible. The material ejected as faeces or pseudo faeces can enter nutrient cycles that may result in either permanent burial in the sediment or removal through chemical processes; i.e. denitrification. Both processes will result in a nutrient extraction service provided by the bivalves that potentially can be used as a mitigation tool by managers seeking means of remediating effects of excess nutrient loading to coastal ecosystems. This can be realized as either bivalve aquaculture or by promoting or restoring natural bivalve populations (Fig. 10.1); e.g. oyster reefs or mussel beds.
2 Nutrient Extraction Through Bivalve Aquaculture
Nutrient extraction through mussel farming or other forms of bivalve aquaculture is based on two simple principles: (1) By providing substrate for mussel or oyster larvae to settle on or by other means of actively increasing recruitment, (e.g. deploying seed from hatcheries) resulting in new bivalve biomass being produced; and (2) mass balance, i.e. the nutrients stored in bivalve tissue are extracted from the water body where bivalves are produced at harvest. Irrespective of the efficiency of the bivalves in transforming particle-bound nutrients into tissue, including the loss of nutrients as dissolved nutrients from the bivalves as excretion (see e.g. Cranford et al. 2007), there will be a net nutrient extraction at the water body scale when the bivalves are harvested (e.g. Holmer et al. 2015; Guyondet et al. 2015).
Bivalve aquaculture for consumption is performed in many different ways, from artisanal seeding of infauna clams to offshore mussel farming on specialized long-line systems. Common for all types is that the main aim is to produce an optimal product suited for human consumption. There is thus less focus on maximum biomass removal or nutrient extraction. However, as long as the culturing activity has resulted in a new production of bivalves and the bivalves are harvested, it can be assumed that nutrients have been removed from the system, or more precisely, have been recycled back to land.
A special application of bivalve aquaculture is bivalve farming aimed at nutrient extraction either as a mitigation tool in relation to general eutrophication, i.e. from diffuse sources, or as a specialized tool in integrated multi trophic aquaculture (IMTA) where bivalve farming is intended to remove particle bound nutrients lost from fish farming (e.g. Chopin 2013). In bivalve farming for nutrient extraction, excess amounts of nutrients in the coastal waters are considered as a resource to be utilized as recycled back to land (Hart 2003; Petersen 2004; Møhlenberg 2007; Lindahl and Kollberg 2009; Weber et al. 2010; Rose et al. 2014, Bricker et al. 2014, Kellogg et al. 2014, Petersen et al. 2016). Nutrients in the marine environment – but originating from land – are taken up by the bivalves as particles, preferably phytoplankton, and returned back to land after harvest. Bivalve farming for nutrient extraction will thus immobilize nutrients lost to the aquatic environment, store them in bivalve tissues, and to a lesser extent in shell (and byssus), and bring them back to land when harvested. Back on land, the bivalves can be used for various purposes, e.g. food, feed, or otherwise; and thus provide additional goods and services. This concept has been termed mitigation, bioremediation, bio-extraction, bio harvesting, agro-aqua recycling, or compensation aquaculture (Petersen et al. 2014); and the whole process is based on a mass balance principle in the recipient water body. Furthermore, there is not necessarily a direct link between the nutrient source and the nutrient extraction process. In IMTA, bivalve farming is physically directed toward marine point sources of nutrients, like aquaculture of fed cultivated animals i.e. fish and shrimps (Chopin 2013; Troell et al. 2009). It should be noted, however, that bivalves only take up nutrients as particulate matter and will thus only be able to directly use the nutrients emitted from a fish farm to a negligible extent. Additionally, due to hydrodynamic constraints, cultured bivalves in IMTA farms will have difficulties filtering major parts of the particulate waste material pool released from fish farms (Cranford et al. 2013; Petersen et al. 2016). Hence, the mitigation of nutrient release from a fish farm in IMTA also works on the mass balance principle, and not as a measure to remove nitrogen and phosphorous molecules released from the fish farm (Cranford et al. 2013; Petersen et al. 2016).
Farming bivalves with the main aim of extracting nutrients from the aquatic environment is different in nature from most commercial bivalve farming, which is mainly performed for human consumption (Farber et al. 2006) or a recent farming practice aimed at providing seed for on-bottom culture of blue mussels (Capelle 2017). Commercial bivalve aquaculture aims for uniform size, high quality and good appearance; where the product is very dependent on the market. Mitigation bivalves are produced to remove as much nutrients as possible at the lowest costs in order to be an efficient tool from a management point of view. The resulting product may not necessarily, or entirely, be suited for human consumption due to its size, heterogeneity, and appearance (Petersen et al. 2016). This has some implications for farming practice, as it for several reasons (e.g. cost-effectiveness), may be preferable to harvest young and small bivalves rather than wait for commercial size:
-
1.
Total bivalve biomass, rather than individual size and quality, matters for harvesting time. Bivalves grow fast in the early stages; relative biomass gain on the production unit will be greater in early stages after recruitment compared to later stages when bivalves are approaching commercial size. Sometime after settling (or deployment), space may become a limiting factor, and density (number of individuals per area settling material) will decrease (e.g. Lahance-Bernard et al. 2010). Biomass may still increase as mussels can grow on top of each other and self-thinning will reduce density without affecting biomass. Ultimately, lack of space or competition for food may become limiting for further biomass increase. In commercial mussel farming, this will result in the farmer either thinning (on net structures) or socking (in long-line units), or losing a part of the crop due to self-thinning as mussels become detached from the settling material (Lahance-Bernard et al. 2010). When the mussels become approximately 1 year old, new spat may start settling on the culture unit; thereby further increasing competition for space. It is thus important in mitigation farming to harvest at the time of maximum biomass.
-
2.
An additional factor may influence harvested biomass: Biofouling. There are many reports on fouling of aquaculture units (see e.g. Locke et al. 2009 and references herein) and the consequences for bivalve aquaculture production (see e.g. Daigle and Herbinger 2009). If bivalve production is affected negatively by fouling, it will affect biomass development and hence the mitigation effect. On the other hand, if the production strategy is designed to promote early harvest, levels of biofouling will be reduced in comparison to present commercial farming practices, due to shorter immersion times of the farm structure. Biofouling may even increase nutrient capture and the subsequent removal when harvested.
-
3.
Total biomass per se is not, however, the only guiding parameter for optimization of farming for nutrient extraction. Nutrient content of the biomass is also important, and different parts of the bivalves contain different amounts of nutrients; for example, the shells of blue mussels have lower nutrient concentrations than blue mussel tissue (Petersen et al. 2014). Tissue weight will fluctuate over the year, resulting in varying total concentrations of nutrients, as tissue content of mussels will depend on size, growth state, and gonadal cycle of the mussels (e.g. Dare and Edwards 1975; Rodhouse et al. 1984). In general, relative tissue content is highest in small, fast-growing bivalves (see e.g. Smaal and Vonck 1997). In addition, blue mussel byssus may add substantially to the total nutrient extraction of blue mussel farming. In a recent experiment in Skive Fjord, Denmark, 12–19% of the nitrogen removal through harvest was from blue mussel byssus (Petersen et al. 2014). In relation to biofouling, nutrient content of the fouling organism will also matter.
-
4.
When bivalve farming is implemented primarily for nutrient extraction, resource allocation for handling the aquaculture unit becomes important in relation to yield in the form of nutrient build-up in the farmed bivalves. Resources include labour, materials like buoys (for keeping the long-lines floating) or on-bottom structures, and boat hours. Beyond a certain point, further investments in labour and/or equipment will not result in increased biomass of bivalves; while preceding that point, the investment will not match the net gain in biomass. Factors determining how long bivalves are to be maintained in the aquaculture unit, rather than being harvested include: relative increase in biomass, tissue content of the bivalves, and environmental conditions like potential ice coverage or increased frequency of storms requiring additional efforts to maintain the biomass.
There is little experimental validation on full scale of bivalve farming with the purpose of nutrient extraction. To our knowledge, the only scientific validation experiment on full production scale of mitigation aquaculture using blue mussels has so far been performed in Skive Fjord, the Limfjorden Denmark (Petersen et al. 2014). Skive Fjord is a shallow estuary with a mean depth of 4.7 m, in the inner part of the Limfjorden (Maar et al. 2010). In the Limfjorden, there are almost no tidal currents and the water column varies between stratified and mixed conditions on a time scale of days to weeks, controlled by differential advection, fresh water input, heating and mixing (Maar et al. 2010; Wiles et al. 2006; Møhlenberg 1999). Skive Fjord is highly nutrient-enriched, characterized by high chlorophyll a concentrations and high primary production throughout the year and seasonal hypoxia occurring in late summer (Møhlenberg 1999; Maar et al. 2010; Holmer et al. 2015). Production of blue mussels (Mytilus edulis) in this trial took place on approx. 90 km of settling material deployed in May 2010 on 90 long-lines in an approximately 19 ha aquaculture unit. During the production period – from deployment of settling material in May 2010 to test harvest in October 2010 and March 2011 and final harvest in May 2011 – there was no intermediate handling (e.g. socking or thinning) of the settled mussels. The only handling of the aquaculture farm during the course of the production period was ordinary maintenance, in particular adding support buoys (buoying up) as mussels grew. By May 2011, approximately 1100 t of fresh mussels could be harvested corresponding to 16 t of N and 0.7 t of P. The efficiency of the aquaculture unit corresponds to a removal of 0.6–0.9 t N ha−1 year−1 and 0.03–0.05 t P ha−1 year−1 (Petersen et al. 2014). This is more area-efficient in nutrient removal compared to most land based abatement measures, such as establishing riparian wetlands or buffer strips, which is estimated to remove 0.1 and 0.04 t N ha−1 year−1, respectively (Petersen et al. 2014). Despite observed depletion of phytoplankton both on the micro scale (close to the mussels) and on the farm scale (Nielsen et al. 2016), there was no evidence of food limitation in the farm (Fig. 10.2). Measurements of spatial variations in mussel biomass throughout the year showed no significant differences in mussel biomass between farm sections as well as between edges, and the centre of the mussel farm. Thus, reduced growth of mussels positioned downstream was not observed in the mussel farm in Skive Fjord, as observed/modelled in other mussel cultivation units (Heasman et al. 1998; Fuentes et al. 2000; Strohmeier et al. 2005; Aure et al. 2007; Petersen et al. 2008a, b; Strohmeier et al. 2008; Rosland et al. 2011). A food depletion model indicated that total mussel filtration rates could be increased by 80–120% without exceeding threshold for the necessary food supply to maintain growth (Nielsen et al. 2016); exhibiting options for further improvement of area efficiency of the mussel production/nutrient extraction (e.g. by approximately doubling the standing stock of mussels within the farm area – if practically possible).
