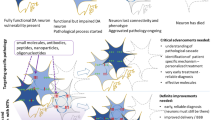
Abstract
Introduction: Parkinson’s disease (PD) is characterized by the progressive loss of midbrain dopamine (DA) neurons that regulate voluntary movement and cognitive functions. Despite notable progress in the development of symptomatic therapy, the development of disease-modifying therapy for PD still holds big challenges. Neurotrophic factors (NTFs) are considered as potential regenerative therapy for PD because they can protect DA neurons and promote neuron survival both in vitro and in vivo. Although preclinical studies with the neurotrophic factors have proved to be efficient in animal PD models with mild lesions, the effect of NTFs on PD patients in clinical trials has been modest. In this review, we discuss the current state of NTFs therapies on PD treatment from preclinical and clinical studies, challenges with growth factors (GFs) therapy, and alternative approaches for further development of disease-modifying therapies. Methods: We formulated the questions and based on that, literature search was carried out using the PUBMED database. Further, we extracted the data, analyzed and summarized the findings. Results: GDNF family ligands GDNF and neurturin and CDNF/MANF neurotrophic factors are extensively studied in preclinical and clinical studies. In preclinical studies, these NTFs have demonstrated neurotrophic activities on DA neurons and have potential disease modifying properties in animal models of PD. However, the effect of NTFs in PD patients has been modest. Importantly, the results from clinical studies show beneficial effects of NTFs including improvements in motor symptoms and quality of life. Conclusion: NTFs still remains a potential candidate in the treatment of PD. However, development of GDNF based treatment has many difficulties related to effective delivery, poor diffusion and optimum dose. Regardless of the challenges, efforts to develop neurotrophic factors-based therapies should be continued towards clinical application in PD.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Airaksinen MS, Saarma M (2002) The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 3:383–394. https://doi.org/10.1038/nrn812
Airavaara M, Harvey BK, Voutilainen MH et al (2012) CDNF protects the nigrostriatal dopamine system and promotes recovery after MPTP treatment in mice. Cell Transplant 21:1213–1223. https://doi.org/10.3727/096368911X600948
Albert K, Raymundo DP, Panhelainen A et al (2021) Cerebral dopamine neurotrophic factor reduces α-synuclein aggregation and propagation and alleviates behavioral alterations in vivo. Mol Ther 29:2821–2840. https://doi.org/10.1016/j.ymthe.2021.04.035
Alexander GE (2004) Biology of Parkinson’s disease: pathogenesis and pathophysiology of a multisystem neurodegenerative disorder. Dialog Clin Neurosci 6:259. https://doi.org/10.31887/DCNS.2004.6.3/galexander
Axelsen TM, Woldbye DPD (2018) Gene therapy for parkinson’s disease, an update. J. Parkinson’s Dis. 8:195–215. https://doi.org/10.3233/JPD-181331
Axten JM, Medina JR, Feng Y et al (2012) Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1H-indol-5-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK). J Med Chem 55:7193–7207. https://doi.org/10.1021/jm300713s
Bäck S, Peränen J, Galli E et al (2013) Gene therapy with AAV2-CDNF provides functional benefits in a rat model of Parkinson’s disease. Brain Behav 3:75–88. https://doi.org/10.1002/brb3.117
Bankiewicz KS, Sudhakar V, Samaranch L et al (2016) AAV viral vector delivery to the brain by shape-conforming MR-guided infusions. J Control Release 240:434–442. https://doi.org/10.1016/j.jconrel.2016.02.034
Bartus RT, Kordower JH, Johnson EM et al (2015) Post-mortem assessment of the short and long-term effects of the trophic factor neurturin in patients with α-synucleinopathies. Neurobiol Dis 78:162–171. https://doi.org/10.1016/j.nbd.2015.03.023
Beck KD, Irwin I, Valverde J et al (1996) GDNF induces a dystonia-like state in neonatal rats and stimulates dopamine and serotonin synthesis. Neuron 16:665–673. https://doi.org/10.1016/s0896-6273(00)80085-9
Bensadoun JC, Déglon N, Tseng JL et al (2000) Lentiviral vectors as a gene delivery system in the mouse midbrain: cellular and behavioral improvements in a 6-OHDA model of Parkinson’s disease using GDNF. Exp Neurol 164:15–24. https://doi.org/10.1006/exnr.2000.7409
Bespalov MM, Saarma M (2007) GDNF family receptor complexes are emerging drug targets. Trends Pharmacol Sci 28:68–74. https://doi.org/10.1016/j.tips.2006.12.005
Bespalov MM, Sidorova YA, Tumova S et al (2011) Heparan sulfate proteoglycan syndecan-3 is a novel receptor for GDNF, neurturin, and artemin. J Cell Biol 192:153–169. https://doi.org/10.1083/jcb.201009136
