Abstract
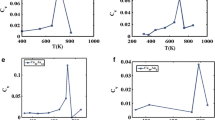
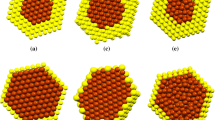
Molecular dynamics (MD) simulation has been done for the melting temperature of gold–copper bimetallic nanostructure with 55 total gold and copper atom numbers and its bulk alloy. The trend of melting temperature for gold–copper bimetallic nanocluster is not the same as the melting temperature for gold–copper bulk alloy. MD simulation shows that the melting temperature of gold–copper bimetallic nanocluster increases with copper atom fraction. Semi-empirical potential within the tight-binding second moment approximation as new application potential model regarding the melting temperature for gold–copper bulk structure shows better result in comparison with EAM, Sutton–Chen potential and quantum Sutton–Chen potential models.
This chapter book is dedicated to my parents due to their endless support and encouragement
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Baletto F, Ferrando R (2005) Structural properties of nanoclusters: energetic, thermodynamic, and kinetic effects. Rev Mod Phys 77:371–423
Ferrando R, Jellinek J, Johnston RL (2008) Nanoalloys: from theory to applications of alloy clusters and nanoparticles. Chem Rev 108:845–910
Huang S-P, Mainardi DS, Balbuena PB (2003) Structure and dynamics of graphite-supported bimetallic nanoclusters. Surf Sci 545:163–179
Bracey CL, Ellis PR, Hutchings GJ (2009) Application of copper–gold alloys in catalysis: current status and future perspectives. Chem Soc Rev 38:2231–2243
Della Pina C, Falletta E, Prati L, Rossi M (2008) Selective oxidation using gold. Chem Soc Rev 37:2077–2095
Yoo W-J, Li C-J (2007) Copper-catalyzed oxidative esterification of alcohols with aldehydes activated by Lewis acids. Tetrahedron Lett 48:1033–1035
Liu X, Wang A, Wang X, Mou C-Y, Zhang T (2008) Au–Cu alloy nanoparticles confined in SBA-15 as a highly efficient catalyst for CO oxidation. Chem Commun 27:3187–3189
Zhang L (2012) A molecular dynamics study of thermal behavior of melting an Au54Cu1 cluster. Procedia Eng 36:207–211
Zhang L, Zhang C-B, Qi Y (2008) Local structure changes of 54-, 55-, 56-atom copper clusters on heating. Phys Lett A 372:2874–2880
Huang W, Ji M, Dong C-D, Gu X, Wang L-M, Gong XG, Wang L-S (2008) Relativistic effects and the unique low-symmetry structures of gold nanoclusters. ACS Nano 2:897–904
Gao Y, Shao N, Pei Y, Zeng XC (2010) Icosahedral crown gold nanocluster Au43Cu12 with high catalytic activity. Nano Lett 10:1055–1062
Häkkinen H, Moseler M, Kostko O, Morgner N, Hoffmann MA, Issendorff BV (2004) Symmetry and electronic structure of noble-metal nanoparticles and the role of relativity. Phys Rev Lett 93:093401
Xing X, Danell RM, Garzón IL, Michaelian K, Blom MN, Burns MM, Parks JH (2005) Size-dependent fivefold and icosahedral symmetry in silver clusters. Phys Rev B 72:081405
Cheng D, Huang S, Wang W (2006) Thermal behavior of core-shell and three-shell layered clusters: melting of Cu1Au54 and Cu12Au43. Phys Rev B 74:064117
Sankaranarayanan SK, Bhethanabotla VR, Joseph B (2005) Molecular dynamics simulation study of the melting of Pd–Pt nanoclusters. Phys Rev B 71:195415
Rodrıguez-López J, Montejano-Carrizales J, José-Yacamán M (2003) Molecular dynamics study of bimetallic nanoparticles: the case of AuxCuy alloy clusters. Appl Surf Sci 219:56–63
Yin J, Shan S, Yang L, Mott D, Malis O, Petkov V, Cai F, Shan Ng M, Luo J, Chen BH (2012) Gold–copper nanoparticles: nanostructural evolution and bifunctional catalytic sites. Chem Mater 24:4662–4674
Taherkhani F, Akbarzadeh H, Abroshan H, Fortunelli A (2012) Dependence of self-diffusion coefficient, surface energy, on size, temperature, and Debye temperature on size for aluminum nanoclusters. Fluid Phase Equilib 335:26–31
Huang S-P, Balbuena PB (2002) Melting of bimetallic Cu–Ni nanoclusters. J Phys Chem B 106:7225–7236
Taherkhani F, Rezania H (2012) Temperature and size dependency of thermal conductivity of aluminum nanocluster. J Nanopart Res 14:1–8
Negreiros F, Taherkhani F, Parsafar G, Fortunelli A (2012) Kinetics of chemical ordering in a Ag–Pt nanoalloy particle via first-principles simulations. J Chem Phys 137:194302
