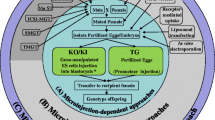
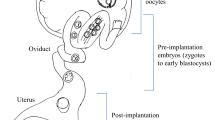
Abstract
Several research centers and pharmaceutical corporations routinely use genetically modified animals (GMAs) in the development of new drugs, in the identification of new drug targets and to test drugs’ efficacy and safety. The most usual methods to produce GMAs are pronuclear microinjection, somatic cell nuclear transfer, retroviral vectors, and recently, embryonic stem cell transgenesis. These methods make use of DNA vectors and present several limitations. Recently, nanomaterials have been applied as an alternative vector for delivery of exogenous DNA into mammalian cells. This chapter addresses the use of carbon nanotubes (CNTs) as a DNA delivery agent for the generation of genetically modified mammals embryos. CNTs can be easily bound to DNA by non-covalent attachement. The DNA strand spontaneously wraps around the carbon nanotubes and DNA molecules can be encapsulated within or around them. The process of interaction of DNA/RNA with CNT favors their protection from degradation by cytoplasmic nucleases, increasing the integration of the transgene into cell nucleus. Thus, the use of CNTs can be far simpler and less laborious when compared to other techniques to produce GMAs.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Wolf E, Schernthaner W, Zakhartchenko V et al (2000) Transgenic technology in farm animals—progress and perspectives. Exp Physiol 85(6):615–625
Harper GS, Brownlee A, Hall TH et al (2006) Global progress toward transgenic food animals: a survey of publicly available information. http://www.foodstandards.govt.nz/publications/documents/Transgenic%20Livestock%20Review%20CSIRO%20FINAL%2012Dec20031.pdf. Accessed 02 June 2013
Maksimenko OG, Deykin AV, Khodarovich YM et al (2013) Use of transgenic animals in biotechnology: prospects and problems. Acta Naturae 5(1):33–46
Bianco A, Wu W, Pastorin G et al (2007) Carbon nanotube-based vectors for delivering immunotherapeutics and drugs. In: Kumar C (ed) Nanomaterials for medical diagnosis and therapy. Nanotechnologies for the life sciences, vol 10. Wiley-VCH, Weinheim, pp 85–142
Campos VF, de Leona PMM, Komninoua ER et al (2011) NanoSMGT: Transgene transmission into bovine embryos using halloysite clay nanotubes or nanopolymer to improve transfection efficiency. Theriogenology 76:1552–1560
Luo D, Saltzman WM (2000) Synthetic DNA delivery systems. Nat Biotech 18:33–37
Houdebine LM (2000) Transgenic animal bioreactors. Transgenic Res 9:305–320
Majundar S, Sahay SSA (2009) Review of: biological and pharmaceutical nanomaterials. Mater Manuf Process 24(4):517–518
Mao HQ, Roy K, Trouq-Le VL et al (2001) Chitosan-DNA nanoparticles as gene carriers: synthesis, characterization and transfection efficiency. J Control Release 70(3):399–421
DeLong RK, Reynolds CM, Malcolm Y et al (2010) Functionalized gold nanoparticles for the binding, stabilization, and delivery of therapeutic DNA, RNA, and other biological macromolecules. Nanotech Sci Appl 3:53–63
Petersen EJ, Tu X, Dizdaroglu M (2013) Protective roles of single-wall carbon nanotubes in ultrasonication-induced DNA base damage. Small 9(2):205–208. doi:10.1002/smll.201201217
Wu Y, Phillips JA, Liu H et al (2008) Carbon Nanotubes protect DNA strands during cellular delivery. ACS Nano 2(10):2023–2028. doi:10.1021/nn800325a
Liu Y, Wu D, Zang W et al (2005) Polyethylenimine-grafted multiwalled carbon nanotubes for secure noncovalent immobilization and efficient delivery of DNA. Angew Chem Int Ed 44:4782–4785. doi:10.1002/anie.200500042