Björklund A, Kirik D, Rosenblad C et al (2000) Towards a neuroprotective gene therapy for Parkinson’s disease: use of adenovirus, AAV and lentivirus vectors for gene transfer of GDNF to the nigrostriatal system in the rat Parkinson model11Published on the World Wide Web on 10 October 2000. Brain Res 886:82–98. https://doi.org/10.1016/S0006-8993(00)02915-2
Bloem BR, Okun MS, Klein C (2021) Parkinson’s disease. Lancet 397:2284–2303. https://doi.org/10.1016/S0140-6736(21)00218-X
Bondarenko O, Saarma M (2021) Neurotrophic factors in Parkinson’s disease: clinical trials, open challenges and nanoparticle-mediated delivery to the brain. Front Cell Neurosci 15:682597. https://doi.org/10.3389/fncel.2021.682597
Borrello MG, Alberti L, Arighi E et al (1996) The full oncogenic activity of Ret/ptc2 depends on tyrosine 539, a docking site for phospholipase Cgamma. Mol Cell Biol 16:2151–2163
Bourque MJ, Trudeau LE (2000) GDNF enhances the synaptic efficacy of dopaminergic neurons in culture. Eur J Neurosci 12:3172–3180. https://doi.org/10.1046/j.1460-9568.2000.00219.x
Bowenkamp KE, Hoffman AF, Gerhardt GA et al (1995) Glial cell line-derived neurotrophic factor supports survival of injured midbrain dopaminergic neurons. J Comp Neurol 355:479–489. https://doi.org/10.1002/cne.903550402
Cass WA, Peters LE (2010) Neurturin effects on nigrostriatal dopamine release and content: comparison with GDNF. Neurochem Res 35:727–734. https://doi.org/10.1007/s11064-010-0128-0
Chmielarz P, Er Ş, Konovalova J et al (2020) GDNF/RET signaling pathway activation eliminates Lewy body pathology in midbrain dopamine neurons. Mov Disord 35:2279–2289. https://doi.org/10.1002/mds.28258
Chu Y, Bartus RT, Manfredsson FP et al (2020) Long-term post-mortem studies following neurturin gene therapy in patients with advanced Parkinson’s disease. Brain 143:960–975. https://doi.org/10.1093/brain/awaa020
Clarkson ED, Zawada WM, Freed CR (1995) GDNF reduces apoptosis in dopaminergic neurons in vitro. NeuroReport 7:145–149
Clarkson ED, Zawada WM, Freed CR (1997) GDNF improves survival and reduces apoptosis in human embryonic dopaminergic neurons in vitro. Cell Tissue Res 289:207–210. https://doi.org/10.1007/s004410050867
Cordero-Llana Ó, Houghton BC, Rinaldi F et al (2015) Enhanced efficacy of the CDNF/MANF family by combined intranigral overexpression in the 6-OHDA rat model of Parkinson’s disease. Mol Ther 23:244–254. https://doi.org/10.1038/mt.2014.206
Cross BCS, Bond PJ, Sadowski PG et al (2012) The molecular basis for selective inhibition of unconventional mRNA splicing by an IRE1-binding small molecule. Proc Natl Acad Sci U S A 109:E869-878. https://doi.org/10.1073/pnas.1115623109
Crupi MJF, Yoganathan P, Bone LN et al (2015) Distinct temporal regulation of RET isoform internalization: roles of Clathrin and AP2. Traffic 16:1155–1173. https://doi.org/10.1111/tra.12315
Danilova T, Belevich I, Li H et al (2019) MANF is required for the postnatal expansion and maintenance of pancreatic β-cell mass in mice. Diabetes 68:66–80. https://doi.org/10.2337/db17-1149
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39:889–909. https://doi.org/10.1016/S0896-6273(03)00568-3
De Lorenzo F, Lüningschrör P, Nam J et al (2023) CDNF rescues motor neurons in models of amyotrophic lateral sclerosis by targeting endoplasmic reticulum stress. Brain. https://doi.org/10.1093/brain/awad087
Decressac M, Kadkhodaei B, Mattsson B, et al (2012) α-Synuclein-induced down-regulation of Nurr1 disrupts GDNF signaling in nigral dopamine neurons. Sci Transl Med 4:163ra156. https://doi.org/10.1126/scitranslmed.3004676
deSouza R-M, Moro E, Lang AE, Schapira AHV (2013) Timing of deep brain stimulation in Parkinson disease: a need for reappraisal? Ann Neurol 73:565–575. https://doi.org/10.1002/ana.23890
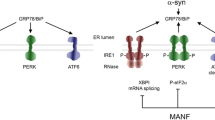
Eesmaa A, Yu L-Y, Göös H et al (2022) CDNF interacts with ER chaperones and requires UPR sensors to promote neuronal survival. Int J Mol Sci 23:9489. https://doi.org/10.3390/ijms23169489
Eesmaa A, Yu L-Y, Göös H et al (2021) The cytoprotective protein MANF promotes neuronal survival independently from its role as a GRP78 cofactor. J Biol Chem 296:100295. https://doi.org/10.1016/j.jbc.2021.100295
Enomoto H, Araki T, Jackman A et al (1998) GFRα1-deficient mice have deficits in the enteric nervous system and kidneys. Neuron 21:317–324. https://doi.org/10.1016/S0896-6273(00)80541-3
Enterría-Morales D, López-López I, López-Barneo J, d’Anglemont de Tassigny X (2020) Role of glial cell line-derived neurotrophic factor in the maintenance of adult mesencephalic catecholaminergic neurons. Mov Disord 35:565–576. https://doi.org/10.1002/mds.27986