Specific measured mussel growth rates (% day−1) (white bars) and the corresponding potential maximal growth derived from DEB modelling (grey bars) calculated for different timespans between biomass sampling dates (from Nielsen et al. 2016)
As the Baltic Sea can be considered highly nutrient enriched and suffering from the negative effects of excess nutrient loading to the marine environment, it is logical that mitigation measures in the recipient water body have been considered in the Baltic Sea (Stadmark and Conley 2011). In the Western Baltic Sea, trials using mussel production for nutrient extraction have been carried out in the municipality of Lysekil, Sweden. In the period 2005–11, the municipality was allowed to purchase ecosystem services in the form of nitrogen removal through blue mussel farming from a mussel farmer producing for human consumption (Lindahl 2011). There is no scientific documentation of production volumes and efficiency of the mitigation measure during the trial period, and the trial was terminated before the trial period had expired due to the mussel farmer’s financial problems (Kollberg, pers. comm.). The municipality achieved acceptance, in relation to the European Community sewage directive, to exchange nitrogen removal in a sewage treatment plant with nutrient removal through mussel production (Lindahl 2011). However, experiences from the trial indicated that when nutrient extraction is tightly connected to mussel production, primarily aiming at other purposes than nutrient extraction, and the payment of ecosystem services amounts to a minor part of the production costs, there is a high risk of non-compliance with the set goals for nutrient extraction. In the Central and Eastern Baltic, nutrient extraction through mussel production is challenged by low salinity, making production of blue mussels suboptimal (e.g. Maar et al. 2015). According to Lindahl (2012), there have been a number of small trials with blue mussel production from the Great Belt in the west (see also Riisgård et al. 2014) to the Åland archipelago in the east. The trials demonstrated that blue mussels settle and can be grown to sizes leading to substantial biomass accumulation, but growth rates are very low. In the BalticSea2020 project on mussel farming as an environmental measure in the Baltic Sea (http://balticsea2020.org/english/), it was shown that in the Åland archipelago, up to 14 kg of mussel ha−1 could be harvested after 2–3 years; however, some trials resulted in less than 10% of this biomass, with mussels of a maximum length of 25 mm. As mussels could be grown on nets up to 4 m in height it was estimated that there is a potential to produce up to 100–150 t blue mussel ha−1 over a 2–3-year period corresponding to 1.2–1.8 t N ha−1 removal (http://balticsea2020.org/english/). These numbers should probably be considered with some care as they are extrapolated from a rather small test sample.
In the Baltic proper, an alternative option that has been proposed is to farm zebra mussels, Dreissena polymorpha, for nutrient extraction. Zebra mussels are widely distributed in the area, from freshwater to brackish and low saline areas, where they can be present in relatively high abundances (Zaiko et al. 2011). The effects of filtration of zebra mussels in freshwater ecosystems are well documented (see e.g. Fahnenstiel et al. 1995; Idrisi et al. 2001; Smith et al. 1998; Caraco et al. 2006; Weber et al. 2010, Pires et al. 2010). From a more theoretical perspective, it has been suggested to use farming of zebra mussels for nutrient extraction (e.g. Stybel et al. 2009; Schernewski et al. 2012). Experiments with farming zebra mussels have been launched in the Oder/Szczecin Lagoon on the border between Germany and Poland and the Curonian Lagoon, Lithuania, but so far with limited data on efficiency (Lindahl 2012). When using zebra mussels for mitigation purposes, and thereby actively taking steps that can result in further proliferation of the species, precautions should be taken that the species is invasive and can cause severe changes to ecosystems. As an invasive species, a large body of scientific literature has documented the changes that zebra mussels can cause in recipient ecosystems. The financial costs preserved by using zebra mussels for mitigation purposes in relation to eutrophication effects may be cancelled-out by increased control of the undesired effects, in systems where they are invasive. The same principle would apply for using the Pacific oyster (Crassostrea gigas) as a mitigation crop in areas, where it is not native. Pacific oysters are today a commercial crop in many countries and one of the largest global aquaculture crops, making a direct comparison with zebra mussels difficult. However, a number of countries still prohibit aquaculture of Pacific oysters; and the damage Pacific oysters can cause in coastal ecosystems are well documented (e.g. Herbert et al. 2016). In areas where they are not endemic, the spread of the Pacific oyster should thus in principle not be enhanced as a means to harvest goods and services from bivalve aquaculture, for the same reasons that apply to zebra mussels.
A special case of nutrient extraction is the potential use of blue mussel spat collectors in Dutch on-bottom culture. In Dutch on-bottom blue mussel production, the mussel seed fishery will (as a consequence of a national compromise between NGOs, industry and the government) gradually be replaced by spat collection on floating spat collectors (Capelle 2017). Spat collection resembles production for nutrient extraction as the primary purpose is to maximize viable mussel spat (i.e. biomass), rather than selectivity for size and quality of the mussels. Thus, to a large degree, Dutch on-bottom culture can be considered as new production, especially in the Oosterschelde where natural spat fall is limited (Capelle 2017). This method generally yields biomass production of 1.5–2.5 kg harvested mussel per kg seeded (Capelle 2017); as such, there is in principle a net nutrient extraction also after a relay period on the bottom. However, in the first approximately 2 months after relay of the seeded mussels, there is a loss of 60–69% of the seeded mussels coming from spat collectors (Capelle et al. 2016). Some of the loss will result in increased nutrient regeneration; therefore, the extraction effect of this aquaculture practice is debatable. If relative biomass production approaches 1, on-bottom culture becomes less relevant in a nutrient extraction perspective, and its primary ecosystem service will be provisioning.
3 Nutrient Extraction Through Altered Nutrient Cycling
The basic principle of nutrient extraction provided by bivalves through altered nutrient cycling is that aggregations of bivalves (e.g. in bivalve beds or in/below aquaculture units) augmenting the capture of organic material. This mechanism leads to altered biogeochemical processes and subsequently loss of nitrogen through enhanced denitrification (Rose et al. 2014). This type of nutrient extraction can further be pursued as goods and services; provided by artificially established or re-established bivalve beds, e.g. oyster reefs (Kellogg et al. 2014) or by bivalve aquaculture (Humphries et al. 2016). Enhanced denitrification is of further interest if the cultured bivalves, as recently demonstrated for Crassostrea virginica and Crassostrea gigas, can contribute to denitrification themselves (Caffrey et al. 2016).
Chemical Reactions in the Sediment
\( {\displaystyle \begin{array}{ll}\mathrm{Nitrification}& {\mathrm{N}\mathrm{H}}_{4+}+2{\mathrm{O}}_2\to {\mathrm{N}\mathrm{O}}_{3-}+{\mathrm{H}}_2\mathrm{O}+2\mathrm{H}\\ {}\mathrm{Denitrification}& {\mathrm{N}\mathrm{O}}_{3-}\to {\mathrm{N}\mathrm{O}}_{2-}\to \mathrm{NO}\to {\mathrm{N}}_2\mathrm{O}\to {\mathrm{N}}_2\\ {}\mathrm{ANAMMOX}& {\mathrm{N}\mathrm{H}}_{4+}+{\mathrm{N}\mathrm{O}}_{2-}\to {\mathrm{N}}_2+{\mathrm{H}}_2\mathrm{O}\\ {}\begin{array}{l}\mathrm{Dissimilatory}\ \mathrm{nitrate}\ \\ {}\mathrm{reducation}\ \mathrm{to}\ \mathrm{ammonium}\end{array}& {\mathrm{N}\mathrm{O}}_{3-}\to {\mathrm{N}\mathrm{O}}_{2-}\to {\mathrm{N}\mathrm{H}}_{4+}\end{array}} \)
Denitrification is a suboxic process. In shallow and often turbulent coastal ecosystems, denitrification is confined to a narrow zone in the surface sediments, typically from a few millimetres to a few centimetres below the sediment surface. Denitrification requires nitrate as an electron acceptor, which is either produced through nitrification, referred to as coupled nitrification-denitrification, or supplied by diffusion from the water column into the sediments. Nitrification only occurs under oxic conditions in the sediments, and rates of nitrification show large seasonal variation controlled by water temperature, ammonium availability, and oxygen concentrations in the sediments. Under eutrophic conditions with high sediment oxygen consumption in the summer, nitrification may be inhibited; thereby diminishing coupled nitrification-denitrification and leading to low rates of denitrification during the summer. Rates of denitrification also exhibit large seasonal variation, but less predictable, as high nitrate concentrations in the water column in the spring may stimulate rates, independent of water temperature and nitrification rates. Both nitrification and denitrification rates typically increase concurrently with transfer of organic matter to the sediments and linked remineralisation and ammonium availability; but only to a certain extent, when nitrification is inhibited due to low oxygen availability in the sediments. In this case, dissimilatory nitrate reduction to ammonium (DNRA) becomes a dominant process, eventually resulting in an elevated transfer of ammonium from the sediments to the water column. Nitrogen cycling in organically enriched sediments (e.g. from sedimentation of biodeposits from bivalve aggregates) may thus be very different from surrounding non-impacted sediments. Organically enriched sediments can be either larger sinks of nitrogen through enhanced denitrification, removing nitrogen from the marine area through N2 production, or larger sources of nitrogen to the water column by enhanced DNRA and NH4 + production. Permanent nitrogen removal from the marine environment, as in denitrification, also occurs in the anammox process, where N2 is formed in the sediments by bacteria using NH4 + and NO2 −. Anammox is, however, most important for nitrogen removal in oligotrophic systems, where it may contribute up to 80% of the nitrogen removal compared to <20% in organic enriched systems (Dalsgaard et al. 2005); and has been found to play only a minor role in N2 removal in bivalve sediments (Minjead et al. 2009; Higgins et al. 2013).
