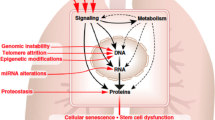
Abstract
Pulmonary function declines during normal aging, whereas pulmonary inflammation increases. The net effect of aging is an increased risk for the development of Chronic Obstructive Pulmonary Disease (COPD) and idiopathic pulmonary fibrosis (IPF) in susceptible individuals. These diseases accelerate the age-related loss in lung function contributing to disability and premature death. Inasmuch as our population is aging, it is important to mechanistically understand the role of aging on the pathobiology of these lung diseases. Lung aging, with its exposure to external factors over many years should be integrated into the complex profiling of COPD and IPF. The understanding of the role played by aging hallmarks in the pathophysiology of COPD and Idiopathic pulmonary fibrosis will be addressed in this chapter. Moreover, future directions and the missing links will be discussed in this chapter.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Vestbo J et al (2013) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 187(4):347–365
Lee J et al (2011) Is the aging process accelerated in chronic obstructive pulmonary disease? Curr Opin Pulm Med 17(2):90–97
Cornwell WD et al (2010) Pathogenesis of inflammation and repair in advanced COPD. Semin Respir Crit Care Med 31(3):257–266
Huiart L, Ernst P, Suissa S (2005) Cardiovascular morbidity and mortality in COPD. Chest 128(4):2640–2646
Rana JS et al (2004) Chronic obstructive pulmonary disease, asthma, and risk of type 2 diabetes in women. Diabetes Care 27(10):2478–2484
Ozge C, Ozge A, Unal O (2006) Cognitive and functional deterioration in patients with severe COPD. Behav Neurol 17(2):121–130
Mannino DM (2003) Chronic obstructive pulmonary disease: definition and epidemiology. Respir Care 48(12):1185–1191; discussion 1191–3
Tashkin DP (2013) Variations in FEV(1) decline over time in chronic obstructive pulmonary disease and its implications. Curr Opin Pulm Med 19(2):116–124
Lamprecht B et al (2011) COPD in never smokers: results from the population-based burden of obstructive lung disease study. Chest 139(4):752–763
Thomsen M et al (2013) Characteristics and outcomes of chronic obstructive pulmonary disease in never smokers in Denmark: a prospective population study. Lancet Respir Med 1(7):543–550
Salvi SS, Barnes PJ (2009) Chronic obstructive pulmonary disease in non-smokers. Lancet 374(9691):733–743
Bosken CH et al (1992) Characterization of the inflammatory reaction in the peripheral airways of cigarette smokers using immunocytochemistry. Am Rev Respir Dis 145(4 Pt 1):911–917
Lams BE et al (1998) Immunopathology of the small-airway submucosa in smokers with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med 158(5 Pt 1):1518–1523
Saetta M et al (1999) CD8 + ve cells in the lungs of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 160(2):711–717
Amin K et al (2003) Relationship between inflammatory cells and structural changes in the lungs of asymptomatic and never smokers: a biopsy study. Thorax 58(2):135–142
Larsson K (2007) Aspects on pathophysiological mechanisms in COPD. J Intern Med 262(3):311–340
Barnes PJ (2008) Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol 8(3):183–192
Henson PM, Cosgrove GP, Vandivier RW (2006) State of the art. Apoptosis and cell homeostasis in chronic obstructive pulmonary disease. Proc Am Thorac Soc 3(6):512–516
Raghu G et al (2011) An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183(6):788–824
Bjoraker JA et al (1998) Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 157(1):199–203
Flaherty KR et al (2002) Clinical significance of histological classification of idiopathic interstitial pneumonia. Eur Respir J 19(2):275–283
Nicholson AG et al (2000) The prognostic significance of the histologic pattern of interstitial pneumonia in patients presenting with the clinical entity of cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med 162(6):2213–2217
American Thoracic, S. and S. European Respiratory (2002) American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 165(2):277–304
Seibold MA et al (2011) A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med 364(16):1503–1512
Selman M et al (2001) Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 134(2):136–151
Bueno M et al (2015) PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J Clin Invest 25(2):521–538
Patel AS et al (2015) Epithelial cell mitochondrial dysfunction and PINK1 are induced by transforming growth factor- beta1 in pulmonary fibrosis. PLoS One 10(3):e0121246
Jain M et al (2013) Mitochondrial reactive oxygen species regulate transforming growth factor-beta signaling. J Biol Chem 288(2):770–777
Sosulski ML et al (2015) Deregulation of selective autophagy during aging and pulmonary fibrosis: the role of TGFbeta1. Aging Cell [Epub ahead of print]
Hecker L et al (2009) NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med 15(9):1077–1081
Griffith B et al (2009) NOX enzymes and pulmonary disease. Antioxid Redox Signal 11(10):2505–2516
Cheresh P et al (2013) Oxidative stress and pulmonary fibrosis. Biochim Biophys Acta 1832(7):1028–1040
Kumar A et al (2014) Genome sequencing of idiopathic pulmonary fibrosis in conjunction with a medical school human anatomy course. PLoS One 9(9):e106744
Peljto AL et al (2015) The MUC5B promoter polymorphism is associated with idiopathic pulmonary fibrosis in a Mexican cohort but is rare among Asian ancestries. Chest 147(2):460–464
Coghlan MA et al (2014) Sequencing of idiopathic pulmonary fibrosis-related genes reveals independent single gene associations. BMJ Open Respir Res 1(1):e000057
Fingerlin TE et al (2013) Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat Genet 45(6):613–620
Noth I et al (2013) Genetic variants associated with idiopathic pulmonary fibrosis susceptibility and mortality: a genome-wide association study. Lancet Respir Med 1(4):309–317
Peng R et al (2013) Bleomycin induces molecular changes directly relevant to idiopathic pulmonary fibrosis: a model for “active” disease. PLoS One 8(4):e59348
Sueblinvong V et al (2012) Predisposition for disrepair in the aged lung. Am J Med Sci 344(1):41–51
Selman M, Pardo A (2014) Revealing the pathogenic and aging-related mechanisms of the enigmatic idiopathic pulmonary fibrosis. an integral model. Am J Respir Crit Care Med 189(10):1161–1172
Gauldie J et al (2006) Smad3 signaling involved in pulmonary fibrosis and emphysema. Proc Am Thorac Soc 3(8):696–702