Eslamboli A, Cummings RM, Ridley RM et al (2003) Recombinant adeno-associated viral vector (rAAV) delivery of GDNF provides protection against 6-OHDA lesion in the common marmoset monkey (Callithrix jacchus). Exp Neurol 184:536–548. https://doi.org/10.1016/j.expneurol.2003.08.007
Espadas-Alvarez AJ, Bannon MJ, Orozco-Barrios CE et al (2017) Regulation of human GDNF gene expression in nigral dopaminergic neurons using a new doxycycline-regulated NTS-polyplex nanoparticle system. Nanomedicine 13:1363–1375. https://doi.org/10.1016/j.nano.2017.02.006
Fan C-H, Ting C-Y, Lin C et al (2016) Noninvasive, targeted, and non-viral ultrasound-mediated GDNF-plasmid delivery for treatment of Parkinson’s disease. Sci Rep 6:19579. https://doi.org/10.1038/srep19579
Galli E, Härkönen T, Sainio MT et al (2016) Increased circulating concentrations of mesencephalic astrocyte-derived neurotrophic factor in children with type 1 diabetes. Sci Rep 6:29058. https://doi.org/10.1038/srep29058
Galli E, Planken A, Kadastik-Eerme L et al (2019) Increased serum levels of mesencephalic astrocyte-derived neurotrophic factor in subjects with Parkinson’s disease. Front Neurosci 13
Garea-Rodríguez E, Eesmaa A, Lindholm P et al (2016) Comparative analysis of the effects of neurotrophic factors CDNF and GDNF in a nonhuman primate model of Parkinson’s disease. PLoS ONE 11:e0149776. https://doi.org/10.1371/journal.pone.0149776
Gartziandia O, Herrán E, Ruiz-Ortega JA et al (2016) Intranasal administration of chitosan-coated nanostructured lipid carriers loaded with GDNF improves behavioral and histological recovery in a partial lesion model of Parkinson’s disease. J Biomed Nanotechnol 12:2220–2280. https://doi.org/10.1166/jbn.2016.2313
Gash DM, Zhang Z, Ai Y et al (2005) Trophic factor distribution predicts functional recovery in parkinsonian monkeys. Ann Neurol 58:224–233. https://doi.org/10.1002/ana.20549
Gash DM, Zhang Z, Ovadia A et al (1996) Functional recovery in parkinsonian monkeys treated with GDNF. Nature 380:252–255. https://doi.org/10.1038/380252a0
Georgievska B, Kirik D, Björklund A (2004) Overexpression of glial cell line-derived neurotrophic factor using a lentiviral vector induces time- and dose-dependent downregulation of tyrosine hydroxylase in the intact nigrostriatal dopamine system. J Neurosci 24:6437–6445. https://doi.org/10.1523/JNEUROSCI.1122-04.2004
Ghosh R, Wang L, Wang ES et al (2014) Allosteric inhibition of the IRE1α RNase preserves cell viability and function during endoplasmic reticulum stress. Cell 158:534–548. https://doi.org/10.1016/j.cell.2014.07.002
Giguère N, Burke Nanni S, Trudeau L-E (2018) On cell loss and selective vulnerability of neuronal populations in Parkinson’s disease. Front Neurol 9:455. https://doi.org/10.3389/fneur.2018.00455
Gill SS, Patel NK, Hotton GR et al (2003) Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med 9:589–595. https://doi.org/10.1038/nm850
Glembotski CC, Thuerauf DJ, Huang C et al (2012) Mesencephalic astrocyte-derived neurotrophic factor protects the heart from ischemic damage and is selectively secreted upon sarco/endoplasmic reticulum calcium depletion. J Biol Chem 287:25893–25904. https://doi.org/10.1074/jbc.M112.356345
Glerup S, Lume M, Olsen D et al (2013) SorLA controls neurotrophic activity by sorting of GDNF and its receptors GFRα1 and RET. Cell Rep 3:186–199. https://doi.org/10.1016/j.celrep.2012.12.011
Grondin R, Cass WA, Zhang Z et al (2003) Glial cell line-derived neurotrophic factor increases stimulus-evoked dopamine release and motor speed in aged rhesus monkeys. J Neurosci 23:1974–1980
Hallett PJ, Deleidi M, Astradsson A et al (2015) Successful function of autologous iPSC-derived dopamine neurons following transplantation in a non-human primate model of Parkinson’s disease. Cell Stem Cell 16:269–274. https://doi.org/10.1016/j.stem.2015.01.018
Han F, Wang W, Chen B et al (2015) Human induced pluripotent stem cell-derived neurons improve motor asymmetry in a 6-hydroxydopamine-induced rat model of Parkinson’s disease. Cytotherapy 17:665–679. https://doi.org/10.1016/j.jcyt.2015.02.001
Hao F, Yang C, Chen S-S et al (2017) Long-term protective effects of AAV9-mesencephalic astrocyte-derived neurotrophic factor gene transfer in parkinsonian rats. Exp Neurol 291:120–133. https://doi.org/10.1016/j.expneurol.2017.01.008
Hayashi T, Wakao S, Kitada M et al (2013) Autologous mesenchymal stem cell-derived dopaminergic neurons function in parkinsonian macaques. J Clin Invest 123:272–284. https://doi.org/10.1172/JCI62516
Heiss JD, Lungu C, Hammoud DA et al (2019) Trial of magnetic resonance-guided putaminal gene therapy for advanced Parkinson’s disease. Mov Disord 34:1073–1078. https://doi.org/10.1002/mds.27724