Due to the increased availability of organic matter and ammonium in or below bivalve aggregations, there is a potential for stimulated nitrification and denitrification. Studies with measurements of rates of nitrification and denitrification demonstrate variable response to bivalve aggregations, as rates can be reduced or enhanced depending on bivalve species, sediment conditions, and environmental factors (Table 10.1). Giles and Pilditch (2006) examined the effects of mussel (Perna canaliculus) aquaculture on sediment oxygen uptake and nutrient fluxes, and found extensive seasonal variation with higher rates of nitrogen release from the sediments at the farm in spring and autumn, but lower during the summer compared to a reference. They suggested that lower nitrogen efflux during the summer was due to enhanced denitrification, reducing the efflux of dissolved inorganic nitrogen compounds, but overall the farm sediments contributed to greater nutrient regeneration compared to the reference site. In this case, denitrification was probably enhanced by the higher organic matter input to the sediments, but without compromising the nitrification rates. So, higher denitrification rates delivered intensified nitrogen regeneration in the sediments. Similarly, studies of hard clams (Mercenaria mercenaria) showed increased fluxes of ammonium and phosphate compared to uncultivated sediments; denitrification rates were also enhanced, but only for parts of the growth season (Table 10.1, Murphy et al. 2016b). In the same study, DNRA was stimulated throughout the growth season and appeared to be the favoured nitrogen cycling process over denitrification, enhancing nitrogen flux to the water column. Welsh and Castadelli (2004) reported enhanced nitrification and coupled nitrification-denitrification from several different bivalves, and suggested that animal-associated nitrogen cycling contributed significantly to nitrogen regeneration in these systems. Furthermore, studies of the manila clam (Ruditapes philippinarum) showed that the clams contributed 64–133% of the total rates of sediment oxygen uptake, nitrogen regeneration, nitrification, and denitrification. This indicates that clam biomass/density play a crucial role in nitrogen cycling in bivalve farming areas (Welsh et al. 2015). Enhanced rates were due to metabolic activity of clams and bacterial activity hosted on the clams. The clam sediments were significant sources of both N2 and N2O gasses through enhanced nitrification and denitrification. Yet, as N2O is a greenhouse gas, this contribution is important to consider in environmental impact assessments of bivalve culturing.
Special attention has been devoted to natural or re-established oyster reefs on the North American east coast (see e.g. Kellogg et al. 2014; Smyth et al. 2015). In a feature paper, Kellogg et al. (2013) estimated annual denitrification rates in restored oyster reefs in the Choptank River, Chesapeake Bay, USA, resulting in removal of approximately 0.5 t N ha−1 year−1 more than at control plots (Kellogg et al. 2013). This corresponds to removal rates from mussel farming as described above. Besides the uncertainty of measuring the effect of oyster reefs (see e.g. Hoellein and Zarnoch 2014; Smyth et al. 2015; Lindemann et al. 2016), there are some caveats in extrapolating this number to larger areas. The rates measured by Kellogg et al. (2013) are in the high end of results when compared to other studies (see Kellogg et al. 2014), and may not be entirely representative for all coastal areas. Further, denitrification rates are variable between reefs/areas; there are large differences between seasons making integration over entire years problematic, and there may also be differences depending on methods (Kellogg et al. 2014; Humphries et al. 2016). The differences between reefs may be explained by their position above or below the euphotic zone (Newell et al. 2005), the actual physical structure of the reef, as well as bioturbation and feeding activities of associated fauna in and around the reefs (e.g. Nizzoli et al. 2006; Smyth et al. 2016). It has generally been concluded that denitrification rates are enhanced in natural or restored oyster reefs compared to rates in oyster aquaculture, probably due to inhibition of nitrification in the more anoxic aquaculture sediments (Higgins et al. 2013; Kellogg et al. 2014; Smyth et al. 2016). However, recent studies have demonstrated comparable denitrification rates in both restored reefs and oyster aquaculture (Humphries et al. 2016). By adding the apparent discrete effect of oysters to denitrification rates (Caffrey et al. 2016), and to a much lesser extent solely empty shells, bivalve mediated denitrification can be considered as an important nutrient extraction service.
4 Additional Mitigation Benefits
Bivalve aggregations or bivalve aquaculture may not only facilitate nutrient extraction either through harvest of bivalves, or as enhanced nitrogen loss to the atmosphere, but may also mitigate effects of excess nutrient loading by filtering the water column and thus removing phytoplankton. This is an important aspect of the ecosystem services provided by bivalves as phytoplankton concentrations directly or indirectly serve as ecosystem health indicator, and high concentrations are seen as an indication of adverse effects. For example, in the EU Water Framework Directive, concentration of chlorophyll a is an intercalibrated indicator in the Baltic eco-region, and high concentrations are also indirectly influencing the depth limit of eelgrass, which is another indicator.
The effects of bivalve suspension feeding on water column phytoplankton concentrations were first described for South San Francisco Bay (Cloern 1982). Using a simple model describing change in phytoplankton concentration, where dispersive transport and zooplankton grazing balance growth rate, calculated concentrations of phytoplankton were much higher than actually observed concentrations in the bay (ibid). By estimating the filtration capacity of benthic suspension-feeders, primarily clams, it was shown that these had the capacity to clear the water column more than once per day. It was further described that invasion of a non-indigenous clam in the northern part of the bay resulted in persistently low levels of phytoplankton (Alpine and Cloern 1992). Since then, a number of studies have demonstrated the impact of grazing exerted by benthic bivalves on the overlaying water column, and on the basin scale (e.g. Hily 1991; Møhlenberg 1995; Ackerman et al. 2001; Petersen et al. 2013). An illustrative example is from Ringkøbing Fjord, Denmark (Petersen et al. 2008a, b), where a small change in sluice practice allowed slightly more saline water from the North Sea to enter the estuary, causing a small increase in salinity. This allowed for massive recruitment of the clam Mya arenaria. With the invasion of clams, benthic grazing became the key feature of the biological structure, causing a sudden regime shift from a bottom-up controlled turbid state, into a top-down controlled clear water state. Mean annual concentration in chlorophyll a dropped concurrently and significantly from 52.3 μg l−1 in the period 1989–94 to 8.7 μg l−1 in 1997–2004 concomitant with the increase in benthic grazing capacity. In the years around the change in sluice management, the change in mean annual concentration of chlorophyll a was especially evident, with a decrease from 64.6 μg l−1 in 1995 to 21.0 μg l−1 in 1996 and 7.6 μg l−1 in 1997. Phytoplankton composition and zooplankton abundance were also affected by the change following the invasion of clams.
The impact of clearance by large populations of bivalves on water column concentrations of phytoplankton/particulate matter at the basin scale will depend not only on the size of the populations and their clearance capacity, but also on water residence time in the basin. Bivalve top-down control of phytoplankton biomass can be achieved when clearance time, i.e. time needed for the bivalve standing stock to clear the entire water column, is shorter than residence time, or primary production time, defined as rate of renewal of the phytoplankton biomass (Dame and Prins 1998). Experimentally it has been demonstrated that under well-mixed conditions, a mussel standing stock with a potential clearance time of 20–35% d−1 of the entire water volume is enough to control phytoplankton biomass under conditions where primary production is not limited by nutrient concentrations (e.g. Cloern 1982; Prins et al. 1995; Prins et al. 1998; Wang and Wang 2011). Similar conclusions can be drawn from modelling exercises (e.g. Herman and Scholten 1990), indicating that increasing nutrient loading under conditions with high suspension-feeding pressure will only marginally change phytoplankton concentrations.
The clearance effects of suspended cultures of bivalves on water column concentrations of phytoplankton have also been documented in the scientific literature (see e.g. Heasman et al. 1998; Cranford et al. 2008; Grant et al. 2008; Petersen et al. 2008a, b; Cranford et al. 2014; Nielsen et al. 2016). In Skive Fjord, where to date the most extensive experiment with farming of mussels for mitigation purposes took place, depletion of phytoplankton could be observed on all scales; from nearby the mussel lines on the micro scale to farm scale (Nielsen et al. 2016). Farm-scale depletion was detected and visualized based on intensive 3D spatial surveys of the distribution of Secchi depth, chlorophyll a and total suspended particulate matter concentrations, both inside and outside the farmed area. Depletion of phytoplankton concentrations within the farm was measured, with average depletion levels of 13–31%; while some areas exhibited >50% depletion. The depletion effects were most pronounced within the farm. Additional model studies showed that summer chlorophyll a concentrations were reduced by 30%, and Secchi depth (Fig. 10.3) was improved by 16% relative to a reference situation without the mussel farm (Nielsen et al. 2016). The environmental effects of mussel clearance were however not only evident on the farm scale, but also on the basin scale. The area affected by mussel clearance reached to the shoreline, thereby potentially increasing areas suitable for submerged vegetation (Petersen et al. 2016). Adding more mitigation farms to the model would increase the effect on chlorophyll a concentration and light attenuation in the Skive Fjord estuary (Timmermann pers. comm.). Thus, given sufficient capacity, farming of bivalves can act as a control mechanism for the effects of nutrient enrichment, like increased phytoplankton biomass, as could the natural population of clams in Ringkøbing Fjord. As such, a strategy for mitigating effects of excess nutrient enrichment can be to establish extractive bivalve aquacultures, especially in relation to internal loading and diffusive sources. In relation to production carrying capacity of mitigation aquacultures – i.e. where mussel productivity is limited by a shortage of phytoplankton – it is not a major concern in mitigation aquacultures in contrast to commercial mussel production. On the contrary, it can be considered as the objective of mitigation mussel farming, as the aim of this type of aquaculture is to remove nutrients and improve water transparency. If carrying capacity on the basin scale becomes an issue, and the production volume decreases and/or environmental parameters like water transparency improves, the purposes of the mitigation farming have been realized, and mitigation aquaculture can be discontinued. In any case, heavily nutrient-enriched systems of interest in this context will require extensive mitigation aquaculture in order to approach limitation of production carrying capacity (Petersen et al. 2016).
