Abstract
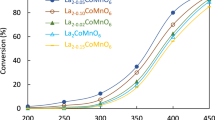
Nickel and cobalt lanthanum-based perovskites prepared by self-combustion method were adequate catalytic precursors in the ammonia decomposition reaction for obtaining hydrogen. The fuel-to-metal nitrates molar ratio, calcination temperature, and the metal substitution clearly affected the catalytic properties of the perovskites. In addition, generating non-precursor species during synthesis and small metal size were two factors which significantly influenced catalytic activity. Thus, with a molar ratio equal to 1, LaNiO3 perovskite can be obtained with few impurities, suitable physicochemical properties, and high basicity. Additionally, a calcination temperature of 650 °C for nickel perovskite led to small and well-dispersed Ni0 after reduction. On the other hand, bimetallic perovskites generated metallic Ni and/or Co in larger size, higher impurities, and lower active sites than pure nickel perovskite, which decreased the ammonia conversion. Self-combustion method was found to be effective and robust synthesis procedure to obtain catalyst precursors to generate very active catalysts after reduction for hydrogen production from NH3 at significantly lower temperature than those reported in bibliography. The nickel perovskite-derived catalyst, calcined at 650 °C, yielded excellent H2 production from ammonia decomposition. In particular, at 450 °C almost 100% of the ammonia was converted over the reduced LaNiO3 under study. Furthermore, these materials displayed admirable performance and stability after 1 day of reaction.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Cechetto, V., Di Felice, L., Medrano, J. A., et al. (2021). H2 production via ammonia decomposition in a catalytic membrane reactor. Fuel Processing Technology, 216, 106772. https://doi.org/10.1016/j.fuproc.2021.106772
Valera-Medina, A., Amer-Hatem, F., Azad, A. K., et al. (2021). Review on ammonia as a potential fuel: From synthesis to economics. Energy and Fuels, 35, 6964–7029.
Lucentini, I., Garcia, X., Vendrell, X., & Llorca, J. (2021b). Review of the decomposition of ammonia to generate hydrogen. Industrial and Engineering Chemistry Research, 60, 18560–18611. https://doi.org/10.1021/acs.iecr.1c00843
Pinzón, M., Avilés-García, O., de la Osa, A. R., et al. (2022a). New catalysts based on reduced graphene oxide for hydrogen production from ammonia decomposition. Sustainable Chemistry and Pharmacy, 25, 100615. https://doi.org/10.1016/j.scp.2022.100615
Pinzón, M., Romero, A., de Lucas, C. A., et al. (2021a). Hydrogen production by ammonia decomposition over ruthenium supported on SiC catalyst. Journal of Industrial and Engineering Chemistry, 94, 326–335. https://doi.org/10.1016/j.jiec.2020.11.003
Pinzón, M., Romero, A., de Lucas-Consuegra, A., et al. (2022b). COx-free hydrogen production from ammonia at low temperature using Co/SiC catalyst: Effect of promoter. Catalysis Today, 390–391, 34–47. https://doi.org/10.1016/j.cattod.2021.12.005
Pinzón, M., Sánchez-Sánchez, A., Sánchez, P., et al. (2021b). Ammonia as a carrier for hydrogen production by using lanthanum based perovskites. Energy Conversion and Management, 246, 114681. https://doi.org/10.1016/j.enconman.2021.114681
Huang, C., Li, H., Yang, J., et al. (2019). Ce0.6Zr0.3Y0.1O2 solid solutions-supported Ni Co bimetal nanocatalysts for NH3 decomposition. Applied Surface Science, 478, 708–716. https://doi.org/10.1016/j.apsusc.2019.01.269
Wu, Z. W., Li, X., Qin, Y. H., et al. (2020). Ammonia decomposition over SiO2-supported Ni–Co bimetallic catalyst for COx-free hydrogen generation. International Journal of Hydrogen Energy, 45, 15263–15269. https://doi.org/10.1016/j.ijhydene.2020.04.007
Muroyama, H., Saburi, C., Matsui, T., & Eguchi, K. (2012). Ammonia decomposition over Ni/La2O3 catalyst for on-site generation of hydrogen. Applied Catalysis A: General, 443–444, 119–124. https://doi.org/10.1016/j.apcata.2012.07.031
Tran, D. T., Nguyen, T. H., Jeong, H., et al. (2022). Recent engineering advances in nanocatalysts for NH3-to-H2 conversion technologies. Nano Energy, 94, 106929.