Hellman M, Arumäe U, Yu L et al (2011) Mesencephalic astrocyte-derived neurotrophic factor (MANF) has a unique mechanism to rescue apoptotic neurons. J Biol Chem 286:2675–2680. https://doi.org/10.1074/jbc.M110.146738
Heuckeroth RO, Enomoto H, Grider JR et al (1999) Gene targeting reveals a critical role for neurturin in the development and maintenance of enteric, sensory, and parasympathetic neurons. Neuron 22:253–263. https://doi.org/10.1016/s0896-6273(00)81087-9
Horger BA, Nishimura MC, Armanini MP et al (1998) Neurturin exerts potent actions on survival and function of midbrain dopaminergic neurons. J Neurosci 18:4929–4937
Hsu J-Y, Crawley S, Chen M et al (2017) Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature 550:255–259. https://doi.org/10.1038/nature24042
Hudson J, Granholm AC, Gerhardt GA et al (1995) Glial cell line-derived neurotrophic factor augments midbrain dopaminergic circuits in vivo. Brain Res Bull 36:425–432. https://doi.org/10.1016/0361-9230(94)00224-o
Huttunen HJ, Booms S, Sjögren M et al (2023) Intraputamenal cerebral dopamine neurotrophic factor in Parkinson’s disease: a randomized, double-blind, multicenter phase 1 trial. Mov Disord 38:1209–1222. https://doi.org/10.1002/mds.29426
Hyndman BD, Crupi MJF, Peng S et al (2017) Differential recruitment of E3 ubiquitin ligase complexes regulates RET isoform internalization. J Cell Sci 130:3282–3296. https://doi.org/10.1242/jcs.203885
Jäntti M, Harvey BK (2020) The trophic activities of the endoplasmic reticulum proteins CDNF and MANF. Cell Tissue Res 382:83–100. https://doi.org/10.1007/s00441-020-03263-0
Jiaming M, Niu C (2015) Comparing neuroprotective effects of CDNF-expressing bone marrow derived mesenchymal stem cells via differing routes of administration utilizing an in vivo model of Parkinson’s disease. Neurol Sci 36:281–287. https://doi.org/10.1007/s10072-014-1929-8
Kells AP, Eberling J, Su X et al (2010) Regeneration of the MPTP-lesioned dopaminergic system after convection-enhanced delivery of AAV2-GDNF. J Neurosci 30:9567–9577. https://doi.org/10.1523/JNEUROSCI.0942-10.2010
Kikuchi T, Morizane A, Doi D et al (2017) Human iPS cell-derived dopaminergic neurons function in a primate Parkinson’s disease model. Nature 548:592–596. https://doi.org/10.1038/nature23664
Kirik D, Georgievska B, Björklund A (2004) Localized striatal delivery of GDNF as a treatment for Parkinson disease. Nat Neurosci 7:105–110. https://doi.org/10.1038/nn1175
Kirik D, Rosenblad C, Björklund A (2000) Preservation of a functional nigrostriatal dopamine pathway by GDNF in the intrastriatal 6-OHDA lesion model depends on the site of administration of the trophic factor. Eur J Neurosci 12:3871–3882. https://doi.org/10.1046/j.1460-9568.2000.00274.x
Kirik D, Rosenblad C, Björklund A, Mandel RJ (2000) Long-term rAAV-mediated gene transfer of GDNF in the rat Parkinson’s model: intrastriatal but not intranigral transduction promotes functional regeneration in the lesioned nigrostriatal system. J Neurosci 20:4686–4700. https://doi.org/10.1523/JNEUROSCI.20-12-04686.2000
Kobori N, Waymire JC, Haycock JW et al (2004) Enhancement of tyrosine hydroxylase phosphorylation and activity by glial cell line-derived neurotrophic factor. J Biol Chem 279:2182–2191. https://doi.org/10.1074/jbc.M310734200
Kopra J, Vilenius C, Grealish S et al (2015) GDNF is not required for catecholaminergic neuron survival in vivo. Nat Neurosci 18:319–322. https://doi.org/10.1038/nn.3941
Kopra JJ, Panhelainen A, Bjerkén S et al (2017) Dampened amphetamine-stimulated behavior and altered dopamine transporter function in the absence of brain GDNF. J Neurosci 37:1581–1590. https://doi.org/10.1523/JNEUROSCI.1673-16.2016
Kordower JH, Emborg ME, Bloch J et al (2000) Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science 290:767–773. https://doi.org/10.1126/science.290.5492.767
Kordower JH, Palfi S, Chen E-Y et al (1999) Clinicopathological findings following intraventricular glial-derived neurotrophic factor treatment in a patient with Parkinson’s disease. Ann Neurol 46:419–424. https://doi.org/10.1002/1531-8249(199909)46:3%3c419::AID-ANA21%3e3.0.CO;2-Q
Kovaleva V, Yu L-Y, Ivanova L et al (2023) MANF regulates neuronal survival and UPR through its ER-located receptor IRE1α. Cell Rep 42:112066. https://doi.org/10.1016/j.celrep.2023.112066
Kramer ER, Aron L, Ramakers GMJ et al (2007) Absence of ret signaling in mice causes progressive and late degeneration of the nigrostriatal system. PLoS Biol 5:e39. https://doi.org/10.1371/journal.pbio.0050039
Kramer ER, Liss B (2015) GDNF-Ret signaling in midbrain dopaminergic neurons and its implication for Parkinson disease. FEBS Lett 589:3760–3772. https://doi.org/10.1016/j.febslet.2015.11.006
