Abstract
Bacillus Calmette-Guérin (BCG) is a live-attenuated vaccine developed over 100 years ago and remains the only vaccine ever licensed in the fight against tuberculosis (TB). It is one of the most widely used vaccines in the world, having been administered to over four billion people, with another 100 million children vaccinated with BCG every year. Despite this, significant debate exists surrounding its efficacy against TB and its place in routine infant vaccination schedules. Severe side effects following BCG administration are rare but may be seen in those with immune system dysfunction. Safer vaccines for use in these individuals would be valuable.
BCG has been shown in some studies to have beneficial effects on mortality and morbidity beyond that attributable to reduction in TB alone. Understanding the immunological mechanisms underpinning these non-specific effects is increasing and appears in part to be due to the induction of trained innate immunity. New vaccines developed against TB will either need to be given as a booster following initial BCG vaccination or be shown to be non-inferior with regard to these off-target effects.
Despite its age, widespread usage, and intensive study, we are still learning how BCG exerts its effects and unpicking what these really are. Alternative routes of administration and recombinant forms of BCG offer promising strategies to further harness the potential of this intriguing vaccine.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 History of BCG
Bacillus Calmette-Guérin (BCG) was named after the French scientists Albert Calmette and Camille Guérin, who developed it for use as a vaccine against tuberculosis (TB) in the early 1900s at the Pasteur Institute in Lille [1]. Calmette was a physician and bacteriologist, who gained initial notoriety for his work developing snake antivenom in Southeast Asia in the 1890s. On returning to France, he was struck by the levels of TB disease amongst the working classes in the crowded, industrial cities of northern France and began to focus his considerable talents on trying to control and prevent the disease. Camille Guérin was a skilled young veterinarian whose father had died from TB. He joined the Pasteur Institute in 1897 and quickly became head of the laboratory [2]. His partnership with Calmette was to prove pivotal in TB vaccine research.
Previous attempts to produce a vaccine against TB, such as by heat or chemical inactivation of tubercle bacilli, had proved ineffective. Use of a live vaccine appeared to be required. Work conducted by the Nobel laureate Emil von Behring in 1902 demonstrated that inoculation with human tubercle bacilli strains could protect cattle against bovine TB. However, potentially infectious viable bacilli were subsequently found to be excreted in milk. Use of a paratuberculosis bacillus isolated from tortoises was also tested in cattle to no avail [3].
Building on this knowledge, Calmette and Guérin began their search for a human vaccine. Whilst attempting to culture tubercle bacilli for experimental use, they found that using standard potato and glycerol culture medium resulted in the unwanted clumping of bacteria. They tested the addition of ox bile as a solution. Serendipitously, this was found not only to reduce clumping but also to result in reduced virulence on subsequent subculture [4].
In 1908, starting with a virulent strain of Mycobacterium bovis (M. bovis), the causative agent of TB in cattle, they began the culture process that would eventually lead to BCG. Utilising their potato, glycerol and ox bile medium, they created new subcultures every 3 weeks, a process also termed passaging. After 30 passages, they had created a strain that was no longer lethal to guinea pigs [3]. In 1913, a vaccination trial in cattle was planned, but this was interrupted by the First World War. Despite the difficulties in obtaining sufficient potatoes and ox bile during the German occupation of Lille, they managed to maintain their cultures. By 1919, their “bile bacillus” had been passaged 230 times and failed to produce TB disease when injected into rabbits, guinea pigs or cattle [4].
At this point, Calmette and Guérin considered the bacilli to be sufficiently attenuated (weakened) that it would not cause disease in humans but might instead stimulate enough of an immune response to confer immunity against TB. The opportunity for the first test in humans came in 1921, courtesy of a Dr. Weil-Hallé, a physician at the Charité Hospital, Paris. He contacted Calmette about a healthy newborn infant whose mother had died from TB shortly after childbirth. On 18 July 1921, the infant became the first human to receive a dose of BCG [3]. Calmette mistakenly believed that the natural route of infection for Mycobacterium tuberculosis (M. tb) was via the gastrointestinal tract, and therefore BCG was initially given orally. No negative sequelae of the vaccination were seen and the child survived to live a TB free life [1].
By the end of 1924, over 600 infants had been vaccinated orally with BCG, with no significant safety concerns identified [3]. Mass production of BCG began and thousands of infants throughout Europe were vaccinated over the next 5 years. BCG was adopted by the Health Committee of the League of Nations, the predecessor of the World Health Organisation, in 1928. Despite this, BCG uptake was initially slow and highly variable between different countries. Scandinavian countries such as Sweden and Norway enthusiastically embraced the new vaccine, and it was here that the now routinely used intradermal route of administration was established [5]. Uptake was much lower in countries such as Great Britain, where articles expressing considerable scepticism about Calmette’s efficacy statistics were published in the medical press [6] and in the USA, where concerns circulated about the potential of the bacilli to regain full virulence [7].
In 1930, the Lübeck disaster nearly brought the history of BCG to a premature end. Around 250 infants in the German city of Lübeck were vaccinated with oral BCG. Tragically, scores of these children went on to develop TB and 72 died from the disease. Confidence in the safety of BCG was profoundly damaged, and Calmette and Guérin found themselves under intense scrutiny. Following an investigation over nearly 2 years, it was ascertained that the BCG stock had been contaminated with a virulent human M. tb strain during preparation in the local TB laboratory. Two of the doctors concerned were sentenced to time in jail for their role in the disaster.
BCG and its creators were fully exonerated, but confidence had been undermined and BCG use declined in many European countries [8]. It was not until the Second World War brought with it a deadly resurgence in TB cases throughout Europe and Asia that BCG use really took off. The advent of lyophilised (freeze dried) BCG around this time helped to facilitate large-scale vaccination programmes.
Subsequently, BCG has become one of the most widely used vaccines in history, with billions of doses delivered worldwide. It has been included in the WHO global extended programme of immunisations (EPI) since 1974 [9]. Current vaccination policies differ by country, but BCG is still widely given at birth in many. In higher income countries, reductions in TB incidence mean that BCG vaccination is now often reserved for specific higher-risk groups, such as migrants and healthcare workers (Fig. 8.1).
Current and historical BCG vaccination strategies by country. (Reprinted with permission from www.bcgatlas.org, which contains an interactive version of this map with additional details including TB incidence and history of BCG vaccination practices for each country [10])
2 Evolution and Genetics of BCG Vaccines
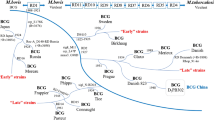
From as early as 1924, seed stock from the original BCG was distributed from the Pasteur Institute in Lille to laboratories across the world. This enabled local production and distribution of the vaccine prior to the advent of lyophilisation techniques and archived seed lots. This has resulted in the existence of numerous “daughter” strains of BCG, which have each undergone additional in vitro evolution and thus contain distinct genetic variations and consequent morphological and phenotypic differences (Fig. 8.2) [11, 14].
The attenuation of BCG was achieved empirically by Calmette and Guérin. Subsequent genomic studies have identified the loss of a 9.5-kb DNA segment, the so-called region of difference 1 (RD1), as the major causative variation. RD1 is present in both virulent M. bovis and M. tb but absent from all sub-strains of BCG. This genetic locus codes for two key immunogenic antigens—early secreted antigenic target 6 (ESAT-6) and culture filtrate protein 10 (CFP-10), plus several parts of their secretion system (ESX-1) [15]. The ESX-1 secretion system is required for full virulence of M. tb and results in disruption of the phagolysosome within host infected macrophages [16]. It should be noted that other variations are also involved in BCG attenuation, as reintroduction of RD1 to BCG-Pasteur or BCG-Russia does not restore full virulence [17].
Numerous other genetic differences, including deletions and duplications of genomic regions, as well as single nucleotide polymorphisms (SNPs), exist between M. tb and BCG. Unlike RD1, these also vary between different BCG strains. For example, RD2 is present in BCG strains derived prior to 1927 (“early” strains) but lost from those propagated subsequently (“late” strains) [18]. Loss of RD2 is implicated in reduced virulence, via disruptions to host innate immune responses, and has been postulated by some to have caused an “over attenuation” of BCG [11]. Several other genomic polymorphisms are identifiable in late BCG strains, including point mutations in genes encoding for mycobacterium protein bovis (MPB) 83 and MPB 70. These antigenic proteins have been implicated in mycobacterial pathogenesis and are found in high levels in strains prior to 1927 but in only very small amounts in later strains [19].
As well as deletions, characteristic duplicated genome sections are seen across BCG strains. These are the so-called tandem duplications DU1 and DU2. DU1 occurs only in BCG-Pasteur. Four main forms of DU2 are seen across different BCG strains (Fig. 8.2). It is postulated that these tandem duplications may have arisen due to the selective pressures of BCG growth on glycerol [11]. The impact these duplicated regions have on the immunogenicity and efficacy of BCG strains remains uncertain.