5 Nutrient Extraction and Nutrient Cycling
The basis of understanding nutrient cycling and potential nutrient extraction in relation to bivalve aggregations – either as aquaculture units or as dense beds/populations – is the trapping of suspended particles in the water column through suspension-feeding, the partitioning of the trapped particles into bivalve tissue (and shell), and as waste products, either dissolved through excretion or as particulate matter as faecal pellets or pseudo faeces. Depending on water transport rate, water depth, and potential resuspension, faecal material will be concentrated in or nearby bivalve aggregations (Chamberlain et al. 2001; McKindsey et al. 2011). Excretion from bivalves is a relatively fast process (compared to regeneration of particle waste products), where particle bound nutrients captured by the bivalves are transformed to dissolved nutrients (mainly ammonia), and thus easily accessible to primary production. Excretion from bivalves is in the order of 10% of the ingested material and has been shown to account for up to 82% of the nitrogen regeneration in mussel farming (Holmer et al. 2015). The turnover of nutrients from excretion is increased compared to release from biodeposits, where decomposition has to take place before nutrients become available in dissolved forms, leading to a slower turnover compared to direct excretion.
Solid waste products from bivalve aggregates will settle on the bottom below or next to the bivalve aggregations. Nutrient regeneration in the surrounding sediments are typically enhanced by the higher quantity (e.g. Hatcher et al. 1994; Grant et al. 1995) and higher quality (e.g. Carlsson et al. 2009) of organic material produced as a result of bivalve digestion, compared to locations lacking larger bivalve populations. Rates of nutrient regeneration reflect the activity of the bivalves, with large seasonal variations controlled by, for example, food availability, water temperatures, and environmental conditions. The regeneration of nitrogen is particularly critical in coastal ecosystems as nitrogen loading from land is high, and are affected by eutrophication with nitrogen as the most important limiting nutrient (Conley et al. 2009; Carstensen et al. 2013; Murphy et al. 2016a). Potential enhanced nitrogen regeneration is thus important to take into account when evaluating the history of coastal areas with major losses of bivalves (e.g. loss of oyster reefs, blue mussel beds; Caffrey et al. 2016), when planning restoration projects (oyster reefs, blue mussel beds; Kellogg et al. 2014, Smyth et al. 2016), or when applying bivalves for mitigation purposes (Stadmark and Conley 2011; Petersen et al. 2012; Petersen et al. 2014). On the other hand, bivalves have a high content of nitrogen in their tissues and shells due to their high protein content and when harvested, significant amounts of nitrogen are permanently removed from the marine environment (Kellogg et al. 2013; Holmer et al. 2015; Petersen et al. 2014). Furthermore, increased denitrification may contribute to a net removal of nitrogen from the ecosystem, particularly if the rates are stimulated during critical periods of the growth season for primary production, such as during summer where nitrogen is the limiting nutrient of phytoplankton production. The general conclusions of the published literature indicate that the regeneration of nitrogen is higher in bivalve aggregates and the nearby surroundings compared to reference sites, and the aggregations/sediments should be considered as net contributors to nitrogen in the water column, when summing up over a production/growth season. There are, however, several important considerations to be taken into account. First, most studies with in situ measurements of denitrification show that rates are enhanced in aggregations/sediments (e.g. Carlsson et al. 2012; Kellogg et al. 2013; Welsh et al. 2015), and the net removal of nitrogen from the marine environment is thus more than just the harvest of bivalves (Table 10.1). The area-specific denitrification rates are typically enhanced, with 25–260% compared to reference conditions (Carlsson et al. 2012). Kellogg et al. (2014) estimated that annual denitrification rates were enhanced from 2.7 to 55.6 g N m−2 year−1 in oyster reefs. These rates can be quite significant in comparison to nitrogen loading from land – e.g. 1.4–60.1 g N m−2 year−1 on the East coast of US (Carmichael et al. 2012) and 0.5–100 g N m−2 year−1 in Danish estuaries (Timmermann pers. comm). Second, bivalve aggregations are concentrators of organic matter (phytoplankton and seston) in the ecosystem, due to their filtering of large volumes of water followed by sedimentation of organic matter in much smaller area, thereby concentrating organic matter enrichment of the ecosystem to a limited area. Biodeposits are heavy and sink to the sediment on the scale of minutes from long-line cultures, and rapidly settle after resuspension events (e.g. Giles and Pilditch 2006; Carlsson et al. 2012). High sedimentation rates thus confine organic enrichment to the immediate vicinity of the aggregations. In contrast, due to increased capture of particles in the bivalve culture, sedimentation will be reduced outside the unit, i.e. on basin scale. The increased particle capture in the bivalve culture will also lead to increased water transparency, promote light penetration and hence reduce nutrient regeneration further afield from bivalve aggregations. This may be beneficial towards the internal loading of marine areas, which is reduced if thresholds of nitrification are not exceeded, allowing coupled nitrification-denitrification to proceed, and remove nitrogen from the area through N2 production (Carlsson et al. 2012) and similarly for the redox-sensitive release of phosphorus (Holmer et al. 2003). Reduction in sedimentation outside bivalve aggregations can be difficult to detect and cannot be deduced from differences in control vs. affected sites in areas where bivalve aggregations are already present; it should be measured before initiating bivalve production in an area. To our knowledge, only a few studies have addressed the potential effects of bivalve aggregations on concentrating sedimentation in hot spots, and comparing these effects with overall basin scale sedimentation and nutrient regeneration outside the bivalve aggregations. Murphy et al. (2016a) suggested that the net import of particles to support hard clam production contributed to increased nitrogen regeneration in the study area. From a mass balance point of view, sedimentation outside the aggregations must, however, be reduced in comparison to a situation without bivalve aggregations. The local effect will depend on a number of factors including water retention time in the specific basin, organic content of the sediments, nutrient input to the basin, water depth, and stratification. One important possible effect of reduced sedimentation, particularly under eutrophic conditions, is minimizing the risk of oxygen depletion events. Oxygen depletion, where the benthic fauna and flora die-off, generally results in high internal nutrient loading. The release of inorganic nitrogen and phosphorous from the sediments to the water column increases the risk of stimulating blooms of phytoplankton or opportunistic macro algae, and initiating a negative feedback loop maintaining high internal loading. By reducing the internal loading at the basin scale, water quality improves, resulting in higher water transparency and growth of benthic vegetation in deeper waters. Such a scenario can be considered a positive feedback on the ecosystem, as benthic vegetation slows the regeneration of nutrients to the water column, particularly during summer months with high productivity in the vegetation. This eventually leads to longer periods of nutrient limitation of phytoplankton, and thus higher ecological quality of the specific area.
Mass balance calculations of the effects of bivalve aggregations on the basin scale are available in the literature (e.g. Cranford et al. 2007; Brigolin et al. 2009; Holmer et al. 2015; Guyondet et al. 2015). These calculations take into account both nutrient removal through harvest and nutrient regeneration in the bivalve structures, as well as the surrounding sediments, but without accounting for reduced sedimentation outside the bivalve aggregations. These studies indicate that natural bivalve reefs and bivalve aquaculture contribute to a net nitrogen removal at the basin scale through harvest and denitrification, despite increased nitrogen regeneration in the water column and sediments (Holmer et al. 2015; Guyondet et al. 2015). The net nitrogen removal capacity, however, varies between studies from negligible (e.g. Cranford et al. 2007) to important (e.g. Guyondet et al. 2015; Holmer et al. 2015). All studies consider the decrease in phytoplankton concentration as the most important effect of bivalve aggregations on ecosystem processes at the basin scale. These studies also highlight the effects of increased sedimentation and stimulated nutrient regeneration in bivalve aggregations, for example, leading to a higher flux of nitrogen to the water column and to the sediments (e.g. Murphy et al. 2016a). Guyondet et al. (2015) observed that the intensive mussel farming in St Peter’s Bay in Eastern Canada maintained phytoplankton biomass at levels corresponding to the 1980s, when aquaculture had not yet developed and nitrogen loading was half of the present level. Basin scale sedimentation in St Peter’s Bay was reduced by 14%, and it was concluded that cultivated mussels play an important role in remediating the negative impacts of land-derived nutrient loading in this area, as the mussel farming in St Peter’s Bay could counteract a doubling in nitrogen loading. Similarly, mussel farming in the eutrophic Limfjorden, Denmark improved water quality and increased light penetration, promoting the light conditions for benthic vegetation in the area (Petersen et al. 2016). In this study, the uptake of nitrogen in the sediments was stimulated, possibly due to high rates of denitrification, and thereby removing a larger fraction of nitrogen from the fjord compared to the absence of mussel farming. The farm thus contributed to water quality improvements by removal of organic bound nitrogen in phytoplankton, as well as stimulating removal of inorganic nitrogen in the sediments (Holmer et al. 2015). Such recent studies suggest that mussel farming under eutrophic conditions has broad potential for mitigation of excess load of nutrients in marine areas; and increase in mussel farming may reduce effects of eutrophication.