Kumar A, Kopra J, Varendi K et al (2015) GDNF Overexpression from the native locus reveals its role in the nigrostriatal dopaminergic system function. PLoS Genet 11:e1005710. https://doi.org/10.1371/journal.pgen.1005710
Lang AE, Gill S, Patel NK et al (2006) Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol 59:459–466. https://doi.org/10.1002/ana.20737
Latge C, Cabral KMS, de Oliveira GAP et al (2015) The solution structure and dynamics of full-length human cerebral dopamine neurotrophic factor and its neuroprotective role against α-synuclein oligomers*. J Biol Chem 290:20527–20540. https://doi.org/10.1074/jbc.M115.662254
Li Z, Wang B, Wu X et al (2005) Identification, expression and functional characterization of the GRAL gene. J Neurochem 95:361–376. https://doi.org/10.1111/j.1471-4159.2005.03372.x
Lin LF, Doherty DH, Lile JD et al (1993) GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 260:1130–1132. https://doi.org/10.1126/science.8493557
Lindahl M, Chalazonitis A, Palm E et al (2020) Cerebral dopamine neurotrophic factor–deficiency leads to degeneration of enteric neurons and altered brain dopamine neuronal function in mice. Neurobiol Dis 134:104696. https://doi.org/10.1016/j.nbd.2019.104696
Lindahl M, Danilova T, Palm E et al (2014) MANF is indispensable for the proliferation and survival of pancreatic β cells. Cell Rep 7:366–375. https://doi.org/10.1016/j.celrep.2014.03.023
Lindgren N, Francardo V, Quintino L et al (2012) A model of GDNF gene therapy in mice with 6-Hydroxydopamine lesions: time course of neurorestorative effects and ERK1/2 activation. J Parkinsons Dis 2:333–348. https://doi.org/10.3233/JPD-012146
Lindholm P, Saarma M (2022) Cerebral dopamine neurotrophic factor protects and repairs dopamine neurons by novel mechanism. Mol Psychiatry 27:1310–1321. https://doi.org/10.1038/s41380-021-01394-6
Lindholm P, Saarma M (2010) Novel CDNF/MANF family of neurotrophic factors. Dev Neurobiol 70:360–371. https://doi.org/10.1002/dneu.20760
Lindholm P, Voutilainen MH, Laurén J et al (2007) Novel neurotrophic factor CDNF protects and rescues midbrain dopamine neurons in vivo. Nature 448:73–77. https://doi.org/10.1038/nature05957
Lindvall O (2013) Developing dopaminergic cell therapy for Parkinson’s disease—give up or move forward? Mov Disord 28:268–273. https://doi.org/10.1002/mds.25378
Liu Y, Zhang J, Jiang M et al (2018) MANF improves the MPP+/MPTP-induced Parkinson’s disease via improvement of mitochondrial function and inhibition of oxidative stress. Am J Transl Res 10:1284–1294
Lo Bianco C, Déglon N, Pralong W, Aebischer P (2004) Lentiviral nigral delivery of GDNF does not prevent neurodegeneration in a genetic rat model of Parkinson’s disease. Neurobiol Dis 17:283–289. https://doi.org/10.1016/j.nbd.2004.06.008
Lonka-Nevalaita L, Lume M, Leppanen S et al (2010) Characterization of the intracellular localization, processing, and secretion of two glial cell line-derived neurotrophic factor splice isoforms. J Neurosci 30:11403–11413. https://doi.org/10.1523/JNEUROSCI.5888-09.2010
Mahato AK, Kopra J, Renko J-M et al (2020) Glial cell line-derived neurotrophic factor receptor rearranged during transfection agonist supports dopamine neurons in Vitro and enhances dopamine release in vivo. Mov Disord 35:245–255. https://doi.org/10.1002/mds.27943
Mahato AK, Sidorova YA (2020) RET receptor tyrosine kinase: role in neurodegeneration, obesity, and cancer. Int J Mol Sci 21:7108. https://doi.org/10.3390/ijms21197108
Mahato AK, Sidorova YA (2020) Glial cell line-derived neurotrophic factors (GFLs) and small molecules targeting RET receptor for the treatment of pain and Parkinson’s disease. Cell Tissue Res 382:147–160. https://doi.org/10.1007/s00441-020-03227-4
Manfredsson FP, Polinski NK, Subramanian T et al (2020) The future of GDNF in Parkinson’s disease. Front Aging Neurosci 12:593572. https://doi.org/10.3389/fnagi.2020.593572
Marks WJ, Bartus RT, Siffert J et al (2010) Gene delivery of AAV2-neurturin for Parkinson’s disease: a double-blind, randomised, controlled trial. The Lancet Neurol 9:1164–1172. https://doi.org/10.1016/S1474-4422(10)70254-4
Marks WJ, Ostrem JL, Verhagen L et al (2008) Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2–neurturin) to patients with idiopathic Parkinson’s disease: an open-label, phase I trial. The Lancet Neurol 7:400–408. https://doi.org/10.1016/S1474-4422(08)70065-6
Menon S, Armstrong S, Hamzeh A et al (2022) Alpha-synuclein targeting therapeutics for Parkinson’s disease and related synucleinopathies. Front Neurol 13:852003. https://doi.org/10.3389/fneur.2022.852003