Mejía-Rosales SJ, Fernández-Navarro C, Pérez-Tijerina E, Montejano-Carrizales JM, José-Yacamán M (2006) Two-stage melting of Au–Pd nanoparticles. J Phys Chem B 110:12884–12889
Mehrer H, Eggersmann M, Gude A, Salamon M, Sepiol B (1997) Diffusion in intermetallic phases of the Fe–Al and Fe–Si systems. Mater Sci Eng A 239:889–898
Holzapfel C, Chakraborty S, Rubie D, Frost D (2009) Fe–Mg interdiffusion in wadsleyite: the role of pressure, temperature and composition and the magnitude of jump in diffusion rates at the 410 km discontinuity. Phys Earth Planet Inter 172:28–33
Lott K, Nirk T, Volobujeva O (2002) Chemical self-diffusion in undoped ZnS and in undoped CdSe. Cryst Eng 5:147–153
Palagin D, Doye PK (2014) Ni-based nanoalloys: towards thermally stable highly magnetic materials. J Chem Phys 141:214302
Smith W, Todorov IT (2006) A short description of DL_POLY. Mol Simul 32:935–943
Qi Y, Çağın T, Kimura Y, Goddard WA III (1999) Molecular-dynamics simulations of glass formation and crystallization in binary liquid metals: Cu–Ag and Cu–Ni. Phys Rev B 59:3527–3533
Qi Y, Çağin T, Kimura Y, Goddard WA III (2001) Viscosities of liquid metal alloys from non-equilibrium molecular dynamics. J Comput Aided Mater 8:233–243
Özdemir KS, Tomak M, Uludoğan M, Çağın T (2004) Liquid properties of Pd–Ni alloys. J Non-Cryst Solids 337:101–108
Cleri F, Rosato V (1993) Tight-binding potentials for transition metals and alloys. Phys Rev B 48:22
Kart H, Tomak M, Çağin T (2005) Thermal and mechanical properties of Cu–Au intermetallic alloys. Model Simul Mater Sci 13:657–669
Lv YJ, Chen M (2011) Thermophysical properties of undercooled alloys: an overview of the molecular simulation approaches. Int J Mol Sci 12:278–316
Akbarzadeh H, Yaghoubi H, Shamkhali AN, Taherkhani F (2013) Effects of gas adsorption on the graphite-supported Ag nanoclusters: a molecular dynamics study. J Phys Chem C 117:26287–26294
Akbarzadeh H, Yaghoubi H, Shamkhali AN, Taherkhani F (2014) CO adsorption on Ag nanoclusters supported on carbon nanotube: a molecular dynamics study. J Phys Chem C 118:9187–9195
Taherkhani F, Freshteh Seresht P (2015) Doping effect on the Janus-like structure of a copper–iron bimetallic nanocluster and its solid–liquid phase transition. PTEP 4:043I01, 1–12
Taherkhani F, Parviz Z, Akbarzadeh H, Fortunelli A (2015) Temperature and doping effect on thermal conductivity of copper–gold icosahedral bimetallic nanoclusters and bulk structures. J Phys Chem C 119:7922–7932
Ala-Nissila T, Ferrando R, Ying S (2002) Collective and single particle diffusion on surfaces. Adv Phys 51:949–1078
Taherkhani F, Negreiros FR, Parsafar G, Fortunelli A (2010) Simulation of vacancy diffusion in a silver nanocluster. Chem Phys Lett 498:312–316
Taherkhani F, Akbarzadeh H, Rezania H (2014) Chemical ordering effect on melting temperature, surface energy of copper–gold bimetallic nanocluster. J Alloy Compd 617:746–750
Akbarzadeh H, Taherkhani F (2013) Cluster size dependence of surface energy of Ni nanocluster: a molecular dynamics study. Chem Phys Lett 558:57–61
Heinonen V, Achim CV, Elder KR, Buyukdagli S, Ala-Nissila T (2014) Phase-field-crystal models and mechanical equilibrium. Phys Rev E 89:032411
Laasonen K, Panizon E, Bochicchio D, Ferrando R (2013) Competition between icosahedral motifs in AgCu, AgNi, and AgCo nanoalloys: a combined atomistic−DFT study. J Phys Chem C 117:26405–26413
Alavi S, Thompson DL (2006) Molecular dynamics simulations of the melting of aluminum nanoparticles. J Phys Chem A 110:1518–1523
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this paper
Cite this paper
Taherkhani, F. (2016). Doping Effect on Melting Temperature for Gold–Copper Bimetallic Nanocluster and Dependency on Bulk Melting Temperature to the Potential Model. In: Ramasami, P., Gupta Bhowon, M., Jhaumeer Laulloo, S., Li Kam Wah, H. (eds) Crystallizing Ideas – The Role of Chemistry. Springer, Cham. https://doi.org/10.1007/978-3-319-31759-5_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-31759-5_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-31758-8
Online ISBN: 978-3-319-31759-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)