Voronina E, Wessel GM (2003) The regulation of oocyte maturatiom. Curr Top Dev Biol 58:53–110. doi:10.1016/S0070-2153(03)58003-6
Van Den Hurk R, Zhao J (2005) Formation of mammalian oocytes and their growth, differentiation and maturation within ovarian follicles. Theriogenology 63:1717–1751. doi:10.1016/j.theriogenology.2004.08.005
Gilchrist RB, Thompson JG (2007) Oocyte maturation: Emerging concepts and technologies to improve developmental potential in vitro. Theriogenology 67:6–15. doi:10.1016/j.theriogenology.2006.09.027
Ringette MJ, Chamberlin ME, Baur AW et al (1988) Molecular analysis of cDNA coding for ZP3, a sperm binding protein of the mouse Zona Pellucida. Dev Biol 127:287–295
Wassarman PM, Liu C, Litscher ES (2004) Constructing the mammalian egg zona pellucida: some new pieces of an old puzzle. J Cell Sci 109:2001–2004
Green DP (1997) Three-dimensional structure of the zona pellucida. Rev Reprod 2:147–156
Jaenisch R, Mintz B (1974) Simian virus 40 DNA sequences in DNA of healthy adult mice derived from preimplantation blastocysts injected with viral DNA. Proc Natl Acad Sci U S A. 71(4):1250–1254
Brownlee C (2004) Biography of Rudolf Jaenisch. PNAS 101(39):13982–13984
GlaxoSmithKline. http://www.gsk.com/content/dam/gsk/globals/documents/pdf/GSK-on-the-role-of-transgenic-animals-in-biomedical-research.pdf. Accessed 02 June 2013
Novartis. http://www.novartis.com/innovation/responsibly-tackling-the-challenging-issues/animal-research/research-at-novartis/transgenic-animals.shtm. Accessed 02 June 1013
Wei L (1997) Transgenic animals as new approaches in pharmacological studies. Annu Rev Pharmacol Toxicol 37:119–141
Houdebine LM (2009) Production of pharmaceutical proteins by transgenic animals. Comp Immunol Microbiol Infect Dis 32:107–121. doi:10.1016/j.cimid.2007.11.005
Reuters. http://www.reuters.com/article/2007/04/18/us-biotech-argentina-diabetes-idUSN1744610320070418. Accessed 10 June 1013
Biosidus: http://www.biosidus.com.ar/animales_transgenicos.php. Accessed 10 June 1013
Australian National Heart and Medical Research Council. http://www.nhmrc.gov.au/health-ethics/ethical-issues/animal-human-transplantation-research-xenotransplantation. Accessed 10 June 1013
Klymiuk N, Aaigner B, Brem G et al (2010) Genetic modification of pigs as organ donors for xenotransplantation. Mol Reprod Dev 77(3):209–221. doi:10.1002/mrd.21127
Godfray HCJ, Beddington JR, Crute IR et al (2010) Food security: the challenge of feeding 9 billion people. Science 327(5967):812–818. doi:10.1126/science.1185383
Alexandratos N (2009) How to feed the world in 2050. http://www.fao.org/fileadmin/templates/wsfs/docs/expert_paper/How_to_Feed_the_World_in_2050.pdf. Accessed 12 June 1013
Tilmana D, Balzer C, Hill J et al (2011) Global food demand and the sustainable intensification of agriculture. Proc Nat Acad Sci USA 108(50):20260–20264. doi:10.1073/pnas.1116437108
Royal Society of London (2009) Reaping the benefits: science and the sustainable intensification of global agriculture. http://royalsociety.org/uploadedFiles/Royal_Society_Content/policy/publications/2009/4294967719.pdf. Accessed 12 June 1013
Lal R (2004) Carbon sequestration impacts on global climate change and food security. Science 304(5677):1623–1627
Gifford JAH, Gifford CA (2013) Role of reproductive biotechnologies in enhancing food security and sustainability. Anim Front 3(3):14–19
Brower V (1998) Nutraceuticals: poised for a healthy slice of the healthcare market? Nat Biotechnol 16(8):728–731