Moore MW, Klein RD, Fariñas I et al (1996) Renal and neuronal abnormalities in mice lacking GDNF. Nature 382:76–79. https://doi.org/10.1038/382076a0
Nadella R, Voutilainen MH, Saarma M et al (2014) Transient transfection of human CDNF gene reduces the 6-hydroxydopamine-induced neuroinflammation in the rat substantia nigra. J Neuroinflammation 11:209. https://doi.org/10.1186/s12974-014-0209-0
Nutt JG, Burchiel KJ, Comella CL et al (2003) Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology 60:69–73. https://doi.org/10.1212/wnl.60.1.69
Othberg A, Odin P, Ballagi A et al (1995) Specific effects of platelet derived growth factor (PDGF) on fetal rat and human dopaminergic neurons in vitro. Exp Brain Res 105:111–122. https://doi.org/10.1007/BF00242187
Pakarinen E, Danilova T, Võikar V, et al (2020) MANF ablation causes prolonged activation of the UPR without neurodegeneration in the mouse midbrain dopamine system. eNeuro 7:ENEURO.0477-19.2019. https://doi.org/10.1523/ENEURO.0477-19.2019
Pakarinen E, Lindholm P, Saarma M, Lindahl M (2022) CDNF and MANF regulate ER stress in a tissue-specific manner. Cell Mol Life Sci 79:124. https://doi.org/10.1007/s00018-022-04157-w
Palgi M, Lindström R, Peränen J et al (2009) Evidence that DmMANF is an invertebrate neurotrophic factor supporting dopaminergic neurons. Proc Natl Acad Sci U S A 106:2429–2434. https://doi.org/10.1073/pnas.0810996106
Paratcha G, Ledda F, Baars L et al (2001) Released GFRα1 potentiates downstream signaling, neuronal survival, and differentiation via a novel mechanism of recruitment of c-ret to lipid rafts. Neuron 29:171–184. https://doi.org/10.1016/S0896-6273(01)00188-X
Paratcha G, Ledda F, Ibáñez CF (2003) The neural cell adhesion molecule NCAM is an alternative signaling receptor for GDNF family ligands. Cell 113:867–879. https://doi.org/10.1016/S0092-8674(03)00435-5
Parkash V, Lindholm P, Peränen J et al (2009) The structure of the conserved neurotrophic factors MANF and CDNF explains why they are bifunctional. Protein Eng Des Sel 22:233–241. https://doi.org/10.1093/protein/gzn080
Pascual A, Hidalgo-Figueroa M, Piruat JI et al (2008) Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci 11:755–761. https://doi.org/10.1038/nn.2136
Paul G, Zachrisson O, Varrone A et al (2015) Safety and tolerability of intracerebroventricular PDGF-BB in Parkinson’s disease patients. J Clin Invest 125:1339–1346. https://doi.org/10.1172/JCI79635
Petrova P, Raibekas A, Pevsner J et al (2003) MANF: a new mesencephalic, astrocyte-derived neurotrophic factor with selectivity for dopaminergic neurons. J Mol Neurosci 20:173–188. https://doi.org/10.1385/jmn:20:2:173
Pfeiffer RF (2016) Non-motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord 22(Suppl 1):S119-122. https://doi.org/10.1016/j.parkreldis.2015.09.004
Piccinini E, Kalkkinen N, Saarma M, Runeberg-Roos P (2013) Glial cell line-derived neurotrophic factor: characterization of mammalian posttranslational modifications. Ann Med 45:66–73. https://doi.org/10.3109/07853890.2012.663927
Pichel JG, Shen L, Sheng HZ et al (1996) Defects in enteric innervation and kidney development in mice lacking GDNF. Nature 382:73–76. https://doi.org/10.1038/382073a0
Poewe W, Seppi K, Tanner CM et al (2017) Parkinson disease. Nat Rev Dis Primers 3:1–21. https://doi.org/10.1038/nrdp.2017.13
Pothos EN, Davila V, Sulzer D (1998) Presynaptic recording of quanta from midbrain dopamine neurons and modulation of the quantal size. J Neurosci 18:4106–4118. https://doi.org/10.1523/JNEUROSCI.18-11-04106.1998
Pruett BS, Salvatore MF (2013) Nigral GFRα1 infusion in aged rats increases locomotor activity, nigral tyrosine hydroxylase, and dopamine content in synchronicity. Mol Neurobiol 47:988–999. https://doi.org/10.1007/s12035-013-8397-7
Rana AQ, Ahmed US, Chaudry ZM, Vasan S (2015) Parkinson’s disease: a review of non-motor symptoms. Expert Rev Neurother 15:549–562. https://doi.org/10.1586/14737175.2015.1038244
Ren X, Zhang T, Gong X et al (2013) AAV2-mediated striatum delivery of human CDNF prevents the deterioration of midbrain dopamine neurons in a 6-hydroxydopamine induced parkinsonian rat model. Exp Neurol 248:148–156. https://doi.org/10.1016/j.expneurol.2013.06.002
Renko J-M, Mahato AK, Visnapuu T et al (2021) Neuroprotective potential of a small molecule RET agonist in cultured dopamine neurons and hemiparkinsonian rats. J Parkinsons Dis 11:1023–1046. https://doi.org/10.3233/JPD-202400
Rossi J, Luukko K, Poteryaev D et al (1999) Retarded growth and deficits in the enteric and parasympathetic nervous system in mice lacking GFRα2, a functional neurturin receptor. Neuron 22:243–252. https://doi.org/10.1016/S0896-6273(00)81086-7
