Abstract
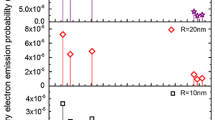
The effective range, maximum deflection angle, interaction parameters, and energy deposition of secondary electrons emitted from a gold nanoparticle irradiated by photon beams were calculated by means of Monte Carlo simulations. Moreover, the low-energy electrons (LEEs) with energy range of 3–20 eV, produced when a gold nanoparticle is irradiated by photon beams, were studied. The author’s group used the Geant4-based computer code was used to simulate and track secondary electrons from a 100 nm diameter gold nanoparticle irradiated at monoenergetic photon beams of 35, 73.3, 660, and 1,200 keV and from 2, 50, and 100 nm diameter gold nanoparticles irradiated at polyenergetic photon beams of 50 kVp, 250 kVp, 1.25 MV, and 6 MV in water. To investigate the LEEs, secondary electrons emitted from a gold nanoparticle with diameter of 100 nm, interacting with photon beams with energies equal to 35, 73.3, and 600 keV, were simulated using the Geant4 code. The phase spaces of the secondary electrons produced by simulations were then used to simulate the LEEs in water using the NOREC Monte Carlo code. All secondary electrons emitted by the gold nanoparticle and all LEEs produced by each secondary electron were tracked in simulations. The author’s group compared the number of secondary electron emitted with and without the gold nanoparticle in water and found that the presence of gold produces more electrons, when irradiated by monoenergetic photon beams with particularly low energy (35 keV). As the photon beam energy was increased from 35 to 1,200 keV, the effective electron range increased from 24.7 to 5,060 μm, but the total number of emitted photoelectric electrons decreased by a factor of 270 per interacting photon. The maximum electron deflection angle relative to the incident beam decreased from 83.1° to 39.2°, and the stopping power of the emitted electron decreased from 1.42 to 0.24 keV/μm. For polyenergetic photon beams with 2, 50, and 100 nm diameter nanoparticles, the author’s group found that both irradiations of the 50 and 250 kVp photon beam caused significantly greater deposited energy surrounding the gold nanoparticle (three orders of magnitude) than the MV beams (approximately five times). The author’s group also found that a larger portion of deposited energy resided within a larger nanoparticle under the photon irradiation. From the LEE results, the author’s group found that the energy distributions of the LEEs from the gold nanoparticle do not vary significantly between different photon beam energies. In addition, the 660 keV photon beam produced more LEEs traveling to a longer range than photon beams of lower energies (35 and 73.3 keV). This higher energy deposition and longer range LEEs produced by the 660 keV photon beam can enhance the cell kill. These simulated results yield important insights concerning the enhancement of tumor cell killing in gold nanoparticle-enhanced radiotherapy. The aim of this chapter is to show that the irradiation of gold nanoparticles at lower monoenergetic and polyenergetic photon energies in the kV range will be more efficient for cell killing. This conclusion is consistent with published studies.
Similar content being viewed by others
References
S.J. McMahon, W.B. Hyland, M.F. Muir, J.A. Coulter, S. Jain, K.T. Butterworth, G. Schettino, G.R. Dickson, A.R. Hounsell, J.M. O’Sullivan, K.M. Prise, D.G. Hirst, F.J. Currell, Biological consequences of nanoscale energy deposition near irradiated heavy atom nanoparticles. Sci Rep (2011). doi:10.1038/srep00018
B.D. Chithrani, S. Jelveh, F. Jalali, M. van Prooijen, C. Allen, R.P. Hill, R. Bristow, D.A. Jaffray, Gold nanoparticles as a radiation sensitizer in cancer therapy. Radiat. Res. 173, 719–728 (2010)
S. Jain, D.G. Hirst, J.M. O’Sulivan, Gold nanoparticles as novel agents for cancer therapy. Br. J. Radiol. 85, 101–113 (2012)