Salamone D, Bevacqua R, Hiriart MI et al (2012) Transgenesis in farm animals. Anim Reprod 9(4):772–776
Dunn DA, Kooyman DL, Pinkert CA (2005) Foundation review: transgenic animals and their impact on the drug discovery industry. Drug Discov Today 10(11):757–767
Ageta-Ishihara N, Yamakado H, Morita T et al (2013) Chronic overload of SEPT4, a parkin substrate that aggregates in Parkinson’s disease, causes behavioral alterations but not neurodegeneration in mice. Mol Brain 6(1):35. doi:10.1186/1756-6606-6-35
Wu X, Ouyang H, Duan B et al (2012) Production of cloned transgenic cow expressing omega-3 fatty acids. Transgenic Res 21:537–543. doi:10.1007/s11248-011-9554-2
Ramírez P, Montoya MJ, Ríos A et al (2005) Prevention of hyperacute rejection in a model of orthotopic liver xenotransplantation from pig to baboon using polytransgenic pig livers (CD55, CD59, and H-Transferase). Transplant Proc 37(9):4103–4106. doi:10.1016/j.transproceed.2005.09.186
Gonzalez-Stawinski GV, Daggett CW, Lau CL et al (2002) Non-anti-Gal alpha1-3Gal antibody mechanisms are sufficient to cause hyperacute lung dysfunction in pulmonary xenotransplantation. J Am Coll Surg 194(6):765–773
Berkel PHC, Welling MM, Geerts M et al (2002) Large scale production of recombinant human lactoferrin in the milk of transgenic cows. Nat Biotechnol 20(5):484–487
Chrenek P, Ryban L, Vetr H et al (2007) Expression of recombinant human factor VIII in milk of several generations of transgenic rabbits. Transgenic Res 16:353–361. doi:10.1007/s11248-007-9070-6
Yekta AA, Dalman A, Eftekhari-Yazdi P et al (2013) Production of transgenic goats expressing human coagulation factor IX in the mammary glands after nuclear transfer using transfected fetal fibroblast cells. Transgenic Res 22:131–142. doi:10.1007/s11248-012-9634-y
Hamada Y, Fujii H, Kitazawa R et al (2009) Thioredoxin-1 overexpression in transgenic mice attenuates streptozotocin-induce diabetic osteopenia: a novel role of oxidative stress and therapeutic implications. Bone 44(5):936–941. doi:10.1016/j.bone.2008.12.011
Huynh K, McMullen JR, Julius TL et al (2010) Cardiac-specific IGF-1 receptor transgenic expression protects against cardiac fibrosis and diastolic dysfunction in a mouse model of diabetic cardiomyopathy. Diabetes 59(6):1512–1520. doi:10.2337/db09-1456
Maga EA, Cullor JS, Smith W et al (2006) Human lysozyme expressed in the mammary gland of transgenic dairy goats can inhibit the growth of bacteria that cause mastitis and the cold-spoilage of milk. Foodborne Pathog Dis 3(4):384–392
Freitas VJF, Serova IA, Andreeva LE et al (2007) Production of transgenic goat (Capra hircus) with human granulocyte colony stimulating factor (hG-CSF) gene in Brazil. An Acad Bras Cienc 79(4):585–592
Tong C, Li P, Wu NL et al (2010) Production of p53 gene knockout rats by homologous recombination in embryonic stem cells. Nature 9:467(7312):211–3. doi: 10.1038/nature09368
Geurts AM, Cost GJ, Freyvert Y et al (2009) Knockout rats via embryo microinjection of zinc-finger nucleases. Science 325(5939):433. doi:10.1126/science.1172447
Tesson L, Usal C, Ménoret S et al (2011) Knockout rats generated by embryo microinjection of TALENs. Nat Biotechnol 29(8):695–696. doi:10.1038/nbt.1940
Sasaki E, Suemizu H, Shimada A et al (2013) Generation of transgenic non-human primates with germline transmission. Nature 459:523–527. doi:10.1038/nature08090
Hu S, Ni W, Sai W et al (2013) Knock down of myostatin Expression by RNAi enhances muscle growth in transgenic sheep. PLoS ONE 8(3):e58521. doi:10.1371/journal.pone.0058521