Runeberg-Roos P, Piccinini E, Penttinen A-M et al (2016) Developing therapeutically more efficient neurturin variants for treatment of Parkinson’s disease. Neurobiol Dis 96:335–345. https://doi.org/10.1016/j.nbd.2016.07.008
Saarenpää T, Kogan K, Sidorova Y et al (2017) Zebrafish GDNF and its co-receptor GFRα1 activate the human RET receptor and promote the survival of dopaminergic neurons in vitro. PLoS ONE 12:e0176166. https://doi.org/10.1371/journal.pone.0176166
Saarma M, Voutilainen MH, Airavaara M et al. (2018) C-terminal CDNF and MANF fragments, pharmaceutical compositions comprising same and uses thereof. United States Patent Application No 17/043028; Publication Date: 01/14/2021 Filing Date: 03/29/2019
Salvatore MF, Ai Y, Fischer B et al (2006) Point source concentration of GDNF may explain failure of phase II clinical trial. Exp Neurol 202:497–505. https://doi.org/10.1016/j.expneurol.2006.07.015
Salvatore MF, Zhang J-L, Large DM et al (2004) Striatal GDNF administration increases tyrosine hydroxylase phosphorylation in the rat striatum and substantia nigra. J Neurochem 90:245–254. https://doi.org/10.1111/j.1471-4159.2004.02496.x
Schapira AHV, Chaudhuri KR, Jenner P (2017) Non-motor features of Parkinson disease. Nat Rev Neurosci 18:435–450. https://doi.org/10.1038/nrn.2017.62
Schuchardt A, D’Agati V, Larsson-Blomberg L et al (1994) Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 367:380–383. https://doi.org/10.1038/367380a0
Shahmoradian SH, Lewis AJ, Genoud C et al (2019) Lewy pathology in Parkinson’s disease consists of crowded organelles and lipid membranes. Nat Neurosci 22:1099–1109. https://doi.org/10.1038/s41593-019-0423-2
Shults CW (2006) Lewy bodies. PNAS 103:1661–1668. https://doi.org/10.1073/pnas.0509567103
Siderowf A, Concha-Marambio L, Lafontant D-E et al (2023) Assessment of heterogeneity among participants in the Parkinson’s progression markers Initiative cohort using α-synuclein seed amplification: a cross-sectional study. The Lancet Neurology 22:407–417. https://doi.org/10.1016/S1474-4422(23)00109-6
Sidorova YA, Saarma M (2020) Can growth factors cure Parkinson’s disease? Trends Pharmacol Sci 41:909–922. https://doi.org/10.1016/j.tips.2020.09.010
Slevin JT, Gash DM, Smith CD et al (2007) Unilateral intraputamenal glial cell line-derived neurotrophic factor in patients with Parkinson disease: response to 1 year of treatment and 1 year of withdrawal. J Neurosurg 106:614–620. https://doi.org/10.3171/jns.2007.106.4.614
Slevin JT, Gerhardt GA, Smith CD et al (2005) Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. J Neurosurg 102:216–222. https://doi.org/10.3171/jns.2005.102.2.0216
Smith AD, Kozlowski DA, Bohn MC, Zigmond MJ (2005) Effect of AdGDNF on dopaminergic neurotransmission in the striatum of 6-OHDA-treated rats. Exp Neurol 193:420–426. https://doi.org/10.1016/j.expneurol.2005.01.010
Subramanian K (2013) Restoration of motor and non-motor functions by neurotrophic factors in nonhuman primates with dopamine depletion. Doctoral Dissertation, University of Pittsburgh. http://d-scholarship.pitt.edu/20301/
Takahashi J (2020) IPS cell-based therapy for Parkinson’s disease: a Kyoto trial. Regen Ther 13:18–22. https://doi.org/10.1016/j.reth.2020.06.002
Tanaka Y, Takenouchi T, Tsukimoto M (2020) Mesencephalic astrocyte-derived neurotrophic factor is a novel radioresistance factor in mouse B16 melanoma. Biochem Biophys Res Commun 524:869–875. https://doi.org/10.1016/j.bbrc.2020.01.167
Tansey MG, Baloh RH, Milbrandt J, Johnson EM (2000) GFRα-mediated localization of RET to lipid rafts is required for effective downstream signaling, differentiation, and neuronal survival. Neuron 25:611–623. https://doi.org/10.1016/S0896-6273(00)81064-8
Tomac A, Widenfalk J, Lin L-FH et al (1995) Retrograde axonal transport of glial cell line-derived neurotrophic factor in the adult nigrostriatal system suggests a trophic role in the adult. Proc Natl Acad Sci USA 92:8274–8278
Tomac AC, Grinberg A, Huang SP et al (1999) Glial cell line-derived neurotrophic factor receptor α1 availability regulates glial cell line-derived neurotrophic factor signaling: evidence from mice carrying one or two mutated alleles. Neurosci 95:1011–1023. https://doi.org/10.1016/S0306-4522(99)00503-5
Tong S-Y, Wang R-W, Li Q et al (2023) Serum glial cell line-derived neurotrophic factor (GDNF) a potential biomarker of executive function in Parkinson’s disease. Front Neurosci 17:1136499. https://doi.org/10.3389/fnins.2023.1136499
Tseng K-Y, Stratoulias V, Hu W-F et al (2023) Augmenting hematoma-scavenging capacity of innate immune cells by CDNF reduces brain injury and promotes functional recovery after intracerebral hemorrhage. Cell Death Dis 14:1–20. https://doi.org/10.1038/s41419-022-05520-2