Today, the most used strains for BCG vaccine production worldwide include BCG-Bulgaria (Sofia SL222), BCG-Denmark (Danish 1331), BCG-Glaxo (Merieux 1007), BCG-Japan (Tokyo 172-1) and BCG-Pasteur (Pasteur 1173 P2) [12].
3 Immunogenicity of BCG
BCG is a whole cell, live-attenuated vaccine and therefore contains a wide variety of mycobacterial antigens, including proteins, lipids and polysaccharides, capable of stimulating an array of immune responses.
3.1 Innate Immune Responses
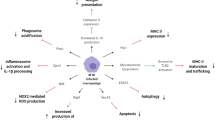
Following initial intradermal inoculation, host epidermal macrophages detect BCG via a number of pattern-recognition receptors (PRRs), including Toll-like receptors 2 and 4 (TLR2, TLR4) and the nucleotide-binding oligomerisation domain 2 (NOD2) receptor [20, 21]. These interact with a variety of BCG pathogen-associated molecular patterns (PAMPs) including mycobacterial cell wall components such as peptidoglycan, mycolic acids and mannosylated lipoarabinomannan [22, 23]. Activation of TLRs stimulates macrophage and dendritic cell (DC) maturation and the secretion of proinflammatory cytokines. Resident DCs phagocytose BCG and migrate to local draining lymph nodes to present BCG-derived antigens to CD4+ T cells and thus initiate adaptive immune responses [24].
Punch biopsies of healthy adult volunteers following intradermal BCG injection have shown that live BCG persists at the site of inoculation for up to 4 weeks. Analysis of the early inflammatory milieu via creation of suction blisters demonstrates the predominant cell type to be CD15+ neutrophils [25]. BCG-infected neutrophils exhibit synergistic co-operation with DCs to stimulate antigen-specific T-cell responses [26].
3.2 Adaptive Immune Response
Effective cellular immunity is known to be critical for adequate immunological control of M. tb, with those lacking key elements of T-cell immunity, for example, people living with HIV, at higher risk of TB disease [27]. BCG vaccination typically induces a T-helper type 1 (Th1)-dominated response, as characterised by production of cytokines such as tumour necrosis factor α (TNFα), interferon γ (IFNγ) and interleukin-2 (IL-2) by CD4+ T cells [28, 29]. BCG vaccination also induces modest increased expression of the cytotoxic markers, granulysin and perforin, by CD8+ T cells [30].
Classically, the immune systems of infants demonstrate a bias towards Th2 regulatory responses following microbial exposure. Despite this, BCG has been shown to induce strong Th1 responses in infants [31]. This may, however, vary by geographical location, with UK infants exhibiting predominantly Th1-driven responses as compared to more Th2 polarised responses elicited in Malawian infants [32]. This observation was not repeated in a more recent study comparing Ugandan and UK infants, with Th1 responses predominating in both cohorts. In this study, no differences were seen in antigen-specific responses to purified protein derivative (PPD), regardless of maternal or infant mycobacterial exposure status [33]. Any true differences in immune responses to BCG vaccination and subsequent efficacy between infant cohorts are likely to be due to other causes, and further work will be needed to elicit these.
The humoral immune system has historically been considered of limited importance in protection against M. tb, but new evidence is emerging that may challenge this view [34]. Highly exposed household contacts of active TB patients who remain tuberculin skin test (TST) and IFNγ release assay (IGRA) negative, so-called resistors, have been shown to possess functionally distinct M. tb-specific antibodies [35]. Increased levels of antigen-specific IgG antibodies and long-lived memory B cells are induced following BCG vaccination and may result in opsonisation and subsequent enhanced phagocytosis of mycobacteria [34, 36].
3.3 Trained Immunity
Whilst immunological memory is classically thought of as the hallmark of the adaptive immune system, innate immune cells can be modified (or “reprogrammed”) to elicit enhanced responses to subsequent homologous and heterologous stimuli, in a type of non-specific immune memory termed trained immunity. Epigenetic changes, such as histone modification and alterations in DNA methylation, are the main drivers of trained immunity [37]. Induction of distinct epigenetic and metabolic modifications in a variety of immune cells may explain the observed effects that BCG exerts on heterologous infections [38].
BCG has been shown to induce epigenetic reprogramming of monocytes, resulting in increased proinflammatory cytokine production in response to unrelated pathogens for at least 3 months post-vaccination [21]. In a mouse model of severe combined immunodeficiency, BCG vaccination leads to increased survival in mice following lethal challenge with Candida albicans, in part mediated by natural killer (NK) cells [39]. BCG induces trained immunity in human NK cells, with increases in proinflammatory cytokine production seen on re-stimulation with mycobacteria as well as unrelated bacterial and fungal pathogens [40].
Until recently, it was unclear what mechanisms underpinned the longevity of these trained immune responses seen in monocytes following BCG, despite the short life span of myeloid cells within the peripheral circulation. It has now been shown that BCG vaccination in humans induces a transcriptional shift within the haematopoietic stem cell compartment of the bone marrow, and this results in persistent epigenetic changes in peripheral monocytes for at least 3 months [41].
4 Efficacy of BCG Against TB
BCG vaccination is provided by 153 countries as part of their standard childhood vaccination programmes, with coverage exceeding 90% in around two thirds of these. Despite this, an estimated ten million new cases of TB disease occurred worldwide in 2020, resulting in around 1.5 million deaths [42]. This highlights the fact that good BCG vaccine coverage is clearly not sufficient to control the current TB pandemic.
Neonatally administered BCG has consistently been shown to offer good protection against TB meningitis and disseminated (miliary) TB in childhood [43, 44]. However, BCG efficacy against the most common form of TB disease, pulmonary TB, is highly variable [45, 46]. In the UK Medical Research Council (MRC) trial in the 1950s, BCG was shown to be over 70% effective in adolescents [47]. In contrast, little or no efficacy in any age group was seen in three large, randomised controlled trials (RCTs) in South India (the Chingleput trial), Brazil and Malawi [48,49,50]. Limited data on the duration of protection exists, although in populations where BCG does show a protective effect, this can be highly durable, lasting for at least 15 years in the UK MRC trial and up to 60 years in native Alaskans [51].
There is now evidence that BCG can protect against M. tb infection, as well as active TB disease, in some settings. This was previously impossible to determine, due to limitations in the tuberculin skin test (TST) as a diagnostic tool, with a positive TST seen due to mycobacterial infection and/or BCG vaccination. T cell-based IGRAs are unaffected by BCG status and therefore allow investigation of the effect that BCG may have on infection. Studies from outbreak settings have shown that BCG vaccination is associated with a reduction in risk of M. tb infection, as defined by positive IGRA, as well as lower rates of TB disease [52, 53].
4.1 Reasons for Variable BCG Efficacy
The underlying reasons for the wide variation in the protection afforded by BCG are still not fully understood (Table 8.1). Several potential explanations have been proposed. Strikingly, BCG efficacy seems to vary by latitude, with the observed protective effect decreasing nearer to the equator [54]. The most widely accepted hypothesis for this discrepancy relates to differences in exposure to non-tuberculous mycobacteria (NTM). Populations living in more tropical climates closer to the equator, particularly in rural areas, are likely to have experienced greater levels of NTM exposure, and this prior mycobacterial sensitisation may limit the protective effect of BCG. Two theories exist to explain this. The blocking hypothesis holds that immune responses elicited by prior NTM exposure prevent protective BCG vaccine effects from developing, potentially by preventing replication of live BCG at the site of inoculation [55, 56]. The masking hypothesis suggests that prior NTM sensitisation itself confers a level of protection against subsequent M. tb exposure that BCG is not able to improve upon [57]. BCG effectiveness in trials is increased if tuberculin skin test-positive individuals (a marker of prior mycobacterial sensitisation) are stringently excluded [45].
Co-infection with any of a variety of helminth species, such as Ascaris (Ascaris lumbricoides), whipworm (Trichuris trichiura) and hookworms (including Ancylostoma duodenale), prevalent in tropical regions, may be associated with reduced immunogenicity of BCG [46, 58]. Viral infections, such as cytomegalovirus (CMV) in the neonatal period may impair the development of BCG-specific immune responses [59].
Other proposed, but less well accepted, explanations for efficacy variations include underlying genetic or microbiome differences between host populations, variability in nutritional status, exposure to ultraviolet light and vitamin D levels, as well as differences in virulence levels of circulating M. tb strains [9, 60, 61]. Timing of BCG vaccination and circadian rhythms may have an impact on immunological responses, potentially influenced by circadian oscillations in macrophage and leukocyte functions. Morning BCG vaccination has been shown to induce stronger trained and adaptive immune responses in humans compared to evening vaccination, with early morning vaccination resulting in the highest levels of cytokine production [62].