Jankowich MD et al (2008) Heterogeneity in combined pulmonary fibrosis and emphysema. Respiration 75(4):411–417
Mahavadi P et al (2010) Epithelial stress and apoptosis underlie Hermansky-Pudlak syndrome-associated interstitial pneumonia. Am J Respir Crit Care Med 182(2):207–219
Tzouvelekis A, Bonella F, Spagnolo P (2015) Update on therapeutic management of idiopathic pulmonary fibrosis. Ther Clin Risk Manag 11:359–370
Chaudhary NI et al (2007) Inhibition of PDGF, VEGF and FGF signalling attenuates fibrosis. Eur Respir J 29(5):976–985
Iyer SN, Gurujeyalakshmi G, Giri SN (1999) Effects of pirfenidone on transforming growth factor-beta gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J Pharmacol Exp Ther 291(1):367–373
Gurujeyalakshmi G, Hollinger MA, Giri SN (1999) Pirfenidone inhibits PDGF isoforms in bleomycin hamster model of lung fibrosis at the translational level. Am J Physiol 276(2 Pt 1):L311–L318
Iyer SN et al (1998) Lung fibrosis is ameliorated by pirfenidone fed in diet after the second dose in a three-dose bleomycin-hamster model. Exp Lung Res 24(1):119–132
Joos L, Pare PD, Sandford AJ (2002) Genetic risk factors of chronic obstructive pulmonary disease. Swiss Med Wkly 132(3–4):27–37
Demeo DL et al (2006) The SERPINE2 gene is associated with chronic obstructive pulmonary disease. Am J Hum Genet 78(2):253–264
Ito K et al (2005) Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med 352(19):1967–1976
Kythreotis P et al (2009) Plasma leptin and insulin-like growth factor I levels during acute exacerbations of chronic obstructive pulmonary disease. BMC Pulm Med 9:11
Fournier M et al (2003) Insulin-like growth factor I prevents corticosteroid-induced diaphragm muscle atrophy in emphysematous hamsters. Am J Physiol Regul Integr Comp Physiol 285(1):R34–R43
Uh ST et al (1998) Morphometric analysis of insulin-like growth factor-I localization in lung tissues of patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 158(5 Pt 1):1626–1635
Ruan W, Ying K (2010) Abnormal expression of IGF-binding proteins, an initiating event in idiopathic pulmonary fibrosis? Pathol Res Pract 206(8):537–543
Honeyman L et al (2013) MicroRNA profiling implicates the insulin-like growth factor pathway in bleomycin-induced pulmonary fibrosis in mice. Fibrogenesis Tissue Repair 6(1):16
Pilewski JM et al (2005) Insulin-like growth factor binding proteins 3 and 5 are overexpressed in idiopathic pulmonary fibrosis and contribute to extracellular matrix deposition. Am J Pathol 166(2):399–407
Yasuoka H, Yamaguchi Y, Feghali-Bostwick CA (2009) The pro-fibrotic factor IGFBP-5 induces lung fibroblast and mononuclear cell migration. Am J Respir Cell Mol Biol 41(2):179–188
Calhoun C et al (2015) Senescent cells contribute to the physiological remodeling of aged lungs. J Gerontol A Biol Sci Med Sci [Epub ahead of print]
Yoshida T et al (2010) Rtp801, a suppressor of mTOR signaling, is an essential mediator of cigarette smoke-induced pulmonary injury and emphysema. Nat Med 16(7):767–773
Nadon AM et al (2014) Rtp801 suppression of epithelial mTORC1 augments endotoxin-induced lung inflammation. Am J Pathol 184(9):2382–2389
Park JS et al (2014) Clinical significance of mTOR, ZEB1, ROCK1 expression in lung tissues of pulmonary fibrosis patients. BMC Pulm Med 14:168
Goc A et al (2011) TGFbeta- and bleomycin-induced extracellular matrix synthesis is mediated through Akt and mammalian target of rapamycin (mTOR). J Cell Physiol 226(11):3004–3013
Patel AS et al (2012) Autophagy in idiopathic pulmonary fibrosis. PLoS One 7(7):e41394
Araya J et al (2013) Insufficient autophagy in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 304(1):L56–69
Nho RS, Hergert P (2014) IPF fibroblasts are desensitized to type I collagen matrix-induced cell death by suppressing low autophagy via aberrant Akt/mTOR kinases. PLoS One 9(4):e94616
Richeldi L et al (2013) Mapping the future for pulmonary fibrosis: report from the 17th International Colloquium on Lung and Airway Fibrosis. Eur Respir J 42(1):230–238
Qi Y et al (2014) Inhibition of AMPK expression in skeletal muscle by systemic inflammation in COPD rats. Respir Res 15(1):156
Park CS et al (2012) Metformin reduces airway inflammation and remodeling via activation of AMP-activated protein kinase. Biochem Pharmacol 84(12):1660–1670
Park S, Mori R, Shimokawa I (2013) Do sirtuins promote mammalian longevity? A critical review on its relevance to the longevity effect induced by calorie restriction. Mol Cells 35(6):474–480
Verdin E (2014) The many faces of sirtuins: coupling of NAD metabolism, sirtuins and lifespan. Nat Med 20(1):25–27
Purushotham A et al (2009) Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab 9(4):327–338
He Q et al (2014) Shorter men live longer: association of height with longevity and FOXO3 genotype in American men of Japanese ancestry. PLoS One 9(5):e94385
Eijkelenboom A et al (2013) FOXO3 selectively amplifies enhancer activity to establish target gene regulation. Cell Rep 5(6):1664–1678
Flachsbart F et al (2009) Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc Natl Acad Sci U S A 106(8):2700–2705
Marinkovic D et al (2007) Foxo3 is required for the regulation of oxidative stress in erythropoiesis. J Clin Invest 117(8):2133–2144
Giannakou ME, Partridge L (2004) The interaction between FOXO and SIRT1: tipping the balance towards survival. Trends Cell Biol 14(8):408–412
Rodgers JT et al (2005) Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 434(7029):113–118
Luo C et al (2013) A cigarette component acrolein induces accelerated senescence in human diploid fibroblast IMR-90 cells. Biogerontology 14(5):503–511
Sundar IK, Yao H, Rahman I (2013) Oxidative stress and chromatin remodeling in chronic obstructive pulmonary disease and smoking-related diseases. Antioxid Redox Signal 18(15):1956–1971
Warburton D, Shi W, Xu B (2013) TGF-beta-Smad3 signaling in emphysema and pulmonary fibrosis: an epigenetic aberration of normal development? Am J Physiol Lung Cell Mol Physiol 304(2):L83–L85
Nakamaru Y et al (2009) A protein deacetylase SIRT1 is a negative regulator of metalloproteinase-9. FASEB J 23(9):2810–2819
Yao H, Rahman I (2012) Perspectives on translational and therapeutic aspects of SIRT1 in inflammaging and senescence. Biochem Pharmacol 84(10):1332–1339
Zerr P et al (2014) Sirt1 regulates canonical TGF-beta signalling to control fibroblast activation and tissue fibrosis. Ann Rheum Dis 205740. Published Online 1 September
Ito K, Barnes PJ (2009) COPD as a disease of accelerated lung aging. Chest 135(1):173–180
