Abstract
Multiple immunological mechanisms interact to protect against Mycobacterium tuberculosis (M.tb) infection and/or tuberculosis (TB) disease. However, development of a much-needed new and effective TB vaccine is hindered by the lack of validated correlates of protection. The identification of correlates of protection would facilitate the rational design, optimisation and evaluation of TB vaccine candidates. In this chapter, we discuss what is currently known about protective immunity against M.tb and potential correlates of protection that have been proposed to date, both including and also looking beyond the central role of IFN-γ producing CD4+ T cells to consider innate and humoral immune parameters. Approaches to identifying and validating correlates of protection will also be reviewed.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
One of the major barriers hampering the development of a new and efficacious TB vaccine is the lack of validated immune correlates of protection, defined as ‘a statistical relation between an immune marker and protection’ [1]. Such correlates can be subdivided into mechanistic (causally responsible for protection) or non-mechanistic (significantly correlated with, but not causally responsible for, protection). Correlates of protection, principally antibody levels, have been instrumental in developing vaccines against a range of infectious diseases including those caused by Haemophilus influenzae type b (Hib), pneumococci, Clostridium tetani, Corynebacterium diphtheriae and several viruses. An immune correlate of protection would similarly be invaluable in rational TB vaccine design, through, for example, facilitating the identification of protective antigens, as well as optimisation of the vaccine delivery system, adjuvant, dose and regimen. It would also provide an early indication of vaccine efficacy, thus expediting clinical TB vaccine trials that currently require very large sample sizes and long follow-up periods to accrue a sufficient number of ‘cases’ meeting clinical endpoint criteria.
However, the field is caught in a dilemma whereby potential correlates of protective immunity can only be validated in clinical trials when a highly effective vaccine is developed, yet the design and evaluation of vaccine candidates are extremely difficult in the absence of a validated correlate. A further challenge is the complexity of clinical manifestations, as correlates of protection from Mycobacterium tuberculosis (M.tb) infection may differ from those of progression to active TB disease (ATB), reactivation or reinfection; and vaccine-induced protection may differ from natural protection from infection, between host compartments, populations or different antigens or vaccine platforms.
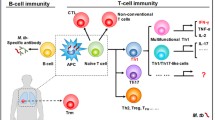
Our understanding of which components of the immune response are necessary, and/or sufficient, to achieve protection from TB is incomplete. It is established that following inhalation of M.tb in aerosols, bacteria are phagocytosed primarily by dendritic cells (DCs) and macrophages. The latter are the main cellular reservoir where M.tb resides and replicates by blocking phagolysosomal fusion. Infected DCs traffic to the local lung-draining mediastinal lymph nodes (LNs) where they present antigen in association with MHC class II. The precise location where M.tb is phagocytosed is thought to play a role in the establishment of potentially protective primary immune responses. DCs sampling the mucosal tissue have faster antigen trafficking to LNs compared to those sampling the lung parenchyma, affecting the activation and proliferation of antigen-specific CD4+ T cells [2] (Fig. 6.1). After activation, CD4+ T cells are primed as T-helper 1 (Th1) cells, expand and migrate to the lung tissue where they become a primary source of IFN-γ and TNF-α production during the acute stage of infection, which stimulates macrophages to kill intracellular mycobacteria by activating downstream pathways including inducible nitric oxide synthase (iNOS). As such, most TB vaccine studies and clinical trials to date have focused on the T-helper 1 (Th1) response—the frequency of IFN-γ producing CD4+ T cells—as the main immunological read-out. Indeed, several studies have confirmed the necessity for CD4+ T cells and IFN-γ in protective immunity. However, other evidence suggests that these parameters alone are not sufficient for, and do not necessarily correlate with, protection (see Sect. 6.3.1.1). An ever-expanding literature supports a role for other cellular subsets, cytokines and immune mechanisms in protection, offering new candidate correlates of protection.