Clear variations in both genotype and phenotype exist between different strains of BCG and in vitro immunological responses have been shown to differ between strains [63]. Despite these clear differences and long held assumptions that this may impact on vaccine efficacy, there is no consistent evidence that efficacy does differ significantly between strains [45].
4.2 BCG Revaccination
It remains unclear whether revaccination with BCG can improve protection. Several studies have previously found that additional doses of BCG do not increase efficacy or duration of protection against TB disease. In the late 1980s, the Karonga Prevention Trial randomised nearly 50,000 individuals across a range of ages in rural Malawi with a visible BCG scar to receive a second dose of intradermal BCG or placebo. The latest 30-year follow-up has confirmed the original study findings that BCG revaccination does not provide additional protection against TB disease in this population. However, subgroup analysis suggests there may be modest benefit in those who are HIV-negative, particularly if the second vaccination occurred in childhood [50, 64].
In the BCG-REVAC study conducted in Brazil, no additional protection was seen from BCG revaccination offered to school aged children. Extended follow-up suggests that a second BCG dose could however offer increased protection in regions with expected lower prevalence of NTM exposure and with earlier age at revaccination [49, 65].
Studies looking at BCG revaccination have generally focused on TB disease as their primary end point. A recent RCT conducted in M. tb uninfected healthy South African adolescents has shown BCG revaccination to have a modest protective effect against sustained M. tb infection. The trial compared the ability of a novel protein-adjuvant subunit vaccine AERAS-404 (comprised of the H4 antigen and IC31 adjuvant) or BCG revaccination to prevent M. tb infection, as defined by positive M. tb-specific IGRA. Whilst neither intervention reached statistical significance for the primary end-point of preventing new initial M. tb infection, BCG revaccination provided statistically significant vaccine efficacy of 45% (p = 0.03) in reducing sustained IGRA conversion (believed to indicate established M. tb infection) [66]. A larger confirmatory trial of these findings is underway (clinicaltrials.gov NCT04152161). If this supports the initial findings, then BCG revaccination could represent a readily available, safe and cost-effective public health intervention to protect selected high-risk populations [66].
4.3 Measuring BCG Protection?
For most licensed vaccines in use today, validated immunological surrogate markers of clinical protection (termed correlates of protection) exist, for example, levels of vaccine-induced antibodies. No such marker exists for BCG, and whilst it is well established that CD4+ T cells and key cytokines including TNFα and IFNγ are essential for controlling mycobacterial infection, it has not been shown that vaccine-induced increases in these immune responses correlate with increased protection [67]. Presence of a visible BCG vaccination scar has historically been taken to represent appropriate vaccine “take”, and in some countries BCG revaccination was routinely recommended for those who did not develop a visible vaccine scar [9].
The proportion of infants undergoing BCG vaccination who go on to develop a visible scar at the sight of inoculation varies in different studies from as low as 52% and as high as 97%, with the 80–90% range most commonly seen [68, 69]. Scar formation may be influenced by a wide variety of factors including training level of the provider and inoculation technique (in particular size of post-vaccination wheal), with scarring more likely to result from intradermal rather than subcutaneous vaccination [70]. Strain of BCG utilised may also be a factor, with BCG-Denmark shown to induce a higher proportion of BCG scars than BCG-Russia [71, 72].
BCG scarring does not appear to correlate in any meaningful way with evidence of BCG efficacy against TB. There is no association between scar size and protection against either TB or leprosy [73]. However, presence of a BCG scar may be associated with increased overall survival in high mortality settings [68]. In West Africa, BCG-vaccinated children with a visible BCG scar at 6 months of age had lower all-cause mortality in the following 12 months than those who did not develop a scar [74].
5 Efficacy of BCG Against Other Infections
5.1 BCG and Other Mycobacterial Infections
Multiple studies have shown that BCG provides greater protection against leprosy, caused by infection with Mycobacterium leprae, than it does against TB. Protection is afforded against both the tuberculoid and more severe lepromatous forms of the disease, with estimates of efficacy ranging from 20–80% [50, 75, 76]. BCG may also offer some protection against infection with Mycobacterium ulcerans, which causes Buruli ulcer disease [77], although this is not consistently seen across all studies [78].
5.2 BCG “Non-Specific” Immunity
In mouse models, BCG has been found to offer protection against a broad range of non-related infections including systemic candidiasis, disseminated schistosomiasis and listeriosis [39, 79, 80]. In humans, BCG vaccination of healthy volunteers leads to an increased production of monocyte-derived proinflammatory cytokines, including TNF-α and IL-1β, not only in response to mycobacterial stimuli but also to heterologous pathogens including Candida albicans and Staphylococcus aureus [21]. BCG has also been shown to protect against an experimental human viral infection, with reduced yellow fever vaccine viremia following BCG vaccination in healthy volunteers seen in correlation with an upregulation of IL-1β, a mediator of trained immunity [81].
Albert Calmette himself noted that, epidemiologically, BCG appeared to reduce childhood mortality to a greater extent than would have been anticipated by the effect on TB disease alone [3]. Observational studies have shown that BCG vaccination reduces all-cause infant mortality across a variety of settings [82, 83]. Combined analysis of three RCTs conducted in West Africa showed that early BCG vaccination of low birthweight infants resulted in a reduction in all-cause mortality by 38% within the neonatal period and by 16% within the first year of life, with effects mainly attributable to reduced deaths from sepsis and respiratory tract infections [84]. However, the same effect was not seen in studies of children in Greenland or Denmark, where BCG vaccination resulted in no detectable reduction in morbidity from infectious diseases other than TB [85, 86]. A systematic review across a variety of settings found that BCG vaccination appeared to be associated with reduction in all-cause mortality, but the authors noted a high risk of bias in several of the published studies [83].
A more recent study conducted in Uganda expands upon these findings. Healthy infants of varying birthweights were randomly assigned to receive BCG on day of birth or at 6 weeks of age and followed up until 10 weeks of age, with investigators and clinicians blinded to allocation. Rates of physician-diagnosed non-tuberculous infectious disease were significantly lower in the early BCG versus delayed vaccination group in the first 6 weeks of life (98 presentations verses 129 presentations, respectively, hazard ratio of 0.71 [95% CI 0.53–0.95], p = 0.23). No difference between the groups was observed after the delayed group had also received BCG. Epigenetic differences in histone trimethylation at the TNF promotor region in peripheral blood mononuclear cells between the groups in the first 6 weeks of life provide evidence that induction of trained immunity may underpin the observed differences in infectious morbidity [87].
At the opposite end of the age spectrum, a recent double-blind RCT has shown that vaccination of elderly patients (aged 65 years and older) with BCG also results in a reduction in infections of the respiratory tract, in the year following BCG vaccination [88].
5.3 BCG and COVID-19 Disease
From the early stages of the coronavirus 2019 (COVID-19) pandemic, BCG was postulated as a possible tool in the fight against the disease. Background rationale included the known potential of BCG to boost trained immunity against heterologous infections, coupled with initial epidemiological data that appeared to suggest less severe COVID-19 outbreaks were seen in countries with a universal BCG vaccination policy [89, 90]. However, as more data has accrued, subsequent analysis with correction for confounding variables including large disparities in COVID-19 testing rates has shown no convincing epidemiological correlation between BCG vaccination policy and COVID-19 spread [91]. Multiple clinical trials have been registered looking at the question of BCG for protection against COVID-19 disease, including the BRACE trial of 10,000 healthcare workers across 5 countries (clinicaltrials.gov NCT04327206) [92]. Results are currently awaited from these studies.
6 BCG and Non-Communicable Diseases
6.1 BCG and Cancer
In 1929, a study carried out at the Johns Hopkins Hospital found a correlation between TB and a lower incidence of cancer at postmortem [93]. This led to the first notion that BCG might be harnessed as a cancer treatment. In 1935, Holmgren utilised intravenous BCG injection as a putative treatment for stomach cancers [94], but around this time, enthusiasm for BCG was severely dampened by the Lübeck disaster and subsequent concerns about BCG safety (see Sect. 8.1). Seminal studies in the 1950s demonstrated clear evidence of BCG-mediated inhibition of cancer tumorigenesis in mice, via the activation of the reticuloendothelial system [95].
Efforts were rekindled to utilise the potential of BCG immunotherapy against cancer in the 1960s and 1970s, with studies showing successful regression of melanoma metastatic to the bladder following intralesional BCG injection [96]. Today, BCG anticancer immunotherapy is most widely used against bladder cancer, with intravesical BCG utilised as a successful treatment for high-grade non-muscle-invasive bladder cancer and carcinoma in situ for many years [97,98,99].