Chun P (2015) Role of sirtuins in chronic obstructive pulmonary disease. Arch Pharm Res 38(1):1–10
Minagawa S et al (2011) Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-beta-induced senescence of human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 300(3):L391–L401
Takasaka N et al (2014) Autophagy induction by SIRT6 through attenuation of insulin-like growth factor signaling is involved in the regulation of human bronchial epithelial cell senescence. J Immunol 192(3):958–968
Liu R et al (2014) Oxidative stress induces endothelial cell senescence via downregulation of Sirt6. Biomed Res Int 2014:902842
Ko CH, Takahashi JS (2006) Molecular components of the mammalian circadian clock. Hum Mol Genet 15(Spec No 2):R271–7
Panda S, Hogenesch JB, Kay SA (2002) Circadian rhythms from flies to human. Nature 417(6886):329–335
Stangherlin A, Reddy AB (2013) Regulation of circadian clocks by redox homeostasis. J Biol Chem 288(37):26505–26511
Gossan N et al (2013) The circadian clock in murine chondrocytes regulates genes controlling key aspects of cartilage homeostasis. Arthritis Rheum 65(9):2334–2345
Patel SA, Velingkaar NS, Kondratov RV (2014) Transcriptional control of antioxidant defense by the circadian clock. Antioxid Redox Signal 20(18):2997–3006
Froy O (2010) Metabolism and circadian rhythms – implications for obesity. Endocr Rev 31(1):1–24
Pekovic-Vaughan V et al (2014) The circadian clock regulates rhythmic activation of the NRF2/glutathione-mediated antioxidant defense pathway to modulate pulmonary fibrosis. Genes Dev 28(6):548–560
Ma D, Lin JD (2012) Circadian regulation of autophagy rhythm through transcription factor C/EBPbeta. Autophagy 8(1):124–125
Eckel-Mahan KL et al (2013) Reprogramming of the circadian clock by nutritional challenge. Cell 155(7):1464–1478
Masri S et al (2013) Circadian acetylome reveals regulation of mitochondrial metabolic pathways. Proc Natl Acad Sci U S A 110(9):3339–3344
Korfhagen TR et al (2009) Rapamycin prevents transforming growth factor-alpha-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 41(5):562–572
Madala SK et al (2011) Rapamycin regulates bleomycin-induced lung damage in SP-C-deficient mice. Pulm Med 2011:653524
Park SJ et al (2012) L-2-oxothiazolidine-4-carboxylic acid or alpha-lipoic acid attenuates airway remodeling: involvement of nuclear factor-kappaB (NF-kappaB), nuclear factor erythroid 2p45-related factor-2 (Nrf2), and hypoxia-inducible factor (HIF). Int J Mol Sci 13(7):7915–7937
Lin CH et al (2014) Resveratrol enhanced FOXO3 phosphorylation via synergetic activation of SIRT1 and PI3K/Akt signaling to improve the effects of exercise in elderly rat hearts. Age (Dordr) 36(5):9705
Eo SH, Cho HS, Kim SJ (2014) Resveratrol regulates type II collagen and COX-2 expression via the ERK, p38 and Akt signaling pathways in rabbit articular chondrocytes. Exp Ther Med 7(3):640–648
Liu M, Liu F (2011) Resveratrol inhibits mTOR signaling by targeting DEPTOR. Commun Integr Biol 4(4):382–384
Harikumar KB, Aggarwal BB (2008) Resveratrol: a multitargeted agent for age-associated chronic diseases. Cell Cycle 7(8):1020–1035
Akgedik R et al (2012) Effect of resveratrol on treatment of bleomycin-induced pulmonary fibrosis in rats. Inflammation 35(5):1732–1741
Sener G et al (2007) Resveratrol alleviates bleomycin-induced lung injury in rats. Pulm Pharmacol Ther 20(6):642–649
Hu YX et al (2013) Resveratrol attenuates left ventricular remodeling in old rats with COPD induced by cigarette smoke exposure and LPS instillation. Can J Physiol Pharmacol 91(12):1044–1054
Li Y et al (2014) Resveratrol attenuates endoplasmic reticulum stress and alveolar epithelial apoptosis in a rat model of chronic obstructive pulmonary disease. Zhonghua Jie He He Hu Xi Za Zhi 37(1):30–35
Knobloch J et al (2010) Resveratrol impairs the release of steroid-resistant inflammatory cytokines from human airway smooth muscle cells in chronic obstructive pulmonary disease. J Pharmacol Exp Ther 335(3):788–798
Ito K, Colley T, Mercado N (2012) Geroprotectors as a novel therapeutic strategy for COPD, an accelerating aging disease. Int J Chron Obstruct Pulmon Dis 7:641–652
Benloucif S, Masana MI, Dubocovich ML (1997) Responsiveness to melatonin and its receptor expression in the aging circadian clock of mice. Am J Physiol 273(6 Pt 2):R1855–R1860
Shin IS et al (2014) Melatonin inhibits MUC5AC production via suppression of MAPK signaling in human airway epithelial cells. J Pineal Res 56(4):398–407
Shin IS et al (2015) Melatonin attenuates neutrophil inflammation and mucus secretion in cigarette smoke-induced chronic obstructive pulmonary diseases via the suppression of Erk-Sp1 signaling. J Pineal Res 58(1):50–60
Bouchecareilh M, Balch WE (2011) Proteostasis: a new therapeutic paradigm for pulmonary disease. Proc Am Thorac Soc 8(2):189–195
Menzies FM, Moreau K, Rubinsztein DC (2011) Protein misfolding disorders and macroautophagy. Curr Opin Cell Biol 23(2):190–197
Koga H, Kaushik S, Cuervo AM (2011) Protein homeostasis and aging: the importance of exquisite quality control. Ageing Res Rev 10(2):205–215
Korfei M et al (2011) Comparative proteomic analysis of lung tissue from patients with idiopathic pulmonary fibrosis (IPF) and lung transplant donor lungs. J Proteome Res 10(5):2185–2205
Tran I et al (2014) Role of cigarette smoke-induced aggresome-formation in COPD-emphysema pathogenesis. Am J Respir Cell Mol Biol 53(2):159–173
Chen ZH et al (2010) Autophagy protein microtubule-associated protein 1 light chain-3B (LC3B) activates extrinsic apoptosis during cigarette smoke-induced emphysema. Proc Natl Acad Sci U S A 107(44):18880–18885
Mizumura K et al (2014) Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD. J Clin Invest 124(9):3987–4003
Ryter SW et al (2012) Autophagy in pulmonary diseases. Annu Rev Physiol 74:377–401
Monick MM et al (2010) Identification of an autophagy defect in smokers’ alveolar macrophages. J Immunol 185(9):5425–5435
Ricci A et al (2013) Decreased expression of autophagic beclin 1 protein in idiopathic pulmonary fibrosis fibroblasts. J Cell Physiol 228(7):1516–1524
Singh KK et al (2015) The essential autophagy gene ATG7 modulates organ fibrosis via regulation of endothelial-to-mesenchymal transition. J Biol Chem 290(5):2547–59
Bernard M et al (2014) Autophagy fosters myofibroblast differentiation through MTORC2 activation and downstream upregulation of CTGF. Autophagy 10(12):2193–207
Hernandez-Gea V, Friedman SL (2012) Autophagy fuels tissue fibrogenesis. Autophagy 8(5):849–850