Understanding the overall effects of natural beds of bivalves and/or aquaculture of bivalves on nitrogen cycling in the local environment can be complicated, as multiple factors affect the cycling of nitrogen in the environment. Removal of nitrogen through harvest is relatively easy to measure and extrapolate from single long-lines/bivalve aggregates to farms/reefs and farming areas, whereas the effects on water quality and nutrient regeneration can be more difficult to document. The net depositional flux of organic matter is a central parameter driving nutrient regeneration in the sediments, but it is difficult to measure in shallow waters due to methodological constraints and dynamic processes, such as resuspension and advection affecting sedimentation on short and long-term time scales. Modelling is therefore becoming ever more important in management of coastal waters (e.g. Cranford et al. 2007; Guyondet et al. 2015). By using a model where sediment trap deployments were combined with a sediment flux model in an area with oysters (Crassostrea virginica), Testa et al. (2015) demonstrated that resuspension and transport effectively removed oyster biodeposits from the studied farms, resulting in limited local environmental impact as there was no long-term sediment accumulation near the oysters, creating hot spots for nutrient recycling. Guyondet et al. (2015) applied a coupled hydrodynamic-biogeochemical model in an area with mussel aquaculture and found that mussel harvest extracts nitrogen resources equivalent to 42% of river inputs and 46.5% of phytoplankton primary production. Based on the limited number of studies at the basin scale, and case studies of individual reefs and individual farms, it is apparent that natural bivalve beds/reefs and/or culturing units of bivalves act as net sinks of nitrogen at the basin scale, due to removal of nitrogen by harvest of bivalve biomass as well as enhanced denitrification. Nonetheless, more mass balance and modelling studies are needed to account for the large spatial and seasonal variation in rates of nitrogen cycling and processes affecting nitrogen cycling measured so far.
6 The Economic Value of Bivalve Nutrient Extraction
The economic value of a natural resource is reflected through the flow of services to people, derived from the resource, and can be thought of as the interest on a natural resource asset. We are now accustomed to call this interest ecosystem services. While the economic interpretation is simple and intuitive, there are a number of challenges in identifying the economic values, and important caveats related to the existing valuation methods. There are primarily two types of ecosystem services derived from bivalves: provisioning and regulating services. Provisioning services are the production value of the bivalves themselves for human consumption and potentially for other purposes like a feed ingredient for fish, pigs, and poultry. These are private goods, in an economic sense, and markets reflect the economic value of the production. It is important to notice that these services are not entirely provided by marine ecosystems, as labour and capital inputs are needed to convert the ecosystem processes to the final economic good. This means that the economic value of the marine space for bivalve production needs to take into account the costs of the inputs in production.
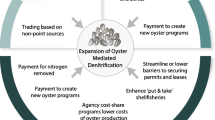
The other important marine ecosystem service that bivalve production provides is the regulation of water quality associated with bivalve filtration of the water column, and the associated nutrient extraction and denitrification. The filtration effects and the extraction of nutrients are not inherently an economic good, but an intermediate service that contributes to improved water quality and the associated increase in human uses and enjoyment of the marine environment. Any economic valuation of bivalve services should reflect the value of the final goods related to production and water quality improvements (Fig. 10.4). Figure 10.4 illustrates that there is a multitude of processes, services and values involved. While they are all interconnected, there is not a 1:1 relationship between the processes, the services, and the values. This implies that when economists seek to value a particular economic good, they select methods to capture the values outlined in the third column of Fig. 10.2. Due to the lack of 1:1 relationships, each study will not capture every economic value aspects of marine ecosystem processes.
Valuation approaches that have frequently been used for marine ecosystem services are the stated-preference methods. Stated-preference methods are environmental valuation methods based on surveys. These surveys collect data on people’s stated rankings of, or choices between, different hypothetical changes in the state of the environment and payments for the change in the associated environmental quality. Such methods have been used to measure the value of clear water by coastal recreationists and other users, but also the more intangible benefits of clear water on biodiversity that do not necessarily depend on recreational use. Clear water also has aesthetic value, which might influence the value from recreation. However, the use of the sea for bivalve production might also be associated with disutility, as the area will not be available for other purposes such as recreation and fisheries. This disutility has not yet, to the author’s knowledge, been studied and quantified. A number of studies have attempted to estimate the use and non-use monetary values by estimating the willingness to pay for water clarity improvements. One example is Söderqvist (1996, updated in Söderqvist and Hasselström 2008), who made such an attempt for the Baltic Sea in the mid 1990’ies. Another is the more recent study of Ahtiainen et al. (2014) who aimed to value achieving good ecological status in 2050 also in the Baltic Sea. Most stated-preference valuation studies have focused on measuring the improvement in clarity of the water or achievement of good ecological status. Attributing these economic values to ecosystem processes, and ultimately production of bivalves, is challenging. Söderqvist’s (1996) study estimates the economic value of a 50% reduction of the nutrient load to the Baltic Sea, which at that time was the load reduction target to achieve good water quality. The study was updated in 2008 (Gren 2008), and is in fact one of the few studies that estimate the value of good water quality in terms of the value per kg N reduced. This value can in turn be used as the value of 1 kg nitrogen assimilated and removed by bivalves, using the measurements of the effect on nitrogen assimilation and denitrification by bivalves. Using the contingent valuation method, Söderqvist (1996) estimated the willingness to pay to be 12–24 € kg−1 N (reported in Gren 2008).
The Söderqvist (1996) study may no longer reflect the present use and non-use value of clear water. Furthermore, the values may vary between locations due to the differences in the number of people exposed and variation in their values and socio-economic characteristics. However, this is only one of the reasons why the economic values of nitrogen reduction are not constant across space. In addition, it is questionable if the biophysical relationships are applicable for all locations, as the nitrogen reduction required to obtain clear water and good ecological status varies between locations. As an example, the nitrogen reduction required to obtain good ecological status in different parts of the Limfjorden (including Skive Fjord) in Denmark varies by a factor 3. Furthermore, as the relationship between the response in water quality and nitrogen reduction might not be linear, this adds further complexity to the valuation task. Overall, these observations imply that the value of reducing nitrogen in different marine ecosystems will likely vary to a substantial degree. This is in sharp contrast to studies valuing reductions in CO2 emissions. For carbon, it is valid to derive unit values of reductions independent of the location where the emissions are reduced. However, the valuation task related to the two types of emission also has similarities as people could still have different willingness to pay for the ecosystem service. In the context of CO2 emissions, this would reflect the difference in peoples’ willingness to give up current consumption to reduce the risk of climate change in the future.
An alternative and frequently used approach, when it is difficult to measure the willingness to pay per kg emission reduced, is cost-based methods. One of these approaches is the substitute cost method, which is based on measurements of the alternative costs of achieving the ecosystem service by using other means, such as reductions in agricultural nutrient loads. This method is appropriate under the assumption that the cost-estimate of achieving an improvement in ecosystem service provision reflects the marginal costs of an optimal investment decision. If this is the case, the cost estimate reflects the sum of the marginal individual willingness to pay for the service. If it is reasonable to assume that the current ecological status is lower than the societal optimal level, cost based estimates can be used as a conservative value estimate. Studies, such as Ahtiainen et al. (2014) support this assumption, as the willingness to pay for clear water and reduced eutrophication up to the level of good ecological status was higher than the costs of obtaining the required nutrient loads in all countries around the Baltic Sea.
Objectives to achieve good ecological status in a coastal area represent a societal level commitment to invest in improved water quality. If the gap between current ecological status and good ecological status (as in the EU Water Framework Directive) can be expressed as targets for nitrogen load reduction, marginal costs of achieving load reductions through land based measures (e.g. agriculture) can be used as estimates of the value of nitrogen extraction using bivalves. The implication of this is that the value of bivalve nutrient extraction is a function of the nutrient load targets and not a constant value. The higher the required nutrient load targets are to achieve good ecological status, the higher the value of bivalve generated nutrient extraction and improvement of water clarity will be. Such marginal value functions have been estimated for the Baltic Sea (e.g. Hasler et al. 2014). They indicate a marginal value of 24 € kg−1 N to obtain load reduction to the level required by HELCOM’s international agreement on nutrient load reductions. This estimate is an average estimate for all countries around the Baltic Sea. Studies at a much more detailed level in the Limfjorden indicate lower marginal costs. Hasler et al. (2015) estimated a cost of 12 EUR € kg−1 N. To estimate the value of nitrogen extraction and removal by mussels, the production costs should be subtracted. The costs of producing mussels for nitrogen mitigation in Skive fjord in Limfjorden is estimated to be in the range 14–20 € kg−1 N (Petersen et al. 2014); i.e. the value of nitrogen removal is negative. It is important to note, however, that in this example it is assumed that a market for the produced mussels does not exist; neither for feed or human consumption. Break-even is reached at a sales price of approximately 0,13 € kg−1 mussel. Furthermore, the filtration effect can be included. In Petersen et al. (2014), the mussel clearance effect on Secchi depth has been calculated; including this effect reduces the costs per kg nitrogen to 2 € kg−1 N. However, as pointed out by Petersen et al. (2014), these estimates should not be included in cost-effectiveness analysis of nitrogen removal, as these indirect effects do not remove nutrients from the ecosystem.
Gren et al. (2009) also estimated marginal costs functions to assess the value of nutrient extraction by mussel farms at the Baltic Sea drainage basin scale. When Gren et al. (2009) subtract the costs of producing the mussels from the value, the results suggest that the value of bivalve nitrogen extraction is still positive; varying between 1.7 and 24.7 € kg−1 N. The range of values depends on the assumption about production costs, whether a market exists for the harvested bivalves, and whether the market is for human consumption or feed. For all scenarios, they assume that 1 kg of live mussels contains between 8.5 and 12 g N, 0.6–0.8 g P and about 40–50 g C with reference to Lutz (1980) and Haamer (1996). In the scenario where no market for the products exists, the value is estimated to be within a range of 0.02–0.11 € kg−1 live mussel, reflecting a value of nitrogen extraction between 1.7 and 2.4 € kg−1 N. When markets exist, the value range is between 0.12 and 0.21 € kg−1 live mussel, reflecting values of nitrogen between 9.2 and 12.9 € kg−1 N. Gren et al. (2009) also distinguishes between high and low production costs for the mussels: In the scenario where mussels are sold on a market, the range is estimated to be between 10.0 and 14.1 € kg−1 N for high production costs, and between 17.5 and 24.7 € kg−1 N for low production costs. They further estimate the value for different parts of the Baltic Sea leading to even larger variations in the value between regions, attributed to differences in the nutrient load reduction targets that vary between sea regions and the differences in the levels of the production costs between the sites. This illustrates that variability in recipient sensitivity to nutrient load levels determines the value of bivalves as extraction aquaculture. Finally, spatial heterogeneity in production costs between locations, due to differences in growth conditions and differences in labour costs also play an important role in determining the economic value of bivalve extraction. This is important, as labour costs constitute the largest part of the production costs (Petersen et al. 2014; Gren et al. 2009).