Huang YJ, Huang Y, Baldassarre H et al (2007) Recombinant human butyrylcholinesterase from milk of transgenic animals to protect against organophosphate poisoning. Proc Natl Acad Sci USA 104(34):13603–13608. doi:10.1073/pnas.0702756104
Gordon JW, Scangos GA, Plotkin DJ et al (1980) Genetic transformation of mouse embryos by microinjection of purified DNA. Proc Natl Acad Sci USA 77:7380–7384
Hogan B, Beddington R, Costantini F et al (1994) Manipulating the Mouse Embryo: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Wheeler MB, Walters EM (2001) Transgenic technology and applications in swine. Theriogenology 56:1345–1369
Wall RJ, Pursel VG, Rammer RE et al (1985) Development of porcine ova that were centrifuged to permit visualization of pronuclei and nuclei. Biol Reprod 32:645–651
Pursel VG, Rexroad CE (1993) Recent progress in the transgenic modification of swine and sheep. Mol Reprod Dev 36(2):251–254
Niemann H, Kues WA (2003) Application of transgenesis in livestock for agriculture and biomedicine. Anim Reprod Sci 79(3–4):291–317
Evans MJ, Kaufman MH (1981) Establishment in culture of pluripotencial cells from mouse embryos. Nature 292:154–156
Bradley A, Evans MJ, Kaufman MH et al (1984) Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature 309:255–256
Koller BH, Hagemann LJ, Doetscheman T et al (1989) Germ-line transmission of a planned alteration made in a hypoxanthine phosphoribosyltransferase gene by homologous recombination in embryonic stem cells. Proc Natl Acad Sci USA 86:8927–8931
Gandolfi F, Panarossa G, Maffei S et al (2012) Why is it so difficult to derive pluripotent stem cells in domestic ungulates? Reprod Domest Anim 47(5):11–17
Lavitrano MA, Camaione A, Fazio VM et al (1989) Sperm cells as vectors for introducing foreign DNA into eggs: genetic transformation of mice. Cell 57:717–723
Celebi C, Guillaudeux T, Auvray P et al (2003) The making of ‘‘transgenic spermatozoa”. Biol Rep 68:1477–1483
Sperandio S, Lulli V, Bacci ML et al (1996) Sperm-mediated DNA transfer in bovine and swine species. Anim Biotechnol 7:59–77
Lavitrano M, Bacci ML, Forni M (2002) Efficient production by sperm-mediated gene transfer of human decay accelerating factor (hDAF) transgenic pigs for xenotransplantation. Proc Natl Acad Sci USA 99:14230–14235
Brinster RL, Sandgren EP, Behringer RR et al (1989) No simple solution for making transgenic mice. Cell 59:239–241
Eghbalsaied S, Ghaedi K, Laible G et al (2013) Exposure to DNA is insufficient for in vitro transgenesis of live bovine sperm and embryos. Reproduction 145:97–108
Chan AWS, Homan EJ, Ballou LU et al (1998) Transgenic cattle produced by reverse-transcribed gene transfer in oocytes. Proc Natl Acad Sci USA 95:14028–14033
Cherry SR, Biniszkiewicz D, Van Parijs L et al (2000) Retroviral expression in embryonic stem cells and hematopoietic stem cells. Mol Cell Biol 20:7419–7426. doi:10.1128/MCB.20.20.7419-7426.2000
Pfeifer A, Ikawa M, Dayn Y et al (2002) Transgenesis by lentiviral vectors: lack of gene silencing in mammalian embryonic stem cells and preimplantation embryos. Proc Natl Acad Sci USA 99(4):2140–2145
Hofmann A (2004) Generation of transgenic cattle by lentiviral gene transfer into oocytes. Biol Reprod 71:405–409
Pauwels K, Gijsbers R, Toelen J et al (2009) State-of-the-art lentiviral vectors for research use: risk assessment and biosafety recommendations. Curr Gene Ther 9:459–474. doi:10.2174/156652309790031120