Del Principe D et al (2013) Fibroblast autophagy in fibrotic disorders. J Pathol 229(2):208–220
Hawkins A et al (2015) A non-BRICHOS SFTPC mutant (SP-CI73T) linked to interstitial lung disease promotes a late block in macroautophagy disrupting cellular proteostasis and mitophagy. Am J Physiol Lung Cell Mol Physiol 308(1):L33–L47
Lam HC et al (2013) Histone deacetylase 6-mediated selective autophagy regulates COPD-associated cilia dysfunction. J Clin Invest 123(12):5212–5230
Tang Z, Zhu M, Zhong Q (2014) Self-eating to remove cilia roadblock. Autophagy 10(2):379–381
Tang Z et al (2013) Autophagy promotes primary ciliogenesis by removing OFD1 from centriolar satellites. Nature 502(7470):254–257
Cloonan SM et al (2014) “Ciliophagy”: the consumption of cilia components by autophagy. Autophagy 10(3):532–534
Yang IV et al (2013) Expression of cilium-associated genes defines novel molecular subtypes of idiopathic pulmonary fibrosis. Thorax 68(12):1114–1121
Moshai EF et al (2014) Targeting the hedgehog-glioma-associated oncogene homolog pathway inhibits bleomycin-induced lung fibrosis in mice. Am J Respir Cell Mol Biol 51(1):11–25
Farout L, Friguet B (2006) Proteasome function in aging and oxidative stress: implications in protein maintenance failure. Antioxid Redox Signal 8(1–2):205–216
Jung T et al (2009) Age-related differences in oxidative protein-damage in young and senescent fibroblasts. Arch Biochem Biophys 483(1):127–135
Sitte N et al (2000) Proteasome inhibition by lipofuscin/ceroid during postmitotic aging of fibroblasts. FASEB J 14(11):1490–1498
Rock KL et al (1994) Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell 78(5):761–771
Torres C, Lewis L, Cristofalo VJ (2006) Proteasome inhibitors shorten replicative life span and induce a senescent-like phenotype of human fibroblasts. J Cell Physiol 207(3):845–853
Chondrogianni N, Gonos ES (2004) Proteasome inhibition induces a senescence-like phenotype in primary human fibroblasts cultures. Biogerontology 5(1):55–61
Breusing N et al (2009) Inverse correlation of protein oxidation and proteasome activity in liver and lung. Mech Ageing Dev 130(11–12):748–753
Weiss CH et al (2010) Proteasomal regulation of pulmonary fibrosis. Proc Am Thorac Soc 7(1):77–83
Baker TA et al (2014) Proteasomes in lungs from organ donors and patients with end-stage pulmonary diseases. Physiol Res 63(3):311–319
Min T et al (2011) Critical role of proteostasis-imbalance in pathogenesis of COPD and severe emphysema. J Mol Med (Berl) 89(6):577–593
Stepaniants S et al (2014) Genes related to emphysema are enriched for ubiquitination pathways. BMC Pulm Med 14:187
Meusser B et al (2005) ERAD: the long road to destruction. Nat Cell Biol 7(8):766–772
Korfei M et al (2008) Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 178(8):838–846
Lawson WE et al (2008) Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am J Physiol Lung Cell Mol Physiol 294(6):L1119–L1126
Roberson EC et al (2012) Influenza induces endoplasmic reticulum stress, caspase-12-dependent apoptosis, and c-Jun N-terminal kinase-mediated transforming growth factor-beta release in lung epithelial cells. Am J Respir Cell Mol Biol 46(5):573–581
Lenna S, Trojanowska M (2012) The role of endoplasmic reticulum stress and the unfolded protein response in fibrosis. Curr Opin Rheumatol 24(6):663–668
Baek HA et al (2012) Involvement of endoplasmic reticulum stress in myofibroblastic differentiation of lung fibroblasts. Am J Respir Cell Mol Biol 46(6):731–739
Bratic A, Larsson NG (2013) The role of mitochondria in aging. J Clin Invest 123(3):951–957
Ott M et al (2007) Mitochondria, oxidative stress and cell death. Apoptosis 12(5):913–922
Bondi CD et al (2010) NAD(P)H oxidase mediates TGF-beta1-induced activation of kidney myofibroblasts. J Am Soc Nephrol 21(1):93–102
Niture SK et al (2010) Nrf2 signaling and cell survival. Toxicol Appl Pharmacol 244(1):37–42
Hybertson BM et al (2011) Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol Aspects Med 32(4–6):234–246
Cho HY, Kleeberger SR (2007) Genetic mechanisms of susceptibility to oxidative lung injury in mice. Free Radic Biol Med 42(4):433–445
Cho HY, Reddy SP, Kleeberger SR (2006) Nrf2 defends the lung from oxidative stress. Antioxid Redox Signal 8(1–2):76–87
Malhotra D et al (2011) Denitrosylation of HDAC2 by targeting Nrf2 restores glucocorticosteroid sensitivity in macrophages from COPD patients. J Clin Invest 121(11):4289–4302
Kumar V et al (2011) Novel chalcone derivatives as potent Nrf2 activators in mice and human lung epithelial cells. J Med Chem 54(12):4147–4159
Soulitzis N et al (2012) Downregulation of lung mitochondrial prohibitin in COPD. Respir Med 106(7):954–961
Bueler H (2010) Mitochondrial dynamics, cell death and the pathogenesis of Parkinson’s disease. Apoptosis 15(11):1336–1353
Hara H et al (2013) Mitochondrial fragmentation in cigarette smoke-induced bronchial epithelial cell senescence. Am J Physiol Lung Cell Mol Physiol 305(10):L737–L746
Hoffmann RF et al (2013) Prolonged cigarette smoke exposure alters mitochondrial structure and function in airway epithelial cells. Respir Res 14:97
Aravamudan B et al (2014) Cigarette smoke-induced mitochondrial fragmentation and dysfunction in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 306(9):L840–L854
Wistuba II, Mao L, Gazdar AF (2002) Smoking molecular damage in bronchial epithelium. Oncogene 21(48):7298–7306
Aoshiba K et al (2012) DNA damage as a molecular link in the pathogenesis of COPD in smokers. Eur Respir J 39(6):1368–1376
Kuwano K et al (1996) P21Waf1/Cip1/Sdi1 and p53 expression in association with DNA strand breaks in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 154(2 Pt 1):477–483
Moyzis RK et al (1988) A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci U S A 85(18):6622–6626
Serra V et al (2003) Extracellular superoxide dismutase is a major antioxidant in human fibroblasts and slows telomere shortening. J Biol Chem 278(9):6824–6830
Tsuji T, Aoshiba K, Nagai A (2006) Alveolar cell senescence in patients with pulmonary emphysema. Am J Respir Crit Care Med 174(8):886–893
Kashima K et al (2006) Decrease of telomeres and increase of interstitial telomeric sites in chromosomes of short-term cultured gastric carcinoma cells detected by fluorescence in situ hybridization. Anticancer Res 26(4B):2849–2855
Houben JM et al (2009) Telomere shortening in chronic obstructive pulmonary disease. Respir Med 103(2):230–236
Armanios MY et al (2007) Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med 356(13):1317–1326