Several other potential uses of BCG within oncology remain under investigation. BCG is utilised as an immunomodulatory adjuvant in a personalised anti-tumour vaccine undergoing phase III clinical testing against colon cancer (clinicaltrials.gov NCT02448173 [100]). Intra-pleural administration of BCG has been associated with possible survival benefit in several lung cancer studies, although high-quality evidence is lacking, and its potential role as an immunotherapeutic agent in malignant pleural mesothelioma is being investigated [101, 102].
As well as immunotherapy of established cancer, BCG may play a role in cancer prevention in some populations. In secondary analysis of long-term follow-up from a historical BCG vaccination trial, a single dose of intradermal BCG in childhood has been shown to be associated with a reduced incidence of lung cancer in American Indian and Alaskan Natives [103].
6.2 BCG and Allergic, Autoimmune and Inflammatory Diseases
Atopic and allergic asthma phenotypes are characterised by an inappropriate imbalance of Th1 verses Th2 immunological responses, with Th2 polarisation and elevated levels of IgE, IL-4 and IL-5 seen. BCG is known to typically induce strong Th1 responses and has been shown in mice to suppress allergen-induced airway inflammation [104]. In a small randomised controlled study, percutaneous BCG vaccination of adults with moderate to severe asthma resulted in improved lung function and reduced medication use. This was associated with suppression of Th2-type cytokine responses in sputum [105]. However, observational studies looking at BCG vaccination and effect on atopy and asthma in humans from a wide range of settings have shown conflicting and inconsistent results [106, 107].
Studies in mice have shown beneficial effects of BCG against a range of other inflammatory and autoimmune conditions, including type 1 diabetes mellitus (T1DM) [108] and multiple sclerosis (MS). In a recent randomised controlled study of adult patients with established T1DM, two doses of BCG have been shown to reduce haemoglobin A1c (HbA1c) levels, a marker of long-term blood sugar control, to near normal levels for at least 5 years. Possible mechanisms for this observed effect include alteration in systemic glucose transport and epigenetic reprogramming of aberrant T regulatory (Treg) cells [109]. A larger, phase II study further exploring the effects of repeated BCG vaccination in T1DM is ongoing (clinicaltrials.gov NCT02081326).
MS is a chronic, neurodegenerative disease characterised by autoimmune central nervous system (CNS) demyelination. Intracutaneous BCG has been shown to reduce active CNS lesions on magnetic resonance scans in a small study of patients with relapsing and remitting MS [110] and to result in a decreased risk of progression to MS following a solitary first demyelinating episode [111]. Studies have suggested that BCG may also play a role in other neuroinflammatory conditions. In a mouse model of Alzheimer’s disease (AD), BCG vaccination reduces neuroinflammation and reverses cognitive behavioural decline [112]. Human observational studies have shown that bladder cancer patients treated with BCG were significantly less likely to develop AD at any age than those who did not receive BCG [113, 114]. The mechanisms underlying the effects of BCG on neuroinflammation remain unknown and further research in this area is needed.
7 Safety and Adverse Effects of BCG
BCG is one of the most widely used vaccines in the world, with around 100 million children newly vaccinated each year [42]. Although considered to be a very safe vaccine, mild local and systemic side effects immediately after vaccination (so-called reactogenicity) are commonly seen, with BCG amongst the most reactogenic vaccines in use today. Reactogenicity is influenced by BCG strain (likely due to differences in residual virulence as a result of genetic variability between strains) with Danish and Pasteur known to be more reactogenic than Tokyo or Glaxo strains [115]. BCG is the only vaccine in modern usage that routinely induces local ulceration and heals with scar formation.
The recommended route of BCG vaccination is via intradermal injection. Following inoculation, a small area of erythema develops, with a raised papule seen several weeks later. Associated mild swelling of ipsilateral axillary lymph nodes may occur. Papule formation is followed by shallow ulceration and healing with scar formation. This may take as long at 3 months in infants. Inadvertent injection into the subcutaneous or intramuscular layers can increase the risk of more severe localised reactions, including local abscess formation and discharging infection of regional draining lymph nodes (suppurative lymphadenitis) [115, 116].
Serious adverse reactions following BCG vaccination are rare, affecting less than 1 in 200,000 individuals [9]. However, more severe local and disseminated side effects may be seen, in particular in immunocompromised infants (Table 8.2). This may be due to primary genetic disorders of the immune system, such as severe combined immunodeficiency (SCID) and chronic granulomatous disease (CGD), or more commonly acquired immunodeficiency, in particular HIV [117].
Spread of BCG infection to distant sites signifies the most serious BCG adverse reactions. BCG infections of the liver, lungs and bones (osteitis and osteomyelitis) have all been documented. Whilst they are most common in immunocompromised individuals, BCG osteitis has been documented in seemingly immunocompetent individuals. Disseminated BCG disease, termed BCGosis, represents haematogenous spread to more than one distant site. Diagnosis can be challenging, and molecular diagnostics are required to distinguish BCG from other mycobacterial infections including TB [118].
Due to the risks of disseminated infection, the WHO recommends BCG should not be given to HIV-infected infants. However, significant improvements in HIV treatment mean that most mothers living with HIV will be taking antiretroviral treatment, infants provided with prophylactic treatment and vertical transmission is near eliminated. Therefore, pragmatically many infants are vaccinated without waiting for HIV testing.
Following intravesical installation of BCG for bladder cancer, systemic symptoms including general malaise and fever are common and affect around a third of patients. Localised bladder irritation with cystitis is seen in over 60% of patients, with visible haematuria in around 20% [119]. Severe side effects are uncommon but may be difficult to diagnose as they can occur at distant sites and be temporally removed from BCG installation. BCG prostatitis, nephritis, osteomyelitis, mycobacterial pneumonia, infection of prosthetic valves and joints and disseminated BCG infections with septicaemia have all been described. Treatment cessation is required in around 8% of patients due to side effects [120].
8 BCG Future Directions
8.1 Recombinant BCG
Despite extensive study, we still do not fully understand the full range of effects that BCG exerts against a variety of infectious and non-infectious diseases. Targeted genetic modification of BCG may allow retention of its positive non-specific effects, whilst aiming to increase the protection it affords against M. tb.
VPM1002 (Vakzine Projekt Management, Serum Institute of India) is a novel, genetically modified live vaccine, formed by recombination of BCG with genes from Listeria monocytogenes that confer phagosomal disruption properties. This aims to improve access of VPM1002 mycobacterial antigens to the host cell cytosol and enhance presentation to T cells via MHC class I molecules. In phase I and II trials, VPM1002 has been shown to be safe and immunogenic, inducing responses in CD4+ and CD8+ T-cell populations thought to be necessary for protection against M. tb. A phase II/III trial is ongoing in India, assessing prevention of relapse following VPM1002 vaccination in recently treated TB patients (clinicaltrial.gov identifier NCT03152903). Preliminary efficacy results are awaited [121, 122].
8.2 Route of Administration
BCG was initially given as an oral vaccine. Use of parental vaccination was pioneered in Norway, and the intradermal route became the most widely used in most countries. In Brazil, oral vaccination with high doses of BCG Moreau was routinely employed until as late as 1974 [10]. Recently, increased interest has focused on whether changing the route of BCG administration might improve levels of protection.
The natural route of M. tb infection is via inhalation of infectious aerosol droplets into the lungs. Delivering a vaccine by aerosol inhalation would allow alignment of the route of vaccination with the route of infection, with potential advantages anticipated particularly in improved local immunogenicity. In non-human primates, local pulmonary mucosal BCG delivery affords greater protection against subsequent M. tb challenge than standard intradermal vaccination and may be the result of superior induction of polyfunctional T-helper 17 cells and increased IgA levels [123]. Clinical studies are ongoing looking at pulmonary and systemic immunological responses following aerosol BCG inhalation in healthy adult volunteers (clinicaltrials.gov NCT02709278, NCT03912207).
Animal models have shown that intravenous (IV) BCG may afford significantly greater protection than vaccination via other routes. Interest in BCG given by the IV route is not new [124] but has been rekindled in recent years. In a study of rhesus macaques, IV BCG resulted in significantly greater protection against subsequent M. tb challenge then either intradermal injection alone or intradermal injection followed by intratracheal boost [125]. A larger study, also in rhesus macaques, showed nine out of ten animals vaccinated with IV BCG were highly protected against M. tb challenge at 6 months. No evidence of established M. tb infection, so-called sterilising immunity, was seen in six out of ten animals. This unexpected result correlated with the expansion of activated mycobacteria-specific lung tissue resident T cells in IV vaccinated animals [126].
Whilst IV BCG is unlikely to represent a widely deployable vaccine strategy in humans, particularly neonates in low- and middle-income countries, the ability to induce sterilising immunity in the non-human primate model paves the way for ongoing interrogation of the immunological mechanisms underpinning these findings. This may in turn advance the search for the illusive immune correlates of protection against TB. If identified, efforts could be focused on developing a vaccine designed to trigger the same protective mechanisms but without the need to be administered intravenously.