Grabowski et al. (2012) estimate the value of ecosystem services provided by another type of bivalves; oyster beds. As part of their study, they report the value of denitrification in the oyster beds, and estimate the denitrification in the oyster beds from literature. The value is estimated using substitute costs, based on the average trading price per kilogram. The data from the trading programme have also been used by Piehler and Smyth (2011) giving a value of 13€ kg−1 in the Nutrient Offset Credit Program. These studies therefore also used the cost-based approach, but only for the denitrification contribution of oysters, i.e. smaller proportion of the potential for nitrogen extraction and removal by bivalves. They calculate the value of a number of ecosystem services delivered by bivalves, including the intermediate service, nitrogen removal, and conclude that this is worth between $1385 and 6716 ha−1 year−1. A major constraint in calculations of the economic value of nutrient extraction through denitrification is that it is difficult to assess a precise amount of nitrogen removed through the denitrification process. Pollack et al. (2013) have also used a cost based approach to estimate the alternative cost of removing nitrogen from the Mission-Aransas Estuary in Texas using biological nitrogen removal processes in a wastewater treatment plant. Unlike extraction through bivalve aquaculture, where the extracted volume can be measured easily, nutrient extraction through denitrification requires measurements and modelling that can be subjected to debate about the actual amount of nitrogen removed.
The overview of existing studies (see also Table 10.2) illustrates that primary studies aiming at valorisation of nutrient extraction services by bivalves are rare. If the ecosystem services provided by bivalves are to be used as an active mitigation measure combatting excess nutrient loading to coastal waters, it is crucial that methods for valorisation and exact accounting of the overall services provided include the effects of potential enhanced nutrient retention and nutrient recycling in the ecosystem. Only then can these services be assessed in an unbiased, reliable, and cost-effective way.
7 Outlook – The Role of Bivalves in Abatement Policies
The value of nutrient regulation by bivalves can be utilized in nutrient reduction policies. This type of mitigation measure requires that bivalve producers be compensated for the direct costs involved in provision of this service, or paid the societal value of improving water quality through nutrient removal. There are different potential institutional set-ups for such compensations, dependent on the actors involved in payments for the services. The arrangement could transpire through negotiations between the public body responsible for meeting nutrient load reductions and the bivalve/mussel producers; or directly through trade between emitters of nutrients from land (farmers, waste water treatment plants) and bivalve producers (often facilitated by a public body). Trade between emitters and bivalve producers involve a purchase of offsets from bivalve producers, allowing emitters to reduce their abatement efforts accordingly. Permits are traded when the price of the offset offered by bivalve producers are lower than the marginal costs of reducing nutrient loads through other measures. The incentives for trade therefore depend on the need for nutrient load reductions in the specific water body, and the costs of alternative measures. Trade mechanisms are rarely used in nutrient regulation in Europe and only a few examples exist worldwide (e.g. Piehler and Smyth 2011; Grabowski et al. 2012; Shortle 2013; Duhon et al. 2015; Ferreira and Bricker 2016). However, there is an increasing focus on this instrument to promote more cost-effective solutions in nutrient regulation. The use of offsets for nutrient abatement by bivalves has been tested in Sweden (Lindahl and Kollberg 2009), and is currently being tested in ongoing experimentation Sweden and Finland (Nutritrade 2017, http://nutritradebaltic.eu/wp-content/uploads/sites/34/2017/06/EUSBSR-Annual-Forum_NutriTrade-pilot-mussel.pdf). There are ample experiences of market based mechanisms in other environmental policy areas (e.g. biodiversity conservation) in both the US and Europe (Pöll et al. 2016). These experiences should be used to explore the risks and potentials of this type of regulation in the aquatic environment.
References
Ackerman JD, Loewen MR, Hamblin PF (2001) Benthic-pelagic coupling over a zebra mussel reef in western Lake Erie. Limnol Oceanogr 46:892–904
Ahtiainen H, Artell J, Czajkowski M, Hasler B et al (2014) Benefits of meeting nutrient reduction targets for the Baltic Sea – a contingent valuation study in the nine coastal states. J Environ Econ Pol 3(3):278–305
Alpine AE, Cloern JE (1992) Trophic interactions and direct physical effects control phytoplankton biomass and production in an estuary. Limnol Oceanogr 37:946–955
Anonymous (1991) Council Directive 91/676/EEC of 12 December 1991 concerning the protection of waters against pollution caused by nitrates from agricultural sources. Off J L375
Anonymous (2000) Directive 200/60/EEC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Off J Eur Commun L327/1
Anonymous (2008) Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 establishing a framework for community action in the field of marine environmental policy (Marine Strategy Framework Directive). Off J Eur Commun L164/19
Aure J, Strohmeier T, Strand Ø (2007) Modelling current speed and carrying capacity in long-line blue mussel (Mytilus edulis) farms. Aquac Res 38:304–312
Bricker SB, Rice KC, Bricker OP III (2014) From headwaters to coast: influence of human activities on water quality of the Potomac River estuary. Aquat Geochem 20(1–2):291–323
Brigolin D, Maschio GD, Rampazzo F, Giani M, Pastres R (2009) An individual-based population dynamic model for estimating biomass yield and nutrient fluxes through an off-shore mussel (Mytilus galloprovincialis) farm. Estuar Coast Shelf Sci 82:365–376
Caffrey JM, Hollibaugh JT, Mortazavi B (2016) Living oysters and their shells as sites of nitrification and denitrification. Mar Pollut Bull 112:86–90. https://doi.org/10.1016/j.marpolbul.2016.08.038
Capelle JJ (2017) Production efficiency of mussel bottom culture. PhD Dissertation, University of Wageningen
Capelle JJ, Scheiberlic G, Weijsman JWM, Smaal A (2016) The role of shore crabs and mussel density in mussel losses at a commercial intertidal mussel plot after seeding. Aquacult Internat 24:1459–1147
Caraco NF, Cole JJ, Strayer DL (2006) Top-down control from the bottom: regulation of eutrophication in a large river by benthic grazing. Limnol Oceanogr 51:664–670
Carlsson MS, Holmer M, Petersen JK (2009) Seasonal and spatial variation of benthic impacts of mussel long-line farming in a eutrophicated Danish fjord, Limfjorden. J Shellfish Res 28(4):791–801
Carlsson MS, Glud RN, Petersen JK (2010) Degradation of mussel (Mytilus edulis) fecal pellets released from hanging long-lines upon sinking and after settling at the sediment. Can J Fish Aquat Sci 67:1376–1387
Carlsson MS, Engström P, Lindahl O, Ljungqvist L, Petersen JK, Svanberg L, Holmer M (2012) Effects of mussel farms on the benthic nitrogen cycle on the Swedish west coast. Aquacult Environ Interact 2:177–191
Carmichael RH, Walton W, Clark H, Ramcharan C (2012) Bivalve-enhanced nitrogen removal from coastal estuaries. Can J Fish Aquat Sci 69:1131–1149
Carstensen J, Krause-Jensen D, Markager S et al (2013) Water clarity and eelgrass responses to nitrogen reductions in the eutrophic Skive Fjord, Denmark. Hydrobiologia 704:293–309
Chamberlain J, Fernandes TF, Read P et al (2001) Impacts of biodeposits from suspended mussel (Mytilus edulis L.) culture on the surrounding surficial sediments. ICES J Mar Sci 58:411–416
Chopin T (2013) Integrated multi-trophic aquaculture. In: Christou P, Savin R, Costa-Pierce BA, Misztal I, Whitelaw CBA (eds) Sustainable food production. Springer, New York, pp 184–205
Cloern JE (1982) Does the benthos control phytoplankton in South San Francisco Bay? Mar Ecol Progr Ser 9:191–202
Cloern JE (2001) Our evolving conceptual model of the coastal eutrophication problem. Mar Ecol Prog Ser 210:223–253
Conley DJ, Paerl HW, Howarth RW et al (2009) Controlling eutrophication: nitrogen and phosphorous. Science 323(5917):1014–1015
Cranford PJ, Strain PM, Dowd M et al (2007) Influence of mussel aquaculture on nitrogen dynamics in a nutrient enriched coastal embayment. Mar Ecol Prog Ser 347:61–78
Cranford PJ, William L, Strand Ø, Strohmeier T (2008) Phytoplankton depletion by mussel aquaculture: high resolution mapping, ecosystem modeling and potential indicators of ecological carrying capacity. ICES CM2008/H:12
Cranford PJ, Reid GK, Robinson SMC (2013) Open water integrated multi-trophic aquaculture: constraints on the effectiveness of mussels as an organic extractive component. Mar Ecol Prog Ser 4:163–173
Cranford PJ, Duarte P, Robinson SMC et al (2014) Suspended particulate matter depletion and flow modification inside mussel (Mytilus galloprovincialis) culture rafts in the Riá de Betanzos, Spain. J Exp Mar Biol Ecol 452:70–81