Tsakiri KD et al (2007) Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci U S A 104(18):7552–7557
Utz JP et al (2005) Usual interstitial pneumonia complicating dyskeratosis congenita. Mayo Clin Proc 80(6):817–821
Chilosi M, Poletti V, Rossi A (2012) The pathogenesis of COPD and IPF: distinct horns of the same devil? Respir Res 13:3
Liu T et al (2007) Telomerase activity is required for bleomycin-induced pulmonary fibrosis in mice. J Clin Invest 117(12):3800–3809
Degryse AL et al (2012) Telomerase deficiency does not alter bleomycin-induced fibrosis in mice. Exp Lung Res 38(3):124–134
Alder JK et al (2011) Telomere length is a determinant of emphysema susceptibility. Am J Respir Crit Care Med 184(8):904–912
Lee J et al (2012) The relationship between telomere length and mortality in chronic obstructive pulmonary disease (COPD). PLoS One 7(4):e35567
Savale L et al (2009) Shortened telomeres in circulating leukocytes of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 179(7):566–571
Muller KC et al (2006) Lung fibroblasts from patients with emphysema show markers of senescence in vitro. Respir Res 7:32
Nyunoya T et al (2006) Cigarette smoke induces cellular senescence. Am J Respir Cell Mol Biol 35(6):681–688
Tsuji T, Aoshiba K, Nagai A (2004) Cigarette smoke induces senescence in alveolar epithelial cells. Am J Respir Cell Mol Biol 31(6):643–649
Kanaji N et al (2014) Fibroblasts that resist cigarette smoke-induced senescence acquire profibrotic phenotypes. Am J Physiol Lung Cell Mol Physiol 307(5):L364–L373
Guzman L et al (2012) Analysis of aberrant methylation on promoter sequences of tumor suppressor genes and total DNA in sputum samples: a promising tool for early detection of COPD and lung cancer in smokers. Diagn Pathol 7:87
Vucic EA et al (2014) DNA methylation is globally disrupted and associated with expression changes in chronic obstructive pulmonary disease small airways. Am J Respir Cell Mol Biol 50(5):912–922
Lange NE et al (2012) Alu and LINE-1 methylation and lung function in the normative ageing study. BMJ Open 2(5)
Boutten A et al (2011) NRF2 targeting: a promising therapeutic strategy in chronic obstructive pulmonary disease. Trends Mol Med 17(7):363–371
Bozinovski S et al (2006) Akt in the pathogenesis of COPD. Int J Chron Obstruct Pulmon Dis 1(1):31–38
Huang SK et al (2010) Hypermethylation of PTGER2 confers prostaglandin E2 resistance in fibrotic fibroblasts from humans and mice. Am J Pathol 177(5):2245–2255
Coward WR et al (2014) A central role for G9a and EZH2 in the epigenetic silencing of cyclooxygenase-2 in idiopathic pulmonary fibrosis. FASEB J 28(7):3183–3196
Sanders YY et al (2008) Thy-1 promoter hypermethylation: a novel epigenetic pathogenic mechanism in pulmonary fibrosis. Am J Respir Cell Mol Biol 39(5):610–618
Robinson CM et al (2012) Hypoxia-induced DNA hypermethylation in human pulmonary fibroblasts is associated with Thy-1 promoter methylation and the development of a pro-fibrotic phenotype. Respir Res 13:74
Dakhlallah D et al (2013) Epigenetic regulation of miR-17 ~ 92 contributes to the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med 187(4):397–405
Gonzalo S (2010) Epigenetic alterations in aging. J Appl Physiol (1985) 109(2):586–97
Pruitt K et al (2006) Inhibition of SIRT1 reactivates silenced cancer genes without loss of promoter DNA hypermethylation. PLoS Genet 2(3):e40
Vaquero A et al (2007) SIRT1 regulates the histone methyl-transferase SUV39H1 during heterochromatin formation. Nature 450(7168):440–444
Fraga MF, Esteller M (2007) Epigenetics and aging: the targets and the marks. Trends Genet 23(8):413–418
Sarg B et al (2002) Postsynthetic trimethylation of histone H4 at lysine 20 in mammalian tissues is associated with aging. J Biol Chem 277(42):39195–39201
Jung JW et al (2010) Histone deacetylase controls adult stem cell aging by balancing the expression of polycomb genes and jumonji domain containing 3. Cell Mol Life Sci 67(7):1165–1176
Cencioni C et al (2013) Oxidative stress and epigenetic regulation in ageing and age-related diseases. Int J Mol Sci 14(9):17643–17663
Szulakowski P et al (2006) The effect of smoking on the transcriptional regulation of lung inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 174(1):41–50
Adenuga D et al (2009) Histone deacetylase 2 is phosphorylated, ubiquitinated, and degraded by cigarette smoke. Am J Respir Cell Mol Biol 40(4):464–473
Yao H, Rahman I (2012) Role of histone deacetylase 2 in epigenetics and cellular senescence: implications in lung inflammaging and COPD. Am J Physiol Lung Cell Mol Physiol 303(7):L557–L566
Raidl M et al (2007) Impaired TNFalpha-induced VEGF expression in human airway smooth muscle cells from smokers with COPD: role of MAPkinases and histone acetylation – effect of dexamethasone. Cell Biochem Biophys 49(2):98–110
Rajendrasozhan S et al (2008) Deacetylases and NF-kappaB in redox regulation of cigarette smoke-induced lung inflammation: epigenetics in pathogenesis of COPD. Antioxid Redox Signal 10(4):799–811
Brunet A et al (2004) Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303(5666):2011–2015
Mahavadi P et al (2014) Altered surfactant homeostasis and alveolar epithelial cell stress in amiodarone-induced lung fibrosis. Toxicol Sci 142(1):285–297
Guo W et al (2009) Abrogation of TGF-beta1-induced fibroblast-myofibroblast differentiation by histone deacetylase inhibition. Am J Physiol Lung Cell Mol Physiol 297(5):L864–L870
Huang SK et al (2013) Histone modifications are responsible for decreased Fas expression and apoptosis resistance in fibrotic lung fibroblasts. Cell Death Dis 4:e621
Bonner JC et al (2002) Susceptibility of cyclooxygenase-2-deficient mice to pulmonary fibrogenesis. Am J Pathol 161(2):459–470
Keerthisingam CB et al (2001) Cyclooxygenase-2 deficiency results in a loss of the anti-proliferative response to transforming growth factor-beta in human fibrotic lung fibroblasts and promotes bleomycin-induced pulmonary fibrosis in mice. Am J Pathol 158(4):1411–1422
Coward WR et al (2009) Defective histone acetylation is responsible for the diminished expression of cyclooxygenase 2 in idiopathic pulmonary fibrosis. Mol Cell Biol 29(15):4325–4339
Sanders YY et al (2014) Histone deacetylase inhibition promotes fibroblast apoptosis and ameliorates pulmonary fibrosis in mice. Eur Respir J 43(5):1448–1458
Williams AE et al (2007) microRNA expression in the aging mouse lung. BMC Genomics 8:172
Gross TJ et al (2014) A microRNA processing defect in smokers’ macrophages is linked to SUMOylation of the endonuclease DICER. J Biol Chem 289(18):12823–12834