9 Conclusions
Despite over a century of use and associated research, BCG continues to be controversial. Outside of its established efficacy against infant disseminated TB, uncertainties remain regarding its ability to protect against TB disease more broadly. New evidence suggests it may yet afford some protection against M. tb infection, but further research is needed to confirm these findings in a range of settings.
BCG is known to exert non-specific effects that may impact a range of disease processes. Knowledge of the immunological mechanisms underpinning these is increasing, but we are still far from fully understanding the wide range of potential effects BCG may induce. Current research looking at BCG revaccination, alternative routes of delivery and recombinant BCG vaccines may provide additional clues as to the best way to harness its potential benefits. Furthering our understanding of this complex vaccine remains important, both in terms of optimising its own use and in its role as a benchmark against which new TB vaccines are measured.
References
Luca S, Mihaescu T. History of BCG vaccine. Maedica (Bucur). 2013;8(1):53–8.
Sakula A. BCG: who were calmette and guérin? Thorax. 1983;38(11):806–12. https://doi.org/10.1136/thx.38.11.806.
Calmette A. Preventive vaccination against tuberculosis with BCG. Proc R Soc Med. 1931;24(11):1481–90.
Calmette A. L’infection bacillaire et la tuberculose chez l’homme et chez les animaux: processus d’infection, et de defense, étude biologique et expérimentale. Masson; 1922.
Wallgren A. BCG inoculation and BCG vaccination. Am J Dis Child. 1948;76(5):485–91. https://doi.org/10.1001/archpedi.1948.02030030498001.
Greenwood M. Professor Calmette’s statistical study of B.C.G vaccination. Br Med J. 1928;1(3514):793–5. https://doi.org/10.1136/bmj.1.3514.793.
Petroff SA, Branch A Jr, WS. A study of bacillus Calmette-Guérin (BCG). Am Rev Tuberc. 1929;19(1):9–46. https://doi.org/10.1164/art.1929.19.1.9.
Fox GJ, Orlova M, Schurr E. Tuberculosis in newborns: the lessons of the “Lübeck disaster” (1929-1933). PLoS Pathog. 2016;12(1):e1005271. https://doi.org/10.1371/journal.ppat.1005271.
Fine PEM, Carneiro IAM, Milstien JB, Clements CJ, World Health Organization. Issues relating to the use of BCG in immunization programmes: a discussion document. Geneva: World Health Organization; 1999.
www.bcgatlas.org. 2021. Accessed 22 Oct 2021.
Brosch R, Gordon SV, Garnier T, Eiglmeier K, Frigui W, Valenti P, et al. Genome plasticity of BCG and impact on vaccine efficacy. Proc Natl Acad Sci. 2007;104(13):5596–601. https://doi.org/10.1073/pnas.0700869104.
Abdallah AM, Hill-Cawthorne GA, Otto TD, Coll F, Guerra-Assunção JA, Gao G, et al. Genomic expression catalogue of a global collection of BCG vaccine strains show evidence for highly diverged metabolic and cell-wall adaptations. Sci Rep. 2015;5:15443. https://doi.org/10.1038/srep15443.
Behr MA, Wilson MA, Gill WP, Salamon H, Schoolnik GK, Rane S, et al. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science. 1999;284(5419):1520–3. https://doi.org/10.1126/science.284.5419.1520.
Mostowy S, Tsolaki AG, Small PM, Behr MA. The in vitro evolution of BCG vaccines. Vaccine. 2003;21(27):4270–4. https://doi.org/10.1016/S0264-410X(03)00484-5.
Lewis KN, Liao R, Guinn KM, Hickey MJ, Smith S, Behr MA, et al. Deletion of RD1 from Mycobacterium tuberculosis mimics bacille Calmette-Guérin attenuation. J Infect Dis. 2003;187(1):117–23. https://doi.org/10.1086/345862.
Houben D, Demangel C, van Ingen J, Perez J, Baldeón L, Abdallah AM, et al. ESX-1-mediated translocation to the cytosol controls virulence of mycobacteria. Cell Microbiol. 2012;14(8):1287–98. https://doi.org/10.1111/j.1462-5822.2012.01799.x.
Pym AS, Brodin P, Brosch R, Huerre M, Cole ST. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol Microbiol. 2002;46(3):709–17. https://doi.org/10.1046/j.1365-2958.2002.03237.x.
Kozak RA, Alexander DC, Liao R, Sherman DR, Behr MA. Region of difference 2 contributes to virulence of Mycobacterium tuberculosis. Infect Immun. 2011;79(1):59–66. https://doi.org/10.1128/iai.00824-10.
Charlet D, Mostowy S, Alexander D, Sit L, Wiker HG, Behr MA. Reduced expression of antigenic proteins MPB70 and MPB83 in Mycobacterium bovis BCG strains due to a start codon mutation in sigK. Mol Microbiol. 2005;56(5):1302–13. https://doi.org/10.1111/j.1365-2958.2005.04618.x.
Heldwein KA, Liang MD, Andresen TK, Thomas KE, Marty AM, Cuesta N, et al. TLR2 and TLR4 serve distinct roles in the host immune response against Mycobacterium bovis BCG. J Leukoc Biol. 2003;74(2):277–86. https://doi.org/10.1189/jlb.0103026.
Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Ifrim DC, Saeed S, et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A. 2012;109(43):17537–42. https://doi.org/10.1073/pnas.1202870109.
Kaufmann SHE, Tsuji S, Matsumoto M, Takeuchi O, Akira S, Azuma I, et al. Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guerin: involvement of toll-like receptors. Infect Immun. 2000;68(12):6883–90. https://doi.org/10.1128/IAI.68.12.6883-6890.2000.
Tsuji S, Matsumoto M, Takeuchi O, Akira S, Azuma I, Hayashi A, et al. Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guérin: involvement of toll-like receptors. Infect Immun. 2000;68(12):6883–90. https://doi.org/10.1128/iai.68.12.6883-6890.2000.
Moliva JI, Turner J, Torrelles JB. Immune responses to bacillus Calmette–Guérin vaccination: why do they fail to protect against Mycobacterium tuberculosis? Front Immunol. 2017;8:407. https://doi.org/10.3389/fimmu.2017.00407.
Minassian AM, Satti I, Poulton ID, Meyer J, Hill AV, McShane H. A human challenge model for Mycobacterium tuberculosis using Mycobacterium bovis bacille Calmette-Guerin. J Infect Dis. 2012;205(7):1035–42. https://doi.org/10.1093/infdis/jis012.
Morel C, Badell E, Abadie V, Robledo M, Setterblad N, Gluckman JC, et al. Mycobacterium bovis BCG-infected neutrophils and dendritic cells cooperate to induce specific T cell responses in humans and mice. Eur J Immunol. 2008;38(2):437–47. https://doi.org/10.1002/eji.200737905.
Geldmacher C, Zumla A, Hoelscher M. Interaction between HIV and Mycobacterium tuberculosis: HIV-1-induced CD4 T-cell depletion and the development of active tuberculosis. Curr Opin HIV AIDS. 2012;7(3):268–75. https://doi.org/10.1097/COH.0b013e3283524e32.
Soares AP, Scriba TJ, Joseph S, Harbacheuski R, Murray RA, Gelderbloem SJ, et al. Bacillus Calmette-Guérin vaccination of human newborns induces T cells with complex cytokine and phenotypic profiles. J Immunol. 2008;180(5):3569–77. https://doi.org/10.4049/jimmunol.180.5.3569.
Smith SM, Malin AS, Pauline T, Lukey, Atkinson SE, Content J, et al. Characterization of human Mycobacterium bovis bacille Calmette-Guérin-reactive CD8+ T cells. Infect Immun. 1999;67(10):5223–30. https://doi.org/10.1128/iai.67.10.5223-5230.1999.
Semple PL, Watkins M, Davids V, Krensky AM, Hanekom WA, Kaplan G, et al. Induction of granulysin and perforin cytolytic mediator expression in 10-week-old infants vaccinated with BCG at birth. Clin Dev Immunol. 2011;2011:438463. https://doi.org/10.1155/2011/438463.
Marchant A, Goetghebuer T, Ota MO, Wolfe I, Ceesay SJ, De Groote D, et al. Newborns develop a Th1-type immune response to Mycobacterium bovis bacillus Calmette-Guérin vaccination. J Immunol. 1999;163(4):2249–55.
Lalor MK, Floyd S, Gorak-Stolinska P, Ben-Smith A, Weir RE, Smith SG, et al. BCG vaccination induces different cytokine profiles following infant BCG vaccination in the UK and Malawi. J Infect Dis. 2011;204(7):1075–85. https://doi.org/10.1093/infdis/jir515.