Pinzón, M., Sánchez-Sánchez, A., Romero, A., et al. (2022c). Self-combustion Ni and Co-based perovskites as catalyst precursors for ammonia decomposition. Effect of Ce and Mg doping. Fuel, 323, 124384. https://doi.org/10.1016/J.FUEL.2022.124384
Da Silva, A. A. A., Da Costa, L. O. O., Mattos, L. V., & Noronha, F. B. (2013). The study of the performance of Ni-based catalysts obtained from LaNiO3 perovskite-type oxides synthesized by the combustion method for the production of hydrogen by reforming of ethanol. Catalysis Today, 213, 25–32. https://doi.org/10.1016/j.cattod.2013.04.033
Sadabadi, H., Allahkaram, S. R., Kordijazi, A., et al. (2021). Structural characterization of LaCoO3 perovskite nanoparticles synthesized by sol–gel autocombustion method. Engineering Reports, 3, 91–96. https://doi.org/10.1002/eng2.12335
Liu, L., Zhang, Z., Das, S., et al. (2020). LaNiO3 as a precursor of Ni/La2O3 for reverse water-gas shift in DBD plasma: Effect of calcination temperature. Energy Conversion and Management, 206, 112475. https://doi.org/10.1016/j.enconman.2020.112475
Sihaib, Z., Puleo, F., Pantaleo, G., et al. (2019). The effect of citric acid concentration on the properties of LaMnO3 as a catalyst for hydrocarbon oxidation. Catalysts, 9, 226. https://doi.org/10.3390/catal9030226
Li, Y., Wen, J., Ali, A. M., et al. (2018). Size structure–catalytic performance correlation of supported Ni/MCF-17 catalysts for COx-free hydrogen production. Chemical Communications, 54, 6364–6367. https://doi.org/10.1039/C8CC01884G
Okura, K., Okanishi, T., Muroyama, H., et al. (2016). Ammonia decomposition over nickel catalysts supported on rare-earth oxides for the on-site generation of hydrogen. ChemCatChem, 8, 2988–2995. https://doi.org/10.1002/cctc.201600610
Li, X., Li, D., Tian, H., et al. (2017). Dry reforming of methane over Ni/La2O3 nanorod catalysts with stabilized Ni nanoparticles. Applied Catalysis B: Environmental, 202, 683–694. https://doi.org/10.1016/j.apcatb.2016.09.071
Civera, A., Pavese, M., Saracco, G., & Specchia, V. (2003). Combustion synthesis of perovskite-type catalysts for natural gas combustion. Catalysis Today, 83, 199–211. https://doi.org/10.1016/S0920-5861(03)00220-7
Omari, E., Makhloufi, S., & Omari, M. (2017). Preparation by sol–gel method and characterization of Co-doped LaNiO3 perovskite. Journal of Inorganic and Organometallic Polymers and Materials, 27, 1466–1472. https://doi.org/10.1007/s10904-017-0604-y
Santos, M. d. S., RCR, N., Noronha, F. B., et al. (2018). Perovskite as catalyst precursors in the partial oxidation of methane: The effect of cobalt, nickel and pretreatment. Catalysis Today, 299, 229–241. https://doi.org/10.1016/j.cattod.2017.06.027
Zhang, T., & Liu, Q. (2020). Mesostructured cellular foam silica supported bimetallic LaNi1-xCoxO3 catalyst for CO2 methanation. International Journal of Hydrogen Energy, 45, 4417–4426. https://doi.org/10.1016/j.ijhydene.2019.12.006
Lucentini, I., García Colli, G., Luzi, C. D., et al. (2021a). Catalytic ammonia decomposition over Ni-Ru supported on CeO2 for hydrogen production: Effect of metal loading and kinetic analysis. Applied Catalysis B: Environmental, 286. https://doi.org/10.1016/j.apcatb.2021.119896
Chang, F., Wu, H., Van Der, P. R., et al. (2019). Effect of pore confinement of NaNH2 and KNH2 on hydrogen generation from ammonia. Journal of Physical Chemistry C, 123, 21487–21496. https://doi.org/10.1021/acs.jpcc.9b03878
Su, Q., Gu, L., Yao, Y., et al. (2017). Layered double hydroxides derived Nix(MgyAlzOn) catalysts: Enhanced ammonia decomposition by hydrogen spillover effect. Applied Catalysis B: Environmental, 201, 451–460. https://doi.org/10.1016/j.apcatb.2016.08.051
Wang, L., Yi, Y., Zhao, Y., et al. (2015). NH3 Decomposition for H2 generation: Effects of cheap metals and supports on plasma-catalyst synergy. ACS Catalysis, 5, 4167–4174. https://doi.org/10.1021/acscatal.5b00728
Ganley, J. C., Thomas, F. S., Seebauer, E. G., & Masel, R. I. (2004). A priori catalytic activity correlations: The difficult case of hydrogen production from ammonia. Catal Letters, 96, 117–122. https://doi.org/10.1023/B:CATL.0000030108.50691.d4
Yu, Y., Gan, Y., Huang, C., et al. (2020). Ni/La2O3 and Ni/MgO–La2O3 catalysts for the decomposition of NH3 into hydrogen. International Journal of Hydrogen Energy, 45, 16528–16539. https://doi.org/10.1016/j.ijhydene.2020.04.127
Acknowledgments
This work was supported by the Regional Government of Castilla-La Mancha and the European Union [FEDER funds SBPLY/21/180501/000165]. M. Pinzón thanks the University of Castilla-La Mancha for the predoctoral contract within the framework of the Plan Propio I + D + i (grant number 2022-PRED-20658).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Pinzón, M., Sánchez-Sánchez, A., Sánchez, P., de la Osa, A.R., Romero, A. (2023). Perovskites as Catalyst Precursor for Hydrogen Production from Ammonia Decomposition. In: Nie, W., Iniewski, K.(. (eds) Metal-Halide Perovskite Semiconductors. Springer, Cham. https://doi.org/10.1007/978-3-031-26892-2_11
Download citation
DOI: https://doi.org/10.1007/978-3-031-26892-2_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-26891-5
Online ISBN: 978-3-031-26892-2
eBook Packages: EnergyEnergy (R0)