Molina-Pinelo S et al (2014) MicroRNA clusters: dysregulation in lung adenocarcinoma and COPD. Eur Respir J 43(6):1740–1749
Chatila WM et al (2014) Blunted expression of miR-199a-5p in regulatory T cells of patients with chronic obstructive pulmonary disease compared to unaffected smokers. Clin Exp Immunol 177(1):341–352
Soeda S et al (2013) Clinical relevance of plasma miR-106b levels in patients with chronic obstructive pulmonary disease. Int J Mol Med 31(3):533–539
Ezzie ME et al (2012) Gene expression networks in COPD: microRNA and mRNA regulation. Thorax 67(2):122–131
Hassan F et al (2012) MiR-101 and miR-144 regulate the expression of the CFTR chloride channel in the lung. PLoS One 7(11):e50837
Christenson SA et al (2013) miR-638 regulates gene expression networks associated with emphysematous lung destruction. Genome Med 5(12):114
Oak SR et al (2011) A micro RNA processing defect in rapidly progressing idiopathic pulmonary fibrosis. PLoS One 6(6):e21253
Milosevic J et al (2012) Profibrotic role of miR-154 in pulmonary fibrosis. Am J Respir Cell Mol Biol 47(6):879–887
Liu G et al (2010) miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med 207(8):1589–1597
Das S et al (2014) MicroRNA-326 regulates profibrotic functions of transforming growth factor-beta in pulmonary fibrosis. Am J Respir Cell Mol Biol 50(5):882–892
Lino Cardenas CL et al (2013) miR-199a-5p Is upregulated during fibrogenic response to tissue injury and mediates TGFbeta-induced lung fibroblast activation by targeting caveolin-1. PLoS Genet 9(2):e1003291
Pandit KV, Milosevic J, Kaminski N (2011) MicroRNAs in idiopathic pulmonary fibrosis. Transl Res 157(4):191–199
Comer BS et al (2015) Epigenetic targets for novel therapies of lung diseases. Pharmacol Ther 147:91–110
Yang S et al (2012) miR-21 regulates chronic hypoxia-induced pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol 302(6):L521–L529
Wang Y et al (2013) miR-375 regulates rat alveolar epithelial cell trans-differentiation by inhibiting Wnt/beta-catenin pathway. Nucleic Acids Res 41(6):3833–3844
Kneidinger N et al (2011) Activation of the WNT/beta-catenin pathway attenuates experimental emphysema. Am J Respir Crit Care Med 183(6):723–733
Hacisuleyman E et al (2014) Topological organization of multichromosomal regions by the long intergenic noncoding RNA Firre. Nat Struct Mol Biol 21(2):198–206
Gong C, Maquat LE (2011) “Alu” strious long ncRNAs and their role in shortening mRNA half-lives. Cell Cycle 10(12):1882–1883
Natoli G, Andrau JC (2012) Noncoding transcription at enhancers: general principles and functional models. Annu Rev Genet 46:1–19
Cao G et al (2013) Differential expression of long non-coding RNAs in bleomycin-induced lung fibrosis. Int J Mol Med 32(2):355–364
Deng N et al (2013) Detecting splicing variants in idiopathic pulmonary fibrosis from non-differentially expressed genes. PLoS One 8(7):e68352
Pilling LC et al (2012) Genomics and successful aging: grounds for renewed optimism? J Gerontol A Biol Sci Med Sci 67(5):511–519
White ES et al (2010) Control of fibroblast fibronectin expression and alternative splicing via the PI3K/Akt/mTOR pathway. Exp Cell Res 316(16):2644–2653
Muro AF et al (2008) An essential role for fibronectin extra type III domain A in pulmonary fibrosis. Am J Respir Crit Care Med 177(6):638–645
Nance T et al (2014) Transcriptome analysis reveals differential splicing events in IPF lung tissue. PLoS One 9(5):e97550
Ito K, Mercado N (2014) STOP accelerating lung aging for the treatment of COPD. Exp Gerontol 59:21–27
Bruunsgaard H et al (2003) Elevated levels of tumor necrosis factor alpha and mortality in centenarians. Am J Med 115(4):278–283
Bruunsgaard H, Pedersen BK (2003) Age-related inflammatory cytokines and disease. Immunol Allergy Clin North Am 23(1):15–39
Gomez CR et al (2008) Innate immunity and aging. Exp Gerontol 43(8):718–728
Brandenberger C et al (2014) Enhanced allergic airway disease in old mice is associated with a Th17 response. Clin Exp Allergy 44(10):1282–1292
Nyenhuis SM et al (2010) Airway neutrophil inflammatory phenotype in older subjects with asthma. J Allergy Clin Immunol 125(5):1163–1165
Wilson JW (2005) Inflammation and remodelling in the ageing airway. Med J Aust 183(1 Suppl):S33–S34
Soler Artigas M et al (2011) Genome-wide association and large-scale follow up identifies 16 new loci influencing lung function. Nat Genet 43(11):1082–1090
Chung HY et al (2009) Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev 8(1):18–30
Kovacs EJ et al (2009) Aging and innate immunity in the mouse: impact of intrinsic and extrinsic factors. Trends Immunol 30(7):319–324
Franceschi C et al (2000) Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 908:244–254
Park GY, Christman JW (2006) Involvement of cyclooxygenase-2 and prostaglandins in the molecular pathogenesis of inflammatory lung diseases. Am J Physiol Lung Cell Mol Physiol 290(5):L797–L805
Canan CH et al (2014) Characterization of lung inflammation and its impact on macrophage function in aging. J Leukoc Biol 96(3):473–480
Ren X et al (2014) Age-related activation of MKK/p38/NF-kappaB signaling pathway in lung: from mouse to human. Exp Gerontol 57:29–40
Chilosi M et al (2013) Premature lung aging and cellular senescence in the pathogenesis of idiopathic pulmonary fibrosis and COPD/emphysema. Transl Res 162(3):156–173
MacNee W (2009) Accelerated lung aging: a novel pathogenic mechanism of chronic obstructive pulmonary disease (COPD). Biochem Soc Trans 37(Pt 4):819–823
Tuder RM, Yun JH, Graham BB (2008) Cigarette smoke triggers code red: p21CIP1/WAF1/SDI1 switches on danger responses in the lung. Am J Respir Cell Mol Biol 39(1):1–6
Provinciali M, Cardelli M, Marchegiani F (2011) Inflammation, chronic obstructive pulmonary disease and aging. Curr Opin Pulm Med 17(Suppl 1):S3–S10
Weiskopf D, Weinberger B, Grubeck-Loebenstein B (2009) The aging of the immune system. Transpl Int 22(11):1041–1050
Vandivier RW, Henson PM, Douglas IS (2006) Burying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest 129(6):1673–1682
Pisetsky DS, Fairhurst AM (2007) The origin of extracellular DNA during the clearance of dead and dying cells. Autoimmunity 40(4):281–284
Mahoney JA, Rosen A (2005) Apoptosis and autoimmunity. Curr Opin Immunol 17(6):583–588
Hogaboam CM, Trujillo G, Martinez FJ (2012) Aberrant innate immune sensing leads to the rapid progression of idiopathic pulmonary fibrosis. Fibrogenesis Tissue Repair 5(Suppl 1 (Proceedings of fibroproliferative disorders: from biochemical analysis to targeted therapies Petro E. Petrides and David Brenner)):S3