A. Mesbahi, A review on gold nanoparticles radiosensitization effect in radiation therapy of cancer. Rep. Pract. Oncol. Radiother. 15, 176–180 (2010)
B. Jeremic, A.R. Aguerri, N. Filipovic, Radiosensitization by gold nanoparticles. Clin. Transl. Oncol. 15, 593–601 (2013)
R.I. Berbeco, W. Nqwa, G.M. Makigiorgos, Localized dose enhancement to tumour blood vessel endothelial cells via megavoltage x-rays and targeted gold nanoparticles : new potential for external beam radiotherapy. Int. J. Radiat. Oncol. Bio. Phys. 81, 270–276 (2011)
J.F. Hainfeld, D.N. Slatkin, H.M. Smilowitz, The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 49, N309–N315 (2004)
J.F. Hainfeld, F.A. Dilmanian, Z. Zhong, D.N. Slatkin, J.A. Kalef-Ezra, H.M. Smilowitz, Gold nanoparticles enhance the radiation therapy of a murine squamous cell carcinoma. Phys. Med. Biol. 55, 3045–3059 (2010)
J.C. Roeske, L. Nunez, M. Hoggarth, E. Labay, R.R. Wicheselbaum, Characterization of the theoretical radiation dose enhancement from nanoparticles. Technol. Cancer Res. Treat. 6, 395 (2007)
S.H. Cho, Estimation of tumour dose enhancement due to gold nanoparticles during typical radiation treatments: a preliminary Monte Carlo study. Phys. Med. Biol. 50, N163–N173 (2005)
S.X. Zhang, J. Gao, T.A. Buchholz, Z. Wang, M.R. Salehpour, R.A. Drezek, T.K. Yu, Quantifying tumor-selective radiation dose enhancements using gold nanoparticles: a monte carlo simulation study. Biomed. Microdevices (2009). doi:10.1007/s10544-009-9309-5
B.L. Jones, S. Krishnan, S.H. Cho, Estimation of microscopic dose enhancement factor around gold nanoparticles by Monte Carlo calculation. Med. Phys. 37, 3809–3816 (2010)
M.K.K. Leung, J.C.L. Chow, B.D. Chithrani, M.J.G. Lee, B. Oms, D.A. Jaffray, Irradiation of gold nanoparticles by x-rays: Monte Carlo simulation of dose enhancements and the spatial properties of the secondary electrons production. Med. Phys. 38, 624–631 (2011)
J.C.L. Chow, M.K.K. Leung, D. Chithrani, M. Lee, B. Oms, D.A. Jaffray, Dosimetry on gold nanoparticle: a macroscopic and macroscopic study using Monte Carlo simulations. Med. Phys. 36, 2512 (2009)
J.C.L. Chow, M.K.K. Leung, D.A. Jaffray, Monte Carlo simulation on gold nanoparticle irradiated by electron beams. Phys. Med. Biol. 57, 3323–3331 (2012)
M.K.K. Leung, J.C.L. Chow, D. Chithrani, M. Lee, B. Oms, D. Jaffray, Characterization of the spatial and energy distribution of electrons emitted from a gold nanoparticle irradiated by x-rays using Monte Carlo simulations. Med. Phys. 36, 2819 (2009)
Q.B. Lu, Electron transfer reaction mechanism of cisplatin with DNA at the molecular level. Mol. Pharm. 4, 624 (2007)
B.D. Chithrani, J. Stewart, C. Allen, D.A. Jaffray, Intracellular uptake, transport, and processing of nanostructures in cancer cells. Nanomedicine 5, 118–127 (2009)
Y. Chen, A. Aleksandrov, T.M. Orlando, Probing low-energy electron induced DNA damage using single photon ionization mass spectrometry. Int. J. Mass Spectrum. 277, 314–320 (2008)
Z. Li et al., Low energy electron induced DNA damage: effects of terminal phosphate and base moieties on the distribution of damage. J. Am. Chem. Soc. 130, 5612–5613 (2008)
Y. Zheng, J.R. Wagner, L. Sanche, DNA damage induced by low-energy electrons: electron transfer and diffraction. Phys. Rev. Lett. 96, 208101 (2006)
Z. Li, C. Pierre, L. Sanche, J.R. Wagner, Low-energy electron-induced DNA damage: effect of base sequence in oligonucleotide trimmers. J. Am. Chem. Soc. 132, 5422–5427 (2010)
S. Agostinelli et al., Geant4 – a simulation toolkit. Nucl. Instrum. Methods Phys. Res. A 506, 250–303 (2003)
J. Spiga, E.A. Siegbahn, E. Bräuer-Krisch, P. Randaccio, A. Bravin, The GEANT4 toolkit for microdosimetry calculations: application to microbeam radiation therapy (MRT). Med. Phys. 34, 4322–4330 (2007)
V.A. Semenenko, J.E. Turner, T.B. Borak, NOREC, a Monte Carlo code for simulating electrons tracks in liquid water. Radiat. Environ. Biophys. 42, 213 (2003)