Mawa PA, Hasso-Agopsowicz M, Lubyayi L, Nabakooza G, Nakibuule M, Blitz R, et al. Immune responses following BCG immunization of infants in Uganda and United Kingdom are similar for purified protein derivative but differ for secretory proteins of Mycobacterium tuberculosis. Front Immunol. 2021;12:637114. https://doi.org/10.3389/fimmu.2021.637114.
Tanner R, Villarreal-Ramos B, Vordermeier HM, McShane H. The humoral immune response to BCG vaccination. Front Immunol. 2019;10:1317. https://doi.org/10.3389/fimmu.2019.01317.
Lu LL, Smith MT, Yu KKQ, Luedemann C, Suscovich TJ, Grace PS, et al. IFN-γ-independent immune markers of Mycobacterium tuberculosis exposure. Nat Med. 2019;25(6):977–87. https://doi.org/10.1038/s41591-019-0441-3.
Chen T, Blanc C, Eder AZ, Prados-Rosales R, Souza AC, Kim RS, et al. Association of human antibodies to arabinomannan with enhanced mycobacterial opsonophagocytosis and intracellular growth reduction. J Infect Dis. 2016;214(2):300–10. https://doi.org/10.1093/infdis/jiw141.
Netea MG, Joosten LAB, Latz E, Mills KHG, Natoli G, Stunnenberg HG, et al. Trained immunity: a program of innate immune memory in health and disease. Science. 2016;352(6284):aaf1098. https://doi.org/10.1126/science.aaf1098.
Covián C, Fernández-Fierro A, Retamal-Díaz A, Díaz FE, Vasquez AE, Lay MK, et al. BCG-induced cross-protection and development of trained immunity: implication for vaccine design. Front Immunol. 2019;10:2806. https://doi.org/10.3389/fimmu.2019.02806.
Kleinnijenhuis J, Quintin J, Preijers F, Joosten LAB, Ifrim DC, Saeed S, et al. Bacille Calmette-Guérin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci. 2012;109(43):17537–42. https://doi.org/10.1073/pnas.1202870109.
Kleinnijenhuis J, Quintin J, Preijers F, Joosten LAB, Jacobs C, Xavier RJ, et al. BCG-induced trained immunity in NK cells: role for non-specific protection to infection. Clin Immunol. 2014;155(2):213–9. https://doi.org/10.1016/j.clim.2014.10.005.
Cirovic B, de Bree LCJ, Groh L, Blok BA, Chan J, van der Velden W, et al. BCG vaccination in humans elicits trained immunity via the hematopoietic progenitor compartment. Cell Host Microbe. 2020;28(2):322–34.e5. https://doi.org/10.1016/j.chom.2020.05.014.
WHO: World Health Organisation: Global Tuberculosis Report 2020. 2020. Accessed 18 Aug 2021.
Rodrigues LC, Diwan VK, Wheeler JG. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int J Epidemiol. 1993;22(6):1154–8. https://doi.org/10.1093/ije/22.6.1154.
Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367(9517):1173–80. https://doi.org/10.1016/s0140-6736(06)68507-3.
Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PE, et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis. 2014;58(4):470–80. https://doi.org/10.1093/cid/cit790.
Abubakar I, Pimpin L, Ariti C, Beynon R, Mangtani P, Sterne JA, et al. Systematic review and meta-analysis of the current evidence on the duration of protection by bacillus Calmette-Guérin vaccination against tuberculosis. Health Technol Assess. 2013;17(37):1–372, v-vi. https://doi.org/10.3310/hta17370.
Hart PD, Sutherland I. BCG and vole bacillus vaccines in the prevention of tuberculosis in adolescence and early adult life. Br Med J. 1977;2(6082):293–5. https://doi.org/10.1136/bmj.2.6082.293.
Anon. Trial of BCG vaccines in south India for tuberculosis prevention: first report—tuberculosis prevention trial. Bull World Health Organ. 1979;57(5):819–27.
Rodrigues LC, Pereira SM, Cunha SS, Genser B, Ichihara MY, de Brito SC, et al. Effect of BCG revaccination on incidence of tuberculosis in school-aged children in Brazil: the BCG-REVAC cluster-randomised trial. Lancet. 2005;366(9493):1290–5. https://doi.org/10.1016/s0140-6736(05)67145-0.
Karonga Prevention Trial Group. Randomised controlled trial of single BCG, repeated BCG, or combined BCG and killed mycobacterium leprae vaccine for prevention of leprosy and tuberculosis in Malawi. Lancet. 1996;348(9019):17–24.
Aronson NE, Santosham M, Comstock GW, Howard RS, Moulton LH, Rhoades ER, et al. Long-term efficacy of BCG vaccine in American Indians and Alaska Natives. A 60-year follow-up study. JAMA. 2004;291(17):2086–91. https://doi.org/10.1001/jama.291.17.2086.
Eisenhut M, Paranjothy S, Abubakar I, Bracebridge S, Lilley M, Mulla R, et al. BCG vaccination reduces risk of infection with Mycobacterium tuberculosis as detected by gamma interferon release assay. Vaccine. 2009;27(44):6116–20. https://doi.org/10.1016/j.vaccine.2009.08.031.
Roy A, Eisenhut M, Harris RJ, Rodrigues LC, Sridhar S, Habermann S, et al. Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: systematic review and meta-analysis. BMJ. 2014;349:g4643. https://doi.org/10.1136/bmj.g4643.
Wilson ME, Fineberg HV, Colditz GA. Geographic latitude and the efficacy of bacillus Calmette-Guérin vaccine. Clin Infect Dis. 1995;20(4):982–91. https://doi.org/10.1093/clinids/20.4.982.
Brandt L, Feino Cunha J, Weinreich Olsen A, Chilima B, Hirsch P, Appelberg R, et al. Failure of the Mycobacterium bovis BCG vaccine: some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infect Immun. 2002;70(2):672–8. https://doi.org/10.1128/iai.70.2.672-678.2002.
Arregui S, Sanz J, Marinova D, Martín C, Moreno Y. On the impact of masking and blocking hypotheses for measuring the efficacy of new tuberculosis vaccines. PeerJ. 2016;4:e1513. https://doi.org/10.7717/peerj.1513.
Palmer CE, Long MW. Effects of infection with atypical mycobacteria on BCG vaccination and tuberculosis. Am Rev Respir Dis. 1966;94(4):553–68. https://doi.org/10.1164/arrd.1966.94.4.553.
Cadmus SI, Akinseye VO, Taiwo BO, Pinelli EO, van Soolingen D, Rhodes SG. Interactions between helminths and tuberculosis infections: implications for tuberculosis diagnosis and vaccination in Africa. PLoS Negl Trop Dis. 2020;14(6):e0008069. https://doi.org/10.1371/journal.pntd.0008069.
Ben-Smith A, Gorak-Stolinska P, Floyd S, Weir RE, Lalor MK, Mvula H, et al. Differences between naive and memory T cell phenotype in Malawian and UK adolescents: a role for cytomegalovirus? BMC Infect Dis. 2008;8:139. https://doi.org/10.1186/1471-2334-8-139.
Lalor MK, Floyd S, Gorak-Stolinska P, Weir RE, Blitz R, Branson K, et al. BCG vaccination: a role for vitamin D? PLoS One. 2011;6(1):e16709. https://doi.org/10.1371/journal.pone.0016709.
Epstein PR. BCG vaccination and nutrition. Lancet. 1990;335(8704):1536–7. https://doi.org/10.1016/0140-6736(90)93087-6.
de Bree LCJ, Mourits VP, Koeken VACM, Moorlag SJCFM, Janssen R, Folkman L, et al. Circadian rhythm influences induction of trained immunity by BCG vaccination. J Clin Invest. 2020;130(10):5603–17. https://doi.org/10.1172/JCI133934.
Ritz N, Hanekom WA, Robins-Browne R, Britton WJ, Curtis N. Influence of BCG vaccine strain on the immune response and protection against tuberculosis. FEMS Microbiol Rev. 2008;32(5):821–41. https://doi.org/10.1111/j.1574-6976.2008.00118.x.
Glynn JR, Fielding K, Mzembe T, Sichali L, Banda L, McLean E, et al. BCG re-vaccination in Malawi: 30-year follow-up of a large, randomised, double-blind, placebo-controlled trial. Lancet Glob Health. 2021;9(10):e1451–e9. https://doi.org/10.1016/s2214-109x(21)00309-0.
Barreto ML, Pereira SM, Pilger D, Cruz AA, Cunha SS, Sant’Anna C, et al. Evidence of an effect of BCG revaccination on incidence of tuberculosis in school-aged children in Brazil: second report of the BCG-REVAC cluster-randomised trial. Vaccine. 2011;29(31):4875–7. https://doi.org/10.1016/j.vaccine.2011.05.023.