Trujillo G et al (2010) TLR9 differentiates rapidly from slowly progressing forms of idiopathic pulmonary fibrosis. Sci Transl Med 2(57):57ra82
Gilani SR et al (2010) CD28 down-regulation on circulating CD4 T-cells is associated with poor prognoses of patients with idiopathic pulmonary fibrosis. PLoS One 5(1):e8959
Sime PJ et al (1997) Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest 100(4):768–776
Mosser DM, Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8(12):958–969
Baran CP et al (2007) Important roles for macrophage colony-stimulating factor, CC chemokine ligand 2, and mononuclear phagocytes in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med 176(1):78–89
Prasse A et al (2006) A vicious circle of alveolar macrophages and fibroblasts perpetuates pulmonary fibrosis via CCL18. Am J Respir Crit Care Med 173(7):781–792
Stahl M et al (2013) Lung collagens perpetuate pulmonary fibrosis via CD204 and M2 macrophage activation. PLoS One 8(11), e81382
Murray LA et al (2011) TGF-beta driven lung fibrosis is macrophage dependent and blocked by Serum amyloid P. Int J Biochem Cell Biol 43(1):154–162
Kiemle-Kallee J et al (1991) Alveolar macrophages in idiopathic pulmonary fibrosis display a more monocyte-like immunophenotype and an increased release of free oxygen radicals. Eur Respir J 4(4):400–406
Prasse A et al (2009) Serum CC-chemokine ligand 18 concentration predicts outcome in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 179(8):717–723
Tiev KP et al (2011) Serum CC chemokine ligand-18 predicts lung disease worsening in systemic sclerosis. Eur Respir J 38(6):1355–1360
Xiao L et al (2003) Evaluation of interleukin-13 in the serum and bronchoalveolar lavage fluid of patients with idiopathic pulmonary fibrosis. Zhonghua Jie He He Hu Xi Za Zhi 26(11):686–688
Sharma G, Hanania NA, Shim YM (2009) The aging immune system and its relationship to the development of chronic obstructive pulmonary disease. Proc Am Thorac Soc 6(7):573–580
Gorska MM et al (2010) Uncoordinated 119 preferentially induces Th2 differentiation and promotes the development of asthma. J Immunol 184(8):4488–4496
Zheng T et al (2000) Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysema. J Clin Invest 106(9):1081–1093
Wang Z et al (2000) Interferon gamma induction of pulmonary emphysema in the adult murine lung. J Exp Med 192(11):1587–1600
Hoshino T et al (2007) Pulmonary inflammation and emphysema: role of the cytokines IL-18 and IL-13. Am J Respir Crit Care Med 176(1):49–62
Maeno T et al (2007) CD8+ T cells are required for inflammation and destruction in cigarette smoke-induced emphysema in mice. J Immunol 178(12):8090–8096
Lambers C et al (2009) T cell senescence and contraction of T cell repertoire diversity in patients with chronic obstructive pulmonary disease. Clin Exp Immunol 155(3):466–475
Grumelli S et al (2004) An immune basis for lung parenchymal destruction in chronic obstructive pulmonary disease and emphysema. PLoS Med 1(1):e8
Di Stefano A et al (2009) T helper type 17-related cytokine expression is increased in the bronchial mucosa of stable chronic obstructive pulmonary disease patients. Clin Exp Immunol 157(2):316–324
Doe C et al (2010) Expression of the T helper 17-associated cytokines IL-17A and IL-17 F in asthma and COPD. Chest 138(5):1140–1147
Weyand CM et al (1998) Functional properties of CD4+ CD28- T cells in the aging immune system. Mech Ageing Dev 102(2–3):131–147
Zhang C et al (2014) Hypomethylation of perforin regulatory elements in CD4+ T cells from rat spleens contributes to the development of autoimmune emphysema. Respirology 19(3):376–381
Sullivan AK et al (2006) Activated oligoclonal CD4+ T cells in the lungs of patients with severe emphysema. Proc Am Thorac Soc 3(6):486
Romero V, Andrade F (2008) Non-apoptotic functions of granzymes. Tissue Antigens 71(5):409–416
Ngan DA et al (2009) The possible role of granzyme B in the pathogenesis of chronic obstructive pulmonary disease. Ther Adv Respir Dis 3(3):113–129
Alkhouri H et al (2014) Regulation of pulmonary inflammation by mesenchymal cells. Pulm Pharmacol Ther 29(2):156–165
Lumsden AB, McLean A, Lamb D (1984) Goblet and Clara cells of human distal airways: evidence for smoking induced changes in their numbers. Thorax 39(11):844–849
Araya J et al (2007) Squamous metaplasia amplifies pathologic epithelial-mesenchymal interactions in COPD patients. J Clin Invest 117(11):3551–3562
Gosselink JV et al (2010) Differential expression of tissue repair genes in the pathogenesis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 181(12):1329–1335
Zhang J et al (2012) Pro-inflammatory phenotype of COPD fibroblasts not compatible with repair in COPD lung. J Cell Mol Med 16(7):1522–1532
Larsson-Callerfelt AK et al (2013) Defective alterations in the collagen network to prostacyclin in COPD lung fibroblasts. Respir Res 14:21
Kitamura H et al (2011) Mouse and human lung fibroblasts regulate dendritic cell trafficking, airway inflammation, and fibrosis through integrin alphavbeta8-mediated activation of TGF-beta. J Clin Invest 121(7):2863–2875
Lindahl GE et al (2013) Microarray profiling reveals suppressed interferon stimulated gene program in fibroblasts from scleroderma-associated interstitial lung disease. Respir Res 14:80
Nagahama KY et al (2013) Oncostatin M modulates fibroblast function via signal transducers and activators of transcription proteins-3. Am J Respir Cell Mol Biol 49(4):582–591
Botelho FM et al (2013) Pulmonary expression of oncostatin M (OSM) promotes inducible BALT formation independently of IL-6, despite a role for IL-6 in OSM-driven pulmonary inflammation. J Immunol 191(3):1453–1464
van der Kraan PM et al (2012) Age-dependent alteration of TGF-beta signalling in osteoarthritis. Cell Tissue Res 347(1):257–265
Doyle KP et al (2010) TGFbeta signaling in the brain increases with aging and signals to astrocytes and innate immune cells in the weeks after stroke. J Neuroinflammation 7:62
Biernacka A, Frangogiannis NG (2011) Aging and cardiac fibrosis. Aging Dis 2(2):158–173
Morty RE, Konigshoff M, Eickelberg O (2009) Transforming growth factor-beta signaling across ages: from distorted lung development to chronic obstructive pulmonary disease. Proc Am Thorac Soc 6(7):607–613
Bonniaud P et al (2004) Smad3 null mice develop airspace enlargement and are resistant to TGF-beta-mediated pulmonary fibrosis. J Immunol 173(3):2099–2108