Lillico S, Vasey D, King T (2011) Lentiviral transgensis in livestock. Transgenic Res 20(3):441–442. doi:10.1007/s11248-010-9448-8
Wilmut I, Schnieke AE, McWhir J et al (1997) Viable offspring derived from fetal and adult mammalian cells. Nature 385:(6619)810–813
Schnieke AE, Kind AJ, Ritchie WA et al (1997) Human factor IX transgenic sheep produced by transfer of nuclei from transfected fetal fibroblasts. Science 278:(5346)2130–2133
Wall RJ, Powell AM, Paape MJ et al (2005) Genetically enhanced cows resist intramammary Staphylococcus aureus infection. Nat Biotechnol 23:445–451
Schellander K, Peli J, Schmoll F et al (1995) Artificial insemination in cattle with DNA-treated sperm. Animal Biotechnol 6:41–50. doi:10.1080/10495399509525831
Powell AM, Talbot NC, Wells KD et al (2004) Cell donor influences success of producing cattle by somatic cell nuclear transfer. Biol Reprod 71:210–216. doi:10.1095/biolreprod.104.027193
Urnov FD, Rebar EJ, Holmes MC et al (2010) Genome editing with engineered zinc finger nucleases. Nat Rev Genet 11(9):636–646. doi:10.1038/nrg2842
Cui X, Ji D, Fisher DA et al (2011) Targeted integration in rat and mouse embryos with zinc-finger nucleases. Nat Biotechnol 29(1):64–67. doi:10.1038/nbt.1731
Carlson DF, Tan W, Lillico SG et al (2012) Efficient TALEN-mediated gene knockout in livestock. Proc Natl Acad Sci USA 109(43):17382–17387. doi:10.1073/pnas.1211446109
Sanz V, Borowiak E, Lukanov P et al (2011) Optimising DNA binding to carbon nanotubes by non-covalent methods. Carbon 49:1775–1781. doi:10.1016/j.carbon.2010.12.064
Rajendra J, Baxendale M, Rap LGD et al (2004) Flow linear dichroism to probe binding of aromatic molecules and DNA to single-walled carbon nanotubes. J Am Chem Soc 1269(36):11182–11188. doi:10.1021/ja048720j
Enyashim AN, Gemming S, Seifert G (2007) DNA-wrapped carbon nanotubes. Nanotechnology 18(24):245702. doi:10.1088/0957-4484/18/24/245702
Gao H, Kong Y (2004) Simulation of DNA-nanotube interactions. Annu Rev Mater Res 34:123–150. doi:10.1146/annurev.matsci.34.040203.120402
Gigliotti B, Sakizzie B, Bethune DS et al (2006) Sequence-independent helical wrapping of single-walled carbon nanotubes by long genomic DNA. Nano Lett 6(2):159–164. doi:10.1021/nl0518775
Li X, Peng Y, Qu X (2006) Carbon nanotubes selective destabilization of duplex and triplex DNA and inducing B-A transition in solution. Nucl Acids Res 34(13):3670–3676. doi:10.1093/nar/gkl513
Hughes ME, Brandin E, Golovchenko JA (2007) Optical absorption of DNA—carbon nanotube structures. Nano Lett 7(5):1191–1194. doi:10.1021/nl062906u
Zhao X, Johnson JK (2007) Simulation of adsorption of DNA on carbon nanotubes. J Am Chem Soc 129:10438–10445. doi:10.1021/ja071844m
Ahmed M, Jiang X, Deng Z et al (2009) Cationic glyco-functionalized single-walled carbon nanotubes as efficient gene delivery vehicles. Bioconjug Chem 20:(11)2017–2022. doi: 10.1021/bc900229v
Pantarotto D, Singh R, McCarthy D et al (2004) Functionalized carbon nanotubes for plasmid DNA gene delivery. Angew Chem Int Ed Engl 43(39):5242–5246. doi:10.1021/bc900229v
Qin W, Yang K, Tang H (2011) Improved GFP gene transfection mediated by polyamidoamine dendrimer-functionalized multi-walled carbon nanotubes with high biocompatibility. Colloids Surf B Biointerfaces 84(1):206–213. doi:10.1016/j.colsurfb.2011.01.001
Wan Y, Liu G, Zhu X et al (2013) pH induced reversible assembly of DNA wrapped carbon nanotubes. Chem Cent J 7(1):14. doi:10.1186/1752-153X-7-14