Nemes E, Geldenhuys H, Rozot V, Rutkowski KT, Ratangee F, Bilek N, et al. Prevention of M. tuberculosis Infection with H4:IC31 Vaccine or BCG revaccination. N Engl J Med. 2018;379(2):138–49.
Satti I, McShane H. Current approaches toward identifying a correlate of immune protection from tuberculosis. Expert Rev Vaccines. 2019;18(1):43–59. https://doi.org/10.1080/14760584.2019.1552140.
Benn CS, Roth A, Garly ML, Fisker AB, Schaltz-Buchholzer F, Timmermann A, et al. BCG scarring and improved child survival: a combined analysis of studies of BCG scarring. J Intern Med. 2020;288(6):614–24. https://doi.org/10.1111/joim.13084.
Rani SH, Vijayalakshmi V, Sunil K, Lakshmi KA, Suman LG, Murthy KJ. Cell mediated immunity in children with scar-failure following BCG vaccination. Indian Pediatr. 1998;35(2):123–7.
Roth A, Sodemann M, Jensen H, Poulsen A, Gustafson P, Gomes J, et al. Vaccination technique, PPD reaction and BCG scarring in a cohort of children born in Guinea-Bissau 2000-2002. Vaccine. 2005;23(30):3991–8. https://doi.org/10.1016/j.vaccine.2004.10.022.
Anderson EJ, Webb EL, Mawa PA, Kizza M, Lyadda N, Nampijja M, et al. The influence of BCG vaccine strain on mycobacteria-specific and non-specific immune responses in a prospective cohort of infants in Uganda. Vaccine. 2012;30(12):2083–9. https://doi.org/10.1016/j.vaccine.2012.01.053.
Frankel H, Byberg S, Bjerregaard-Andersen M, Martins CL, Aaby P, Benn CS, et al. Different effects of BCG strains—a natural experiment evaluating the impact of the Danish and the Russian BCG strains on morbidity and scar formation in Guinea-Bissau. Vaccine. 2016;34(38):4586–93. https://doi.org/10.1016/j.vaccine.2016.07.022.
Sterne JA, Fine PE, Pönnighaus JM, Sibanda F, Munthali M, Glynn JR. Does bacille Calmette-Guérin scar size have implications for protection against tuberculosis or leprosy? Tuber Lung Dis. 1996;77(2):117–23. https://doi.org/10.1016/s0962-8479(96)90025-8.
Garly M-L, Martins CL, Balé C, Baldé MA, Hedegaard KL, Gustafson P, et al. BCG scar and positive tuberculin reaction associated with reduced child mortality in West Africa: a non-specific beneficial effect of BCG? Vaccine. 2003;21(21):2782–90. https://doi.org/10.1016/S0264-410X(03)00181-6.
Stanley SJ, Howland C, Stone MM, Sutherland I. BCG vaccination of children against leprosy in Uganda: final results. J Hyg (Lond). 1981;87(2):233–48. https://doi.org/10.1017/s002217240006945x.
Pönnighaus JM, Fine PE, Sterne JA, Wilson RJ, Msosa E, Gruer PJ, et al. Efficacy of BCG vaccine against leprosy and tuberculosis in northern Malawi. Lancet. 1992;339(8794):636–9. https://doi.org/10.1016/0140-6736(92)90794-4.
Smith PG, Revill WD, Lukwago E, Rykushin YP. The protective effect of BCG against Mycobacterium ulcerans disease: a controlled trial in an endemic area of Uganda. Trans R Soc Trop Med Hyg. 1976;70(5–6):449–57. https://doi.org/10.1016/0035-9203(76)90128-0.
Zimmermann P, Finn A, Curtis N. Does BCG vaccination protect against nontuberculous mycobacterial infection? A systematic review and meta-analysis. J Infect Dis. 2018;218(5):679–87. https://doi.org/10.1093/infdis/jiy207.
Tribouley J, Tribouley-Duret J, Appriou M. Effect of bacillus Callmette Guerin (BCG) on the receptivity of nude mice to Schistosoma mansoni. C R Seances Soc Biol Fil. 1978;172(5):902–4.
van Dissel JT, Stikkelbroeck JJ, van den Barselaar MT, Sluiter W, Leijh PC, van Furth R. Divergent changes in antimicrobial activity after immunologic activation of mouse peritoneal macrophages. J Immunol. 1987;139(5):1665–72.
Arts RJW, Moorlag SJCFM, Novakovic B, Li Y, Wang S-Y, Oosting M, et al. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe. 2018;23(1):89–100.e5. https://doi.org/10.1016/j.chom.2017.12.010.
Kristensen I, Aaby P, Jensen H. Routine vaccinations and child survival: follow up study in Guinea-Bissau, West Africa. BMJ. 2000;321(7274):1435–8. https://doi.org/10.1136/bmj.321.7274.1435.
Higgins JPT, Soares-Weiser K, López-López JA, Kakourou A, Chaplin K, Christensen H, et al. Association of BCG, DTP, and measles containing vaccines with childhood mortality: systematic review. BMJ. 2016;355:i5170. https://doi.org/10.1136/bmj.i5170.
Biering-Sørensen S, Aaby P, Lund N, Monteiro I, Jensen KJ, Eriksen HB, et al. Early BCG-denmark and neonatal mortality among infants weighing <2500 g: a randomized controlled trial. Clin Infect Dis. 2017;65(7):1183–90. https://doi.org/10.1093/cid/cix525.
Haahr S, Michelsen SW, Andersson M, Bjorn-Mortensen K, Soborg B, Wohlfahrt J, et al. Non-specific effects of BCG vaccination on morbidity among children in Greenland: a population-based cohort study. Int J Epidemiol. 2016;45(6):2122–30. https://doi.org/10.1093/ije/dyw244.
Stensballe LG, Sørup S, Aaby P, Benn CS, Greisen G, Jeppesen DL, et al. BCG vaccination at birth and early childhood hospitalisation: a randomised clinical multicentre trial. Arch Dis Child. 2017;102(3):224–31. https://doi.org/10.1136/archdischild-2016-310760.
Prentice S, Nassanga B, Webb EL, Akello F, Kiwudhu F, Akurut H, et al. BCG-induced non-specific effects on heterologous infectious disease in Ugandan neonates: an investigator-blind randomised controlled trial. Lancet Infect Dis. 2021;21(7):993–1003. https://doi.org/10.1016/S1473-3099(20)30653-8.
Giamarellos-Bourboulis EJ, Tsilika M, Moorlag S, Antonakos N, Kotsaki A, Domínguez-Andrés J, et al. Activate: randomized clinical trial of BCG vaccination against infection in the elderly. Cell. 2020;183(2):315–23.e9. https://doi.org/10.1016/j.cell.2020.08.051.
Shet A, Ray D, Malavige N, Santosham M, Bar-Zeev N. Differential COVID-19-attributable mortality and BCG vaccine use in countries. MedRxiv. 2020; https://doi.org/10.1101/2020.04.01.20049478.
Hamiel U, Kozer E, Youngster I. SARS-CoV-2 rates in BCG-vaccinated and unvaccinated young adults. JAMA. 2020;323(22):2340–1. https://doi.org/10.1001/jama.2020.8189.
Hensel J, McAndrews KM, McGrail DJ, Dowlatshahi DP, LeBleu VS, Kalluri R. Protection against SARS-CoV-2 by BCG vaccination is not supported by epidemiological analyses. Sci Rep. 2020;10(1):18377. https://doi.org/10.1038/s41598-020-75491-x.
Pittet LF, Messina NL, Gardiner K, Orsini F, Abruzzo V, Bannister S, et al. BCG vaccination to reduce the impact of COVID-19 in healthcare workers: protocol for a randomised controlled trial (BRACE trial). BMJ Open. 2021;11(10):e052101. https://doi.org/10.1136/bmjopen-2021-052101.
Pearl R. Cancer and tuberculosis. Am J Epidemiol. 1929;9(1):97–159. https://doi.org/10.1093/oxfordjournals.aje.a121646.
Holmgren I. Employment of BCG, especially in intravenous injection. Acta Med Scand. 1936;90(S78):350–61. https://doi.org/10.1111/j.0954-6820.1936.tb15958.x.
Old LJ, Clarke DA, Benacerraf B. Effect of bacillus Calmette-Guérin infection on transplanted tumours in the mouse. Nature. 1959;184(4682):291–2. https://doi.org/10.1038/184291a0.
Silverstein MJ, deKernion J, Morton DL. Malignant melanoma metastatic to the bladder: regression following intratumor injection of BCG vaccine. JAMA. 1974;229(6):688. https://doi.org/10.1001/jama.1974.03230440046032.