Lopez-Otin C et al (2013) The hallmarks of aging. Cell 153(6):1194–1217
Heintz C, Mair W (2014) You are what you host: microbiome modulation of the aging process. Cell 156(3):408–411
Tsujino K et al (2012) Tetraspanin CD151 protects against pulmonary fibrosis by maintaining epithelial integrity. Am J Respir Crit Care Med 186(2):170–180
Zhao ZJ, Liu XM, Zhou GP (2008) Changes of matrix metalloproteinase 2,9 and tissue inhibitor of metalloproteinase 1, 2, 3 expression level in aged rat lung. Beijing Da Xue Xue Bao 40(1):101–104
Wagner DE et al (2014) Comparative decellularization and recellularization of normal versus emphysematous human lungs. Biomaterials 35(10):3281–3297
Booth AJ et al (2012) Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med 186(9):866–876
McAloon CJ et al (2009) Matrix metalloprotease polymorphisms are associated with gas transfer in alpha 1 antitrypsin deficiency. Ther Adv Respir Dis 3(1):23–30
Elkington PT, Friedland JS (2006) Matrix metalloproteinases in destructive pulmonary pathology. Thorax 61(3):259–266
Dancer RC, Wood AM, Thickett DR (2011) Metalloproteinases in idiopathic pulmonary fibrosis. Eur Respir J 38(6):1461–1467
Suga M et al (2000) Characteristic elevation of matrix metalloproteinase activity in idiopathic interstitial pneumonias. Am J Respir Crit Care Med 162(5):1949–1956
Gooptu B, Ekeowa UI, Lomas DA (2009) Mechanisms of emphysema in alpha1-antitrypsin deficiency: molecular and cellular insights. Eur Respir J 34(2):475–488
Pardo A, Selman M (2012) Role of matrix metaloproteases in idiopathic pulmonary fibrosis. Fibrogenesis Tissue Repair 5(Suppl 1 (Proceedings of fibroproliferative disorders: from biochemical analysis to targeted therapies Petro E Petrides and David Brenner)):S9
Vegeto E et al (2010) Estrogen receptor-alpha as a drug target candidate for preventing lung inflammation. Endocrinology 151(1):174–184
Corbo GM et al (2014) Serum level of testosterone, dihydrotestosterone and IGF-1 during an acute exacerbation of COPD and their relationships with inflammatory and prognostic indices: a pilot study. Minerva Med 105(4):289–294
Debigare R et al (2003) Catabolic/anabolic balance and muscle wasting in patients with COPD. Chest 124(1):83–89
Balasubramanian V, Naing S (2012) Hypogonadism in chronic obstructive pulmonary disease: incidence and effects. Curr Opin Pulm Med 18(2):112–117
Pan L et al (2014) Effects of anabolic steroids on chronic obstructive pulmonary disease: a meta-analysis of randomised controlled trials. PLoS One 9(1):e84855
Podzolkov VI et al (2012) Comparative characteristic of the hormonal profile in men with stable obstructive pulmonary disease and smokers. Klin Med (Mosk) 90(6):26–30
Mendoza-Milla C et al (2013) Dehydroepiandrosterone has strong antifibrotic effects and is decreased in idiopathic pulmonary fibrosis. Eur Respir J 42(5):1309–1321
Gumral N et al (2009) Antioxidant enzymes and melatonin levels in patients with bronchial asthma and chronic obstructive pulmonary disease during stable and exacerbation periods. Cell Biochem Funct 27(5):276–283
Unlu M et al (2006) Effects of melatonin on the oxidant/antioxidant status and lung histopathology in rabbits exposed to cigarette smoke. Respirology 11(4):422–428
Zhao H et al (2014) Melatonin inhibits endoplasmic reticulum stress and epithelial-mesenchymal transition during bleomycin-induced pulmonary fibrosis in mice. PLoS One 9(5):e97266
Kajstura J et al (2011) Evidence for human lung stem cells. N Engl J Med 364(19):1795–1806
Gong X et al (2014) Isolation and characterization of lung resident mesenchymal stem cells capable of differentiating into alveolar epithelial type II cells. Cell Biol Int 38(4):405–411
Lama VN et al (2007) Evidence for tissue-resident mesenchymal stem cells in human adult lung from studies of transplanted allografts. J Clin Invest 117(4):989–996
Majka SM et al (2005) Identification of novel resident pulmonary stem cells: form and function of the lung side population. Stem Cells 23(8):1073–1081
Butler JP et al (2012) Evidence for adult lung growth in humans. N Engl J Med 367(3):244–247
Blyszczuk P et al (2011) Profibrotic potential of prominin-1+ epithelial progenitor cells in pulmonary fibrosis. Respir Res 12:126
Murphy S et al (2011) Human amnion epithelial cells prevent bleomycin-induced lung injury and preserve lung function. Cell Transplant 20(6):909–923
Zhen G et al (2008) Mesenchymal stem cells transplantation protects against rat pulmonary emphysema. Front Biosci 13:3415–3422
Ortiz LA et al (2003) Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A 100(14):8407–8411
Guan XJ et al (2013) Mesenchymal stem cells protect cigarette smoke-damaged lung and pulmonary function partly via VEGF-VEGF receptors. J Cell Biochem 114(2):323–335
McQualter JL et al (2014) Harnessing the potential of lung stem cells for regenerative medicine. Int J Biochem Cell Biol 56:82–91
Ruiz EJ, Oeztuerk-Winder F, Ventura JJ (2014) A paracrine network regulates the cross-talk between human lung stem cells and the stroma. Nat Commun 5:3175
Bonner JC (2010) Mesenchymal cell survival in airway and interstitial pulmonary fibrosis. Fibrogenesis Tissue Repair 3:15
Pierro M, Thebaud B (2010) Mesenchymal stem cells in chronic lung disease: culprit or savior? Am J Physiol Lung Cell Mol Physiol 298(6):L732–L734
Yan X et al (2007) Injured microenvironment directly guides the differentiation of engrafted Flk-1(+) mesenchymal stem cell in lung. Exp Hematol 35(9):1466–1475
Walker N et al (2011) Resident tissue-specific mesenchymal progenitor cells contribute to fibrogenesis in human lung allografts. Am J Pathol 178(6):2461–2469
Todd JL, Palmer SM (2011) Bronchiolitis obliterans syndrome: the final frontier for lung transplantation. Chest 140(2):502–508
Acknowledgments
Dr. Sanchez work was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number P20GM103629-04 and the American Thoracic Society and Scleroderma Foundation award 552114G1.
Editor: Anthony Punturieri, National Heart, Lung and Blood Institute (NHLBI), NIH.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing
About this chapter
Cite this chapter
Sanchez, C.G. (2016). Aging in COPD and Idiopathic Pulmonary Fibrosis. In: Sierra, F., Kohanski, R. (eds) Advances in Geroscience. Springer, Cham. https://doi.org/10.1007/978-3-319-23246-1_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-23246-1_15
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-23245-4
Online ISBN: 978-3-319-23246-1
eBook Packages: MedicineMedicine (R0)