Ladeira MS, Andrade VA, Gomes ERM et al (2010) Highly efficient siRNA delivery system into human and murine cells using single-wall carbon nanotubes. Nanotechnology 21(38):385101. doi:10.1088/0957-4484/21/38/385101
Brandão HM, Pereira MM, Carvalho BC et al. Multiwalled carbon nanotubos delivery of the GFP gene into bovine embryos. Unpublished manuscript
Nagano M, Shinohara T, Avarbock MR et al (2000) Retrovirus-mediated gene delivery into male germ line stem cells. FEBS Lett 475(1):7–10
Mittermeyer G, Christine CW, Rosenbluth CH et al (2012) Long-term evaluation of a phase 1 study of AADC gene therapy for Parkinson’s disease. Hum Gene Ther 23(4):377–381. doi:10.1089/hum.2011.220
Schirmer JM, Miyagi N, Rao VP et al (2007) Recombinant adeno-associated virus vector for gene transfer to the transplanted rat heart. Transpl Int 20(6):550–557. doi:10.1111/j.1432-2277.2007.00479.x
Kamstock D, Guth A, Elmslie R et al (2006) Liposome-DNA complexes infused intravenously inhibit tumor angiogenesis and elicit antitumor activity in dogs with soft tissue sarcoma. Cancer Gene Ther 13(3):306–317. doi:10.1038/sj.cgt.7700895
Rieth A, Pothier F, Sirard M (2000) Electroporation of bovine spermatozoa to carry DNA containing highly repetitive sequences into oocytes and detection of homologous recombination events. Mol Reprod Dev 57:338–345 I:10.1002/1098-2795(200012)57:4<338:AID-MRD5>3.0.CO;2-K
Toledo JR, Prieto Y, Oramas N et al (2009) Polyethylenimine-based transfection method as a simple and effective way to produce recombinant lentiviral vectors. Appl Biochem Biotechnol 157(3):538–544. doi:10.1007/s12010-008-8381-2
Guo H, Hao R, Wei Y et al (2012) Optimization of electrotransfection conditions of mammalian cells with different biological features. J Membr Biol 245(12):789–795. doi:10.1007/s00232-012-9480-0
Nunes A, Amsharov N, Guo C et al (2010) Hybrid polymer-grafted multiwalled carbon nanotubes for in vitro gene delivery. Small 6(20):2281–2291. doi:10.1002/smll.201000864
Li S, Huang L (2000) Nonviral gene therapy: promises and challenges. Gene Ther 7(1):31–34
Trimaille T, Chaix C, Pichot C et al (2003) Polymer functionalized submicrometric emulsions as potential synthetic DNA vectors. J Colloid and Interface Sci 258(1):135–145. doi:10.1016/S0021-9797(02)00069-3
Lesage D, Cao A, Briane D et al (2002) Evaluation and optimization of DNA delivery into gliosarcoma 9L cells by a cholesterol-based cationic liposome. Biochim Biophys Acta 1564(2):393–402
Wong SY, Pelet JM, Putnam D (2007) Polymer systems for gene delivery-past, present, and future. Prog Polym Sci 32:799–837. doi:10.1016/j.progpolymsci.2007.05.007
Mintzer MA, Simanek EE (2009) Nonviral vectors for gene delivery. Chem Rev 109:259–302
Joshi RP, Schoenbach KH (2002) Mechanism for membrane electroporation irreversibility under high-intensity, ultrashort electrical pulse conditions. Phys Rev E: Stat, Nonlin, Soft Matter Phys 66:052901. doi:10.1103/PhysRevE.66.052901
Rittner K, Benavente A, Bompard-Sorlet A et al (2002) New basic membrane-destabilizing peptides for plasmid-based gene delivery in vitro and in vivo. Mol Ther 5(2):104–114. doi:10.1006/mthe.2002.0523
Cai D, Mataraza JM, Qin ZH et al (2005) Highly efficient molecular delivery into mammalian cells using carbon nanotube spearing. Nat Methods 2(6):449–454
Singh R, Pantarotto D, McCarthy D et al (2005) Binding and condensation of plasmid DNA onto functionalized carbon nanotubes: toward the construction of nanotube-based gene delivery vectors. J Am Chem Soc 127(12):4388–4396. doi:10.1021/ja0441561