Daigle R, Herbinger CM (2009) Ecological interactions between the vase tunicate (Ciona intestinalis) and the farmed blue mussel (Mytilus edulis) in Nova Scotia, Canada. Aquat Invasions 4:177–187
Dalsgaard T, Thamdrup B, Canfield DE (2005) Anaerobic ammonium oxidation (anammox) in the marine environment. Res Microbiol 156:457–464
Dame RF (2012) Ecology of marine bivalves: an ecosystem approach. CRC Press, London
Dame RF, Prins TC (1998) Bivalve carrying capacity in coastal ecosystems. Aquat Ecol 31:409–421
Dare PJ, Edwards BD (1975) Seasonal changes in flesh weight and biochemical composition of mussels (M. edulis L.) in Conway estuary, North Wales. J Exp Mar Biol Ecol 18:89–97
Duarte CM, Conley DJ, Carstensen J, Sanchez-Camacho M (2009) Return to neverland: shifting baselines affect eutrophication restoration targets. Estuar Coasts 32:29–36
Duhon M, Mcdonald H, Kerr S (2015) Nitrogen trading in Lake Taupo: an analysis and evaluation of an innovative water management policy. Motu working paper 15-07. Motu Economic and Public Policy Research
Fahnenstiel GL, Lang GA, Nalepa TF, Jahnengen TH (1995) Effects of zebra mussel (Dreissena polymorpha) colonization on water quality parameters in Saginaw Bay, Lake Huron. J Great Lakes Res 21(4):435–448
Farber S, Costanza R, Childers DL et al (2006) Linking ecology and economics for ecosystem management. Bioscience 56:121–133
Ferreira JG, Bricker SB (2016) Goods and services of extensive aquaculture: shellfish culture and nutrient trading. Aquacu Int 24:803–825
Fuentes J, Gregorio V, Giráldez R, Molares J (2000) Within-raft variability of the growth rate of mussels, Mytilus galloprovincialis, cultivated in the Ría de Arousa (NW Spain). Aquaculture 189:39–52
Giles H, Pilditch CA (2006) Effects of mussel (Perna canaliculus) biodeposit decomposition on benthic respiration and nutrient fluxes. Mar Biol 150:261–271
Grabowski JH, Brumbaugh RD, Conrad RF et al (2012) Economic valuation of ecosystem services provided by oyster reefs. Bioscience 62:900–909
Grant J, Hatcher A, Scott DB et al (1995) A multidisciplinary approach to evaluating impacts of shellfish aquaculture on benthic communities. Estuaries 18(1A):124–144
Grant J, Bacher C, Cranford PJ et al (2008) A spatially explicit ecosystem model of seston depletion in dense mussel culture. J Mar Sys 73:155–168
Gren I-M (2008). Costs and benefits from nutrient reductions to the Baltic Sea. The Swedish Environmental Protection Agency, Stockholm, Sweden. http://www.naturvardsverket.se/Documents/publikationer/978–91–620–5877–7.pdf
Gren I-M, Lindahl O, Lindqvist M (2009) Values of mussel farming for combating eutrophication: an application to the Baltic Sea. Ecol Engineer 35:935–945
Guyondet T, Comeau LA, Bacher C et al (2015) Climate change influences carrying capacity in a coastal embayment dedicated to shellfish aquaculture. Estuar Coasts 38:1593–1618
Haamer J (1996) Improving water quality in a eutrophied fjord system with mussel farming. Ambio 25:356–362
Hart R (2003) Dynamic pollution control – time lags and optimal restoration of marine ecosystems. Ecol Econom 47:9–93
Hasler B, Smart JCR, Fonnesbech-Wulff A et al (2012) Regional cost-effectiveness in transboundary water quality management for the Baltic Sea. http://www.worldwaterweek.org/documents/.../IWREC/BeritHasler.pdf
Hasler B, Smart JCR, Fonnesbech-Wulff A et al (2014) Hydro-economic modelling of cost-effective transboundary water quality management in the Baltic Sea. Water Res Econom 5:1–23
Hasler B, Hansen LB, Andersen HE, Konrad M (2015) Modellering af omkostningseffektive reduktioner af kvælstoftilførslerne til Limfjorden: Dokumentation af model og resultater. Aarhus University, DCE - Danish Centre for Environment and Energy (in Danish)
Hatcher A, Grant J, Schofield B (1994) Effects of suspended mussel culture (Mytilus spp.) on sedimentation, benthic respiration and sediment nutrient dynamics in a coastal bay. Mar Ecol Prog Ser 115(3):219–235
Heasman KG, Pitcher GC, Mcquaid CD, Hecht T (1998) Shellfish mariculture in the Benguela system: raft culture of Mytilus galloprovincialis and the effect of rope spacing on food extraction, growth rate, production, and condition of mussels. J Shellfish Res 17:33–39
Herbert RJH, Humphreys JH, Davies CJ et al (2016) Ecological impacts of non-native Pacific oysters (Crassostrea gigas) and management measures for protected areas in Europe. Biodivers Conserv 25(14):2835–2865
Herman PMJ, Scholten H (1990) Can suspension-feeders stabilise estuarine ecosystems. In: Barnes MA, Gibson RN (eds) Trophic relations in the marine environment. Aberdeen University Press, Aberdeen, pp 104–116
Higgins CB, Tobias C, Piehler M et al (2013) Effect of aquacultured oyster biodeposition on sediment N2 production in Chesapeake Bay. Mar Ecol Prog Ser 473:7–27
Hily C (1991) Is the activity of benthic suspension feeders a factor controlling water quality in the Bay of Brest. Mar Ecol Prog Ser 69:179–188
Hoellein TJ, Zarnoch CB (2014) Effect of eastern oysters (Crassostrea virginica) on sediment carbon and nitrogen dynamics in an urban estuary. Ecol Appl 24(2):271–286
Holmer M, Ahrensberg N, Jørgensen NP (2003) Impacts of mussel dredging on sediment dynamics in an eutrophic Danish fjord. Chem Ecol 19:343–362
Holmer M, Thorsen SW, Carlsson MS, Petersen JK (2015) Pelagic and benthic nutrient regeneration processes in mussel cultures (Mytilus edulis) in a eutrophic coastal area (Skive Fjord, Denmark). Estuar Coasts 38(5):1629–1641
Holyoke RR (2008) Biodeposition and biogeochemical processes in shallow mesohaline sediments of Chesapeake Bay. Ph.D. Dissertation, University of Maryland, College Park
Humphries AT, Ayvazian SG, Carey JC et al (2016) Directly measured denitrification reveals oyster aquaculture and restored oyster reefs remove nitrogen at comparable high rates. Front Mar Sci 3:74. https://doi.org/10.3389/fmars.2016.00074
Idrisi N, Mills EL, Rudstam LG, Stewart DJ (2001) Impact of zebra mussels (Dreissena polymorpha) on the pelagic lower trophic levels of Oneida Lake, New York. Can J Fish Aquat Sci 58:1430–1441
Kellogg ML, Cornwell JC, Owens MS, Paynter KT (2013) Denitrification and nutrient assimilation on a restored oyster reef. Mar Ecol Prog Ser 480:1–19
Kellogg ML, Smyth AR, Luckenbach MW et al (2014) Use of oysters to mitigate eutrophication in coastal waters. Estuar Coast Shelf Sci 151:156–168
Lahance-Bernard M, Daigle G, Himmelman JH, Frechette M (2010) Biomass–density relationships and self-thinning of blue mussels (Mytilus spp.) reared on self-regulated longlines. Aquaculture 308:34–43
Lindahl O (2011) Mussel farming as a tool for re-eutrophication of coastal waters: experiences from Sweden. In: Shumway S (ed) Shellfish aquaculture and the environment. Wiley, London, pp 217–237
Lindahl O (ed) (2012) Mussel cultivation. In: Submariner compendium: an assessment of innovative and sustainable uses of Baltic marine resources. Maritime Institute in Gdansk. ISBN: 978-83-62438-14-3. https://www.submariner-network.eu/images/downloads/submariner_compendium_web.pdf
Lindahl O, Kollberg S (2009) Can the EU agri-environmental aid program be extended into the coastal zone to combat eutrophication? Hydrobiologia 629:59–64
Lindemann S, Zarnoch CB, Castignetti D, Hoellein TJ (2016) Effect of eastern oysters (Crassostrea virginica) and seasonality on nitrite reductase gene abundance (nirS, nirK, nrfA) in an urban estuary. Estuar Coasts 39:218–232
Locke A, Hanson JM, Macnair NG, Smith AH (2009) Rapid response to non-indigenous species. 2. Case studies of invasive tunicates in Prince Edward Island. Aquat Invasions 4(1):249–258
Lutz RA (ed) (1980) Mussel culture and harvest: a north American perspective. Elsevier Scientific Publishing, Amsterdam
Maar M, Timmermann K, Petersen JK et al (2010) A model study of the regulation of blue mussels by nutrient loadings and water column stability in a shallow estuary, the Limfjorden. J Sea Res 64:322–333
Maar M, Saurel C, Landes A et al (2015) Growth potential of blue mussels (M. edulis) exposed to different salinities evaluated by a dynamic energy budget model. J Mar Sys 148:48–55
McKindsey CW, Archambault P, Callier MD, Olivier F (2011) Influence of suspended and off-bottom mussel culture on the sea bottom and benthic habitats: a review. Can J Zool 89:622–646
Minjead L, Michotey VD, Garcia N, Bonin PC (2009) Seasonal variation in di-nitrogen fluxes and associated processes (denitrification, anammox and nitrogen fixation) in sediment subject to shellfish farming influences. Aquat Sci 71:425–435
Møhlenberg F (1995) Regulating mechanisms of phytoplankton growth and biomass in a shallow estuary. Ophelia 42(1):239–256
Møhlenberg F (1999) Effect of meteorology and nutrient load on oxygen depletion in a Danish micro-tidal estuary. Aquat Ecol 33:55–64
Møhlenberg SJ (2007) Blue mussel cultivation for nitrogen removal in fjords. Assessment of an alternative measure to comply with the water framework directive using Odense Fjord as a case study. Msc Dissertation, Copenhagen University, Denmark
Mortazavi B, Ortmann AC, Wang L et al (2015) Evaluating the impact of oyster (Crassostrea virginica) gardening on sediment nitrogen cycling in a subtropical estuary. Bull Mar Sci 91(3):323–341