Tsui CC, Pierchala BA (2010) The differential axonal degradation of Ret accounts for cell-type-specific function of glial cell line-derived neurotrophic factor as a retrograde survival factor. J Neurosci 30:5149–5158. https://doi.org/10.1523/JNEUROSCI.5246-09.2010
Turconi G, Kopra J, Võikar V et al (2020) Chronic 2-fold elevation of endogenous GDNF levels is safe and enhances motor and dopaminergic function in aged mice. Mol Ther Methods Clin Dev 17:831–842. https://doi.org/10.1016/j.omtm.2020.04.003
Vieira P, Thomas-Crusells J, Vieira A (2003) Internalization of glial cell-derived neurotrophic factor receptor GFRα1 in the absence of the ret tyrosine kinase coreceptor. Cell Mol Neurobiol 23:43–55. https://doi.org/10.1023/A:1022593001155
Voutilainen MH, Bäck S, Peränen J et al (2011) Chronic infusion of CDNF prevents 6-OHDA-induced deficits in a rat model of Parkinson’s disease. Exp Neurol 228:99–108. https://doi.org/10.1016/j.expneurol.2010.12.013
Voutilainen MH, Bäck S, Pörsti E et al (2009) Mesencephalic astrocyte-derived neurotrophic factor is neurorestorative in rat model of Parkinson’s disease. J Neurosci 29:9651–9659. https://doi.org/10.1523/JNEUROSCI.0833-09.2009
Voutilainen MH, De Lorenzo F, Stepanova P et al (2017) Evidence for an Additive Neurorestorative Effect of Simultaneously Administered CDNF and GDNF in hemiparkinsonian rats: implications for different mechanism of action. eNeuro 4:ENEURO.0117-16.2017. https://doi.org/10.1523/ENEURO.0117-16.2017
Walter P, Ron D (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334:1081–1086. https://doi.org/10.1126/science.1209038
Wang L, Wang Z, Zhu R et al (2017) Therapeutic efficacy of AAV8-mediated intrastriatal delivery of human cerebral dopamine neurotrophic factor in 6-OHDA-induced parkinsonian rat models with different disease progression. PLoS ONE 12:e0179476. https://doi.org/10.1371/journal.pone.0179476
Warren Olanow C, Bartus RT, Baumann TL et al (2015) Gene delivery of neurturin to putamen and substantia nigra in Parkinson disease: A double-blind, randomized, controlled trial. Ann Neurol 78:248–257. https://doi.org/10.1002/ana.24436
Whone A, Luz M, Boca M et al (2019) Randomized trial of intermittent intraputamenal glial cell line-derived neurotrophic factor in Parkinson’s disease. Brain 142:512–525. https://doi.org/10.1093/brain/awz023
Whone AL, Boca M, Luz M et al (2019) Extended treatment with glial cell line-derived neurotrophic factor in Parkinson’s disease. J Parkinsons Dis 9:301–313. https://doi.org/10.3233/JPD-191576
Yagi T, Asada R, Kanekura K et al (2020) Neuroplastin modulates anti-inflammatory effects of MANF. iScience 23:101810. https://doi.org/10.1016/j.isci.2020.101810
Yan Y, Rato C, Rohland L et al (2019) MANF antagonizes nucleotide exchange by the endoplasmic reticulum chaperone BiP. Nat Commun 10:541. https://doi.org/10.1038/s41467-019-08450-4
Yu L-Y, Selberg S, Teino I et al (2023) Small molecule activation of m6A mRNA methylation as a novel approach for neuroprotection. 2023.07.05.547860
Zachrisson O, Zhao M, Andersson A et al (2011) Restorative effects of platelet derived growth factor-BB in rodent models of Parkinson’s disease. J Parkinsons Dis 1:49–63. https://doi.org/10.3233/JPD-2011-0003
Zahavi EE, Ionescu A, Gluska S et al (2015) A compartmentalized microfluidic neuromuscular co-culture system reveals spatial aspects of GDNF functions. J Cell Sci 128:1241–1252. https://doi.org/10.1242/jcs.167544
Zhang Y, Xiang Y, Wang X et al (2019) Cerebral dopamine neurotrophic factor protects microglia by combining with AKT and by regulating FoxO1/mTOR signaling during neuroinflammation. Biomed Pharma 109:2278–2284. https://doi.org/10.1016/j.biopha.2018.11.028
Acknowledgements
We are grateful to Päivi Lindholm-Pulkkila and Vera Kovaleva for their valuable comments on the manuscript. This work was supported by Jane and Aatos Erkko Foundation, Sigrid Juselius Foundation, Academy of Finland (grant number 1343299) and Enterprise Estonia. Figures 4.1 and 4.2 were created with BioRender.
Disclaimer Statement
MS is a founder and shareholder at Herantis Pharma Plc. MS and AKM report unrelated research support from the GeneCode Ltd. and ArgoBio Studio.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Mahato, A.K., Saarma, M. (2024). Neurotrophic Factors in Parkinson’s Disease: Clinical Trials. In: Peplow, P.V., Martinez, B., Gennarelli, T.A. (eds) Regenerative Medicine and Brain Repair. Stem Cell Biology and Regenerative Medicine, vol 75. Springer, Cham. https://doi.org/10.1007/978-3-031-49744-5_4
Download citation
DOI: https://doi.org/10.1007/978-3-031-49744-5_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-49743-8
Online ISBN: 978-3-031-49744-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)