J.E. Turner, R.N. Hamm, M.L. Souleyrette, D.E. Martz, T.A. Rhea, D.W. Schmidt, Calculations for beta dosimetry using Monte Carlo code (OREC) for electron transport in water. Health Phys. 55, 741 (1988)
F.H. Attix, Introduction to radiological physics and radiation dosimetry (Wiley-VCH Verlag GmbH & Co. KgaA, Weinheim, 2004)
R. Kulkarmi, B. Sramek, A. Lisle, E. Boote, C. Caldwell, K. Katti, Enhancement of cell killing by x-ray irradiation in the presence of gold nanoparticles. Med. Phys. 34, 2602 (2007)
D.M. Herold, I.J. Das, C.C. Stobbe, R.V. Iyer, J.D. Chapman, Gold microspheres: a selective technique for producing biologically effective dose enhancement. Int. J. Radiat. Biol. 76, 1357–1364 (2000)
M.K.K. Leung, J.C.L. Chow, D. Chithrani, M. Lee, D. Jaffray, Comparison of the physical characteristics of secondary electrons and dose enhancement from x-ray irradiation of gold nanoparticles using Monte Carlo simulation. Med. Phys. 38, 3643 (2011)
K. Song, P. Xu, Y. Meng, F. Geng, J. Li, Z. Li, J. Xing, J. Chen, B. Kong, Smart gold nanoparticles enhance killing effect on cancer cells. Int. J. Oncol. 42, 597–608 (2013)
D.K. Kirui, I. Khalidov, Y. Wang, C.A. Batt, Targeted near-IR hybrid magnetic nanoparticles for in vivo cancer therapy and imaging. Nanomed. Nanotechnol. Biol. Med. 9, 702–711 (2013)
D. Lapotko, Therapy with gold nanoparticles and lasers: what really kills the cells. Nanomedicine 4, 253–256 (2009)
X. Liu, M.C. Lloyd, I.V. Fedorenko, P. Bapat, T. Zhukov, Q. Huo, Enhanced imaging and accelerated photothermalysis of A549 human lung cancer cells by gold nanoparticles. Nanomedicine 3, 617–626 (2008)
E.Y. Hleb, J.H. Hafner, J.N. Myers et al., LANTCET: elimination of solid tumour cells with photothermal bubbles generated around clusters of gold nanoparticles. Nanomedicine 3, 647–667 (2008)
C.M. Pitsillides, E.K. Joe, X. Wei, R.R. Anderson, C.P. Lin, Selective cell targeting with light-absorbing microparticles and nanoparticles. Biophys. J. 84, 4023–4032 (2003)
B. Bodia, P. Cloutier, D. Hunting, M.A. Huels, L. Sanche, Resonant formation of DNA strand breaks by low-energy (3 to 20 keV). Science 287, 1658 (2000)
L. Sanche, Low energy electron-driven damage in biomolecules. Eur. Phys. J. D 35, 367 (2005)
S. Cho, O. Vassiliev, S. Jang, S. Krishnan, Modifications of megavoltage photon beams for gold nanoparticle-aided radiation therapy (GNRT): a Monte Carlo study. Med. Phys. 33, 2121 (2006)
S. Cho, O. Vassiliev, S. Krishnan, Microscopic estimation of tumour dose enhancement during gold nanoparticle-aided radiation therapy (GNRT) using diagnostic energy range x-rays. Med. Phys. 34, 2468 (2007)
Acknowledgments
The author would like to thank Michael Leung of the University of Toronto and Sean Fahey of the Ryerson University for their assistances in the Geant4 and NOREC programming and Monte Carlo simulations. The author would also like to acknowledge the SciNet HPC Consortium in the University of Toronto for providing computing support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this entry
Cite this entry
Chow, J.C. (2015). Characteristics of Secondary Electrons from Irradiated Gold Nanoparticle in Radiotherapy. In: Aliofkhazraei, M. (eds) Handbook of Nanoparticles. Springer, Cham. https://doi.org/10.1007/978-3-319-13188-7_10-1
Download citation
DOI: https://doi.org/10.1007/978-3-319-13188-7_10-1
Received:
Accepted:
Published:
Publisher Name: Springer, Cham
Online ISBN: 978-3-319-13188-7
eBook Packages: Springer Reference Chemistry and Mat. ScienceReference Module Physical and Materials ScienceReference Module Chemistry, Materials and Physics
Publish with us
Chapter history
-
Latest
Characteristics of Secondary Electrons from Irradiated Gold Nanoparticle in Radiotherapy- Published:
- 29 June 2015
DOI: https://doi.org/10.1007/978-3-319-13188-7_10-2
-
Original
Characteristics of Secondary Electrons from Irradiated Gold Nanoparticle in Radiotherapy- Published:
- 25 May 2015
DOI: https://doi.org/10.1007/978-3-319-13188-7_10-1