Kamat AM, Flaig TW, Grossman HB, Konety B, Lamm D, O’Donnell MA, et al. Expert consensus document: consensus statement on best practice management regarding the use of intravesical immunotherapy with BCG for bladder cancer. Nat Rev Urol. 2015;12(4):225–35. https://doi.org/10.1038/nrurol.2015.58.
Gandhi NM, Morales A, Lamm DL. Bacillus Calmette-Guérin immunotherapy for genitourinary cancer. BJU Int. 2013;112(3):288–97. https://doi.org/10.1111/j.1464-410X.2012.11754.x.
Morales A, Eidinger D, Bruce AW. Intracavitary bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116(2):180–3. https://doi.org/10.1016/s0022-5347(17)58737-6.
Vermorken JB, Claessen AM, van Tinteren H, Gall HE, Ezinga R, Meijer S, et al. Active specific immunotherapy for stage II and stage III human colon cancer: a randomised trial. Lancet. 1999;353(9150):345–50. https://doi.org/10.1016/s0140-6736(98)07186-4.
Bibby AC, Walker S, Maskell NA. Are intra-pleural bacterial products associated with longer survival in adults with malignant pleural effusions? A systematic review. Lung Cancer. 2018;122:249–56. https://doi.org/10.1016/j.lungcan.2018.06.002.
Bibby AC, Maskell NA. Current treatments and trials in malignant pleural mesothelioma. Clin Respir J. 2018;12(7):2161–9. https://doi.org/10.1111/crj.12938.
Usher NT, Chang S, Howard RS, Martinez A, Harrison LH, Santosham M, et al. Association of BCG vaccination in childhood with subsequent cancer diagnoses: a 60-year follow-up of a clinical trial. JAMA Netw Open. 2019;2(9):e1912014. https://doi.org/10.1001/jamanetworkopen.2019.12014.
Erb KJ, Holloway JW, Sobeck A, Moll H, Le Gros G. Infection of mice with Mycobacterium bovis-bacillus Calmette-Guérin (BCG) suppresses allergen-induced airway eosinophilia. J Exp Med. 1998;187(4):561–9. https://doi.org/10.1084/jem.187.4.561.
Choi IS, Koh YI. Therapeutic effects of BCG vaccination in adult asthmatic patients: a randomized, controlled trial. Ann Allergy Asthma Immunol. 2002;88(6):584–91. https://doi.org/10.1016/S1081-1206(10)61890-X.
Kowalewicz-Kulbat M, Locht C. BCG for the prevention and treatment of allergic asthma. Vaccine. 2021;39(50):7341–52. https://doi.org/10.1016/j.vaccine.2021.07.092.
Arnoldussen DL, Linehan M, Sheikh A. BCG vaccination and allergy: a systematic review and meta-analysis. J Allergy Clin Immunol. 2011;127(1):246–53.e21. https://doi.org/10.1016/j.jaci.2010.07.039.
Kowalewicz-Kulbat M, Locht C. BCG and protection against inflammatory and auto-immune diseases. Expert Rev Vaccines. 2017;16(7):699–708. https://doi.org/10.1080/14760584.2017.1333906.
Kühtreiber WM, Tran L, Kim T, Dybala M, Nguyen B, Plager S, et al. Long-term reduction in hyperglycemia in advanced type 1 diabetes: the value of induced aerobic glycolysis with BCG vaccinations. NPJ Vaccines. 2018;3(1):23. https://doi.org/10.1038/s41541-018-0062-8.
Ristori G, Buzzi MG, Sabatini U, Giugni E, Bastianello S, Viselli F, et al. Use of Bacille Calmette-Guèrin (BCG) in multiple sclerosis. Neurology. 1999;53(7):1588–9. https://doi.org/10.1212/wnl.53.7.1588.
Ristori G, Romano S, Cannoni S, Visconti A, Tinelli E, Mendozzi L, et al. Effects of Bacille Calmette-Guerin after the first demyelinating event in the CNS. Neurology. 2014;82(1):41–8. https://doi.org/10.1212/01.wnl.0000438216.93319.ab.
Zuo Z, Qi F, Yang J, Wang X, Wu Y, Wen Y, et al. Immunization with bacillus Calmette-Guérin (BCG) alleviates neuroinflammation and cognitive deficits in APP/PS1 mice via the recruitment of inflammation-resolving monocytes to the brain. Neurobiol Dis. 2017;101:27–39. https://doi.org/10.1016/j.nbd.2017.02.001.
Gofrit ON, Klein BY, Cohen IR, Ben-Hur T, Greenblatt CL, Bercovier H. Bacillus Calmette-Guérin (BCG) therapy lowers the incidence of Alzheimer’s disease in bladder cancer patients. PLoS One. 2019;14(11):e0224433. https://doi.org/10.1371/journal.pone.0224433.
Klinger D, Hill BL, Barda N, Halperin E, Gofrit ON, Greenblatt CL, et al. Bladder cancer immunotherapy by BCG is associated with a significantly reduced risk of Alzheimer’s disease and Parkinson’s disease. Vaccines (Basel). 2021;9(5):491. https://doi.org/10.3390/vaccines9050491.
Milstien JB, Gibson JJ. Quality control of BCG vaccine by WHO: a review of factors that may influence vaccine effectiveness and safety. Bull World Health Organ. 1990;68(1):93–108.
Department of Health. Immunisation against infectious disease (green book). London: Department of Health; 2018.
Boisson-Dupuis S, Bustamante J, El-Baghdadi J, Camcioglu Y, Parvaneh N, El Azbaoui S, et al. Inherited and acquired immunodeficiencies underlying tuberculosis in childhood. Immunol Rev. 2015;264(1):103–20. https://doi.org/10.1111/imr.12272.
Hesseling AC, Rabie H, Marais BJ, Manders M, Lips M, Schaaf HS, et al. Bacille Calmette-Guérin vaccine-induced disease in HIV-infected and HIV-uninfected children. Clin Infect Dis. 2006;42(4):548–58. https://doi.org/10.1086/499953.
Oddens J, Brausi M, Sylvester R, Bono A, van de Beek C, van Andel G, et al. Final results of an EORTC-GU cancers group randomized study of maintenance bacillus Calmette-Guérin in intermediate- and high-risk ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. Eur Urol. 2013;63(3):462–72. https://doi.org/10.1016/j.eururo.2012.10.039.
Decaestecker K, Oosterlinck W. Managing the adverse events of intravesical bacillus Calmette-Guérin therapy. Res Rep Urol. 2015;7:157–63. https://doi.org/10.2147/rru.S63448.
Loxton AG, Knaul JK, Grode L, Gutschmidt A, Meller C, Eisele B, et al. Safety and immunogenicity of the recombinant Mycobacterium bovis BCG vaccine VPM1002 in HIV-unexposed newborn infants in South Africa. Clin Vaccine Immunol. 2017;24(2):e00439–16. https://doi.org/10.1128/cvi.00439-16.
Nieuwenhuizen NE, Kulkarni PS, Shaligram U, Cotton MF, Rentsch CA, Eisele B, et al. The recombinant Bacille Calmette-Guerin vaccine VPM1002: ready for clinical efficacy testing. Front Immunol. 2017;8:1147.
Dijkman K, Sombroek CC, Vervenne RAW, Hofman SO, Boot C, Remarque EJ, et al. Prevention of tuberculosis infection and disease by local BCG in repeatedly exposed rhesus macaques. Nat Med. 2019;25(2):255–62. https://doi.org/10.1038/s41591-018-0319-9.
Barclay WR, Anacker RL, Brehmer W, Leif W, Ribi E. Aerosol-induced tuberculosis in subhuman primates and the course of the disease after intravenous BCG vaccination. Infect Immun. 1970;2(5):574–82. https://doi.org/10.1128/iai.2.5.574-582.1970.
Sharpe S, White A, Sarfas C, Sibley L, Gleeson F, McIntyre A, et al. Alternative BCG delivery strategies improve protection against Mycobacterium tuberculosis in non-human primates: protection associated with mycobacterial antigen-specific CD4 effector memory T-cell populations. Tuberculosis (Edinb). 2016;101:174–90. https://doi.org/10.1016/j.tube.2016.09.004.
Darrah PA, Zeppa JJ, Maiello P, Hackney JA, Wadsworth MH 2nd, Hughes TK, et al. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature. 2020;577(7788):95–102. https://doi.org/10.1038/s41586-019-1817-8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Morrison, H., McShane, H. (2023). BCG: Past, Present and Future Direction. In: Christodoulides, M. (eds) Vaccines for Neglected Pathogens: Strategies, Achievements and Challenges . Springer, Cham. https://doi.org/10.1007/978-3-031-24355-4_8
Download citation
DOI: https://doi.org/10.1007/978-3-031-24355-4_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-24354-7
Online ISBN: 978-3-031-24355-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)