Murphy AE, Anderson IC, Smyth AR, Luckenbach MW (2016a) Microbial nitrogen processing in hard clam (Mercenaria mercenaria) aquaculture sediments: the relative importance of denitrification and dissimilatory nitrate reduction to ammonium (DNRA). Limnol Oceanogr 61:1589–1604
Murphy AE, Emery KA, Anderson IC et al (2016b) Quantifying the effects of commercial clam aquaculture on C and N cycling: an integrated ecosystem approach. Estuar Coasts 39:1746–1761
Newell RIE, Fisher TR, Holyoke RR, Cornwell JC (2005) Influence on eastern oysters on nitrogen andphosphorous regenration in Chesapeake Bay, USA. In: Dame R, Olenin S (eds) The comparative role of suspension feeders in ecosystems. NATO science series IV. Earth and environmental sciences, vol 47. Springer, Amsterdam, pp 93–120
Nielsen P, Cranford PJ, Maar M, Petersen JK (2016) Magnitude, spatial scale and optimization of ecosystem services from a nutrient extraction mussel farm in the eutrophic Skive Fjord, Denmark. Aquacult Environ Interact 8:311–329
Nizzoli D, Welsh DT, Fano EA, Viaroli P (2006) Impact of clam and mussel farming on benthic metabolism and nitrogen cycling, with emphasis on nitrate reduction pathways. Mar Ecol Prog Ser 315:151–165
Petersen JK (2004) Grazing on pelagic primary producers - the role of benthic suspension feeders in estuaries. In: Nielsen SL, Banta G, Pedersen MF (eds) Estuarine nutrient cycling: the influence of primary producers. Kluwer Academic, Dordrecht, pp 129–152
Petersen JK, Hansen JW, Laursen MB et al (2008a) Regime shift in a coastal marine ecosystem. Ecol Appl 18(2):497–510
Petersen JK, Nielsen TG, van Duren L, Maar M (2008b) Depletion of plankton in a raft culture of Mytilus galloprovincialis in Riá de Vigo, NW Spain. I. Phytoplankton. Aquat Biol 4:113–125
Petersen JK, Timmermann K, Carlsson MS et al (2012) Mussel farming can be used as mitigation tool – a reply. Mar Pollut Bull 64:452–454
Petersen JK, Maar M, Ysebart T, Herman PMJ (2013) Near-bed gradients in particles and nutrients above a mussel bed in the Limfjorden: influence of physical mixing and mussel activity. Mar Ecol Prog Ser 490:137–146
Petersen JK, Hasler B, Timmermann K et al (2014) Mussels as a tool for mitigation in the marine environment. Mar Pollut Bull 82:137–143
Petersen JK, Saurel C, Nielsen P, Timmermann K (2016) The use of shellfish for eutrophication control. Aquacult Internat 24(3):857–878
Piehler MF, Smyth AR (2011) Habitat-specific distinctions in estuarine denitrification affect both ecosystem function and services. Ecosphere 2:12
Pires LMD, Ibeling BW, van Donk E (2010) Zebra mussels as a potential tool in the restoration of eutrophic shallow lakes dominated by toxic cyanobacteria. In: van der Velde G, Rajagopal S, Bij de Vaate A (eds) The zebra mussel in Europe. Backhuys Publishers, Leiden, pp 331–342
Pöll CE, Willner W, Wrbka T (2016) Challenging the practice of biodiversity offsets: ecological restoration success evaluation of a large-scale railway project. Landsc Ecol Eng 12:85–97. https://doi.org/10.1007/s11355-015-0282-2
Pollack JB, Yoskowitz D, Hae-Cheol K, Montagna PA (2013) Role and value of nitrogen regulation provided by oysters (Crassostrea virginica) in the mission-Aransas estuary, Texas, USA. PLoS One 8(6):e65314. https://doi.org/10.1371/journal.pone.0065314
Prins TC, Escaravage V, Smaal AC, Peeters JCH (1995) Nutrient cycling and phytoplankton dynamics in relation to mussel grazing in a mesocosm experiment. Ophelia 41:289–315
Prins TC, Smaal AC, Dame RF (1998) A review of the feedbacks between bivalve grazing and ecosystem processes. Aquat Ecol 31:349–359
Riisgård HU, Lundgreen K, Larsen PS (2014) Potential for production of ‘mini-mussels’ in Great Belt (Denmark) evaluated on basis of actual and modelled growth of young mussels Mytilus edulis. Aquacult Internat 22:859–885
Rodhouse PG, Roden CM, Hensey MP, Ryan TH (1984) Resource allocation in Mytilus edulis on the shore and in suspended culture. Mar Biol 84(1):27–34
Rose JM, Bricker SB, Tedesco MA, Wikfors GH (2014) A role for shellfish aquaculture in coastal nitrogen management. Environ Sci Technol 48(5):2519–2525
Rosland R, Bacher C, Strand Ø et al (2011) Modelling growth variability in longline mussel farms as a function of stocking density and farm design. J Sea Res 66:318–330
Schernewski G, Stybel N, Neumann T (2012) Zebra mussel farming in the Szczecin (Oder) Lagoon: water-quality objectives and cost-effectiveness. Ecol Soc 17:4 https://doi.org/10.5751/ES-04644-170204
Shortle J (2013) Economics and environmental markets: lessons from water-quality trading. Agricult Res Econ Rev 42(1):57–74
Sisson GM, Kellogg ML, Luckenbach MW et al (2011) Assessment of oyster reefs in Lynnhaven River as a Chesapeake Bay TDML best management practice. Technical report. Virginia Institute of Marine Science, Gloucester Point, VA
Smaal AC, Vonck APMA (1997) Seasonal variation in C, N and P budgets and tissue composition of the mussel Mytilus edulis. Mar Ecol Prog Ser 153:167–179
Smith TE, Stevenson RJ, Caraco NF, Cole JJ (1998) Changes in phytoplankton community structure during the zebra mussel (Dreissena polymorpha) invasion of the Hudson River (New York). J Plankton Res 20(8):1567–1579
Smyth AR, Thompson SP, Siporin KN et al (2013) Assessing nitrogen dynamics throughout the estuarine landscape. Estuar Coasts 36:44–55
Smyth AR, Piehler MF, Grabowski JH (2015) Habitat context influences nitrogen removal by restored oyster reefs. J Appl Ecol 52(3):716–725
Smyth AR, Geraldi NR, Thompson SP, Piehler MF (2016) Biological activity exceeds biogenic structure in influencing sediment nitrogen cycling in experimental oyster reefs. Mar Ecol Prog Ser 560:173–183
Söderqvist T (1996). Contingent valuation of a less eutrophicated Baltic Sea. Beijer discussion paper series no. 88, Beijer International Institute of Eco logical Economics, The Royal Swedish Academy of Sciences, Stockholm
Söderqvist T, Hasselström L (2008). The economic value of ecosystem services provided by the Baltic Sea. Swedish Environmental Protection Agency, Stockholm, Sweden
Stadmark J, Conley DJ (2011) Mussel farming as a nutrient reduction measure in the Baltic Sea: consideration of nutrient biogeochemical cycles. Mar Pollut Bull 62:1385–1388
Strohmeier T, Aure J, Duinker A et al (2005) Flow reduction, seston depletion, meat content and distribution of diarrhetic shellfish toxins in a long-line blue mussel (Mytilus edulis) farm. J Shellfish Res 24:15–23
Strohmeier T, Duinker A, Strand Ø, Aure J (2008) Temporal and spatial variation in food availability and meat ratio in a longline mussel farm (Mytilus edulis). Aquaculture 276:83–90
Stybel N, Fenske C, Schernewski G (2009) Mussel cultivation to improve water quality in the Szczecin Lagoon. J Coast Res SI56:1459–1463
Testa JM, Brady DC, Cornwell JC et al (2015) Modeling the impact of floating oyster (Crassostrea virginica) aquaculture on sediment-water nutrient and oxygen fluxes. Aquacult Environ Interact 7:205–222
Troell M, Joyce A, Chopin T et al (2009) Ecological engineering in aquaculture – potential for integrated multi-trophic aquaculture (IMTA) in marine offshore systems. Aquaculture 297:1–9
Wang B, Wang Z (2011) Long-term variations in chlorophyll a and primary productivity in Jiaozhou Bay, China. J Mar Biol 2011:1–7
Weber A, Smit MGD, Collombon MT (2010) Eutrophication and algal blooms: zebra mussels as a weapon. In: van der Velde G, Rajagopal S, Bij de Vaate A (eds) The zebra mussel in Europe. Backhuys Publishers, Leiden, pp 343–347
Welsh DT, Castadelli G (2004) Bacterial nitrification activity directly associated with isolated benthic marine animals. Mar Biol 144:1029–1037
Welsh DT, Nizzoli D, Fano EA, Viaroli P (2015) Direct contribution of clams (Ruditapes philippinarum) to benthic fluxes, nitrification, denitrification and nitrous oxide emission in a farmed sediment. Estuar Coast Shelf Sci 154:84–93
Wiles PJ, van Duren LA, Häse C, Larsen J, Simpson JH (2006) Stratification and mixing in the Limfjorden in relation to mussel culture. J Mar Sys 60:129–143
Zaiko A, Lehtiniemi M, Narscius A, Olenin S (2011) Assessment of bioinvasion impacts on regional scale: a comparative approach. Biol Invasions 13:1739–1765
Acknowledgements
This manuscript was supported by Innovation Fund Denmark project 6150-00008B MuMiPro “Mussel farming – mitigation and protein source for organic husbandry”. This manuscript was also a result of the BONUS OPTIMUS project supported by BONUS (Art 185), funded jointly by EU and Innovation Fund Denmark.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this book are included in the book's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the book's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2019 The Author(s)
About this chapter
Cite this chapter
Petersen, J.K., Holmer, M., Termansen, M., Hasler, B. (2019). Nutrient Extraction Through Bivalves. In: Smaal, A., Ferreira, J., Grant, J., Petersen, J., Strand, Ø. (eds) Goods and Services of Marine Bivalves. Springer, Cham. https://doi.org/10.1007/978-3-319-96776-9_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-96776-9_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-96775-2
Online ISBN: 978-3-319-96776-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)