Delogu GL, Magrini A, Bergamaschi A et al (2009) Conjugation of antisense oligonucleotides to PEGylated carbon nanotubes enables efficient knockdown of PTPN22 in T lymphocytes. Bioconjug Chem 20(3):427–431. doi:10.1021/bc800540j
Kam SWN, Liu Z, Dai H (2006) Carbon nanotubes as intracellular transporters for proteins and DNA: an investigation of the uptake mechanism and pathway. Angew Chem Int Ed Engl 45:577–581. doi:10.1002/ange.200503389
Yang K, Qin W, Tang H et al (2011) Polyamidoamine dendrimer-functionalized carbon nanotubes-mediated GFP gene transfection for HeLa cells:effects of different types of carbon nanotubes. J Biomed Mater Res A 99(2):231–239
Vanroose G, Nauwynck H, Soom AV et al (2000) Structural aspects of the zona pellucida of in vitro-produced bovine embryos: a scanning electron and confocal laser scanning microscopic study. Biol Reprod 62(2):463–469. doi:10.1095/biolreprod62.2.463
van Berlo D, Clift MJD, Albrecht C et al (2012) Carbon nanotubes: an insight into the mechanisms of their potential genotoxicity. Swiss Med Wkly 142:w13698. doi:10.4414/smw.2012.13698
Toyokuni S (2013) Genotoxicity and carcinogenicity risk of carbon nanotubes. Adv Drug Deliv Rev S0169–409X(13):00149-X. doi:10.1016/j.addr.2013.05.011
Boczkowski J, Lanone S (2012) Genotoxicity and carcinogenicity risk of carbon nanotubes. Adv Drug Deliv Rev 64(15):1694–1699. doi:10.1016/j.addr.2012.05.011
Roman D, Yasmeen A, Mireuta M et al (2013) Significant toxic role for single-walled carbon nanotubes during normal embryogenesis. Nanomedicine 9(7):945–950. doi:10.1016/j.nano.2013.03.010
Cheng J, Cheng SH (2012) Influence of carbon nanotube length on toxicity to zebrafish embryos. Int J Nanomedicine. 7:3731–37319
Cheng J, Flahaut E, Cheng SH (2007) Effect of carbon nanotubes on developing zebrafish (Danio rerio) embryos. Environ Toxicol Chem 26(4):708–716
Cheng J, Chan CM, Veca LM et al (2009) Acute and long-term effects after single loading of functionalized multi-walled carbon nanotubesinto zebrafish (Danio rerio). Toxicol Appl Pharmacol 235(2):216–225
Pacurari M, Yin XJ, Zhao J et al (2008) Raw single-wall carbon nanotubes induce oxidative stress and activate MAPKs, AP-1, NF-kappaB, and Akt in normal and malignant human mesothelial cells. Environ Health Perspect 116(9):1211–1217. doi:10.1289/ehp.10924
Yang H, Liu C, Yang D et al (2009) Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: the role of particle size, shape and composition. J Appl Toxicol 29(1):69–78. doi:10.1002/jat.1385
Lindberg HK, Falck GC, Suhonen S et al (2009) Genotoxicity of nanomaterials: DNA damage and micronuclei induced by carbon nanotubes and graphite nanofibres in human bronchial epithelial cells in vitro. Toxicol Lett 186(3):166–173. doi:10.1016/j.toxlet.2008.11.019
Pan S, Sardesai NP, Liu H et al (2013) Assessing DNA damage from enzyme-oxidized single-walled carbon nanotubes. Toxicol Res 2:375–378. doi:10.1039/c3tx50022e
Di Giorgio ML, Di Bucchianico S, Ragnelli AM et al (2011) Effects of single and multi walled carbon nanotubes on macrophages: cyto and genotoxicity and electron microscopy. Mutat Res 722(1):20–31. doi:10.1016/j.mrgentox.2011.02.008
Kisin ER, Murray AR, Sargent L et al (2011) Genotoxicity of carbon nanofibers: are they potentially more or less dangerous than carbon nanotubes or asbestos? Toxicol Appl Pharmacol 252(1):1–10. doi:10.1016/j.taap.2011.02.001
Patlolla AK, Hussain SM, Schlager JJ et al (2010) Comparative study of the clastogenicity of functionalized and nonfunctionalized multiwalled carbon nanotubes in bone marrow cells of Swiss-Webster mice. Environ Toxicol 25(6):608–621. doi:10.1002/tox.20621
Ema M, Masumori S, Kobayashi N et al (2013) In vivo comet assay of multi-walled carbon nanotubes using lung cells of rats intratracheally instilled. J Appl Toxicol 33(10):1053–1060. doi:10.1002/jat.2810
Naya M, Kobayashi N, Endo S et al (2012) In vivo genotoxicity study of single-wall carbon nanotubes using comet assay following intratracheal instillation in rats. Regul Toxicol Pharmacol 64(1):124–129. doi:10.1016/j.yrtph.2012.05.020
Yamashita K, Yoshioka Y, Higashisaka K et al (2010) Carbon nanotubes elicit DNA damage and inflammatory response relative to their size and shape. Inflammation 33(4):276–280. doi:10.1007/s10753-010-9182-7
He X, Young S, Fernback JE et al (2012) Single-Walled Carbon Nanotubes induce fibrogenic effect by disturbing mitochondrial oxidative stress and activating NF-κB signaling. J Clinic Toxicol S:5. doi:10.4172/2161-0495.S5-005
Jacobsen NR, Pojana G, White P et al (2008) Genotoxicity, cytotoxicity, and reactive oxygen species induced by single-walled carbon nanotubes and C(60) fullerenes in the FE1-Mutatrade markMouse lung epithelial cells. Environ Mol Mutagen 49(6):476–487. doi:10.1002/em.20406
Muller J, Huaux F, Fonseca A et al (2008) Structural defects play a major role in the acute lung toxicity of multiwall carbon nanotubes: toxicological aspects. Chem Res Toxicol 21(9):1698–1705. doi:10.1021/tx800101p
Lindberg HK, Falcka GC, Singh R et al (2013) Genotoxicity of short single-wall and multi-wall carbon nanotubes in human bronchial epithelial and mesothelial cells in vitro. Toxicology 313(1):24–37. doi:10.1016/j.tox.2012.12.008
Ponti J, Broggi F, Mariani V et al (2013) Morphological transformation induced by multiwall carbon nanotubes on Balb/3T3 cell model as an in vitro end point of carcinogenic potential. Nanotoxicol 7(2):221–233. doi:10.3109/17435390.2011.652681
Wu P, Yuan S, Ho C et al (2013) Focal amplification of HOXD-Harboring Chromosome Region is implicated in Multiple-Walled Carbon Nanotubes-induced carcinogenicity. Nano Lett 13(10):4632–4641. doi:10.1021/nl401658c
Catalán J, Järventaus H, Vippola M et al (2012) Induction of chromosomal aberrations by carbon nanotubes and titanium dioxide nanoparticles in human lymphocytes in vitro. Nanotoxicol 6(8):825–836. doi:10.3109/17435390.2011.625130
Mohiuddin Keka IS, Evans TJ et al (2013) A novel genotoxicity assay of carbon nanotubes using functional macrophage receptor with collagenous structure (MARCO)-expressing chicken B lymphocytes. Arch Toxicol. doi:10.1007/s00204-013-1084-7
Manshian BB, Jenkins GJS, Williams PM (2013) Single-walled carbon nanotubes: differential genotoxic potential associated with physico-chemical properties. Nanotoxicol 7(2):144–156. doi:10.3109/17435390.2011.647928
Kato T, Totsuka Y, Ishino K et al (2013) Genotoxicity of multi-walled carbon nanotubes in both in vitro and in vivo assay systems. Nanotoxicol 7(4):452–461. doi:10.3109/17435390.2012.674571
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
de Mello Brandão, H. et al. (2016). Carbon Nanotubes as a DNA Delivery Agent for Generation of Genetically Modified Mammals Embryos. In: Jorio, A. (eds) Bioengineering Applications of Carbon Nanostructures. Nanomedicine and Nanotoxicology. Springer, Cham. https://doi.org/10.1007/978-3-319-25907-9_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-25907-9_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-25905-5
Online ISBN: 978-3-319-25907-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)