Immune response to M.tb infection. Alveolar macrophages and DCs phagocytose aerosolised M.tb that reaches the lung. Antigen location may lead to different speeds of DC trafficking through the lymph vessels. Upon arrival at the LNs, DCs present M.tb antigen which activates specific T cells. Days after the initial infection, adaptive immune cells arrive at the site of infection and secrete IFN-γ, TNF-α and IL-17 amongst other cytokines which stimulate macrophages to kill intracellular mycobacteria. Activated specific B cells secrete antibodies that may increase opsonization, phagocytosis and other potentially protective responses mediated by T cells and NK cells. DC dendritic cell, LN lymph node, NK natural killer. (Created with BioRender.com)
2 Innate Immunity
2.1 Early Clearance and a Role for Innate Immunity
M.tb case-contact studies demonstrate that up to 55% of people exposed to M.tb do not develop a traceable adaptive immune response, as detected by interferon gamma release assay (IGRA) or tuberculin skin test (TST) [3, 4]. Persistent IGRA-negative M.tb contacts have low risk of progression to TB disease, suggesting that M.tb is cleared in these individuals [3, 5, 6]. This ‘early clearance’ could be evidence of protective innate immune mechanisms independent of an adaptive immune response [7]. However, this phenomenon has not been proven, and an early efficient protective adaptive immune response could simply result in rapid elimination and IGRA reversion [8, 9]. Alternatively, persistently IGRA-negative individuals may have developed an adaptive response, and infection may even persist but is never detected on systemic IGRA, due to T cell anergy, IFN-γ-independent mechanisms or because the response is restricted to the local lung mucosa [6, 10,11,12] (Fig. 6.2).
Hypothesized relationship between M.tb clearance and immune mechanisms following natural exposure in humans. IGRA interferon gamma release assay, TST tuberculin skin test. (Created with BioRender.com)
Despite this uncertainty, as early clearance is a model of protective immunity against M.tb, further investigation into the underlying immune mechanisms is warranted, as it could lead to new insights into correlates of protection. In addition to this putative protective role independent of adaptive immunity, evidence suggests that the innate immune response is crucial in defining adaptive immune polarisation, for example, to a Th1 or Th2 response [13,14,15]. This further highlights the central role the innate immune response may play in protection against M.tb infection and TB disease.
2.2 Mononuclear Phagocytes
Mononuclear phagocytes (monocytes, DCs and resident macrophages) are likely key in the development of a protective immune response against M.tb infection [16, 17]. Human monocyte and DC primary immunodeficiency syndromes are characterised by disseminated mycobacterial infection after intradermal (ID) BCG vaccination [18]. DCs are professional antigen-presenting cells connecting the innate and adaptive immune response [19]. Macrophages are professional phagocytes, likely amongst the first cells to encounter M.tb on infection, but also the main niche in which the obligate intracellular mycobacteria resides [14, 20].
Delayed DC migration to the draining LNs and dysfunctional DC-mediated antigen presentation increase risk of overwhelming M.tb infection in mice, likely due to the delay in the development of an effective adaptive immune response [21,22,23]. Further, DC depletion in mice delays M.tb-specific CD4+ T cell activation and results in overwhelming M.tb infection [24]. In humans, CXCL10+ CD14dim monocytes are associated with BCG growth inhibition in a functional ex vivo mycobacterial growth inhibition assay (MGIA) [25]. CXCL10 is a chemokine facilitating homing to the infection site, regulated by IFN-γ production [26]. Genetic inability to produce IFN-γ in humans is characterised by mycobacterial susceptibility and associated with disseminated BCG disease [27, 28]. Conversely, systemic monocytosis and a high monocyte to lymphocyte (ML) ratio are associated with poorly controlled TB disease and increased risk of developing TB disease [29,30,31,32,33]. It may be that only certain monocyte effector subtypes are protective or, alternatively, that chronic but not acute monocytosis reduces protective efficacy.
2.3 Natural Killer Cells
Human natural killer (NK) cells can directly recognise M.tb infected cells in vitro and induce apoptosis within 24 hours via perforin and granzyme proteases [34, 35]. They also directly lyse infected cells via antibody-dependent cell-mediated cytotoxicity (ADCC). Circulating cytotoxic NK cells are associated with reduced risk of M.tb infection in healthy TB contacts [36]. Additionally, IFN-γ+ TNF-α+ NK cells derived from historically BCG-vaccinated adults improve BCG growth inhibition in the MGIA [37, 38]. A low level of systemic cytotoxic NK cells increases the risk of progression to active TB disease. While NK cells alone do not mediate protection in M.tb challenge mouse models, depletion of both NK cells and T cells increases susceptibility to TB, compared with depletion of T cells alone, and adoptive transfer of NK cells reduces M.tb burden [39].
2.4 Neutrophils
Neutrophils have the potential to be key players in early defence against M.tb infection. They can migrate to the site of infection within minutes, are professional phagocytes and release potent anti-microbial oxidative granules which kill M.tb in vitro [40,41,42]. Depletion or enhanced recruitment of neutrophils in mice leads to increased or decreased mycobacterial burden, respectively, and a high neutrophil count is protective against M.tb infection in contacts of TB patients [43,44,45]. However, this protective effect may only be upon initial exposure, as neutrophilia is a hallmark of active TB disease in humans and uncontrolled M.tb infection in susceptible mice [46,47,48,49,50].
2.5 Donor Unrestricted T Cells
Donor unrestricted T cells (DURTs) are innate CD3+ T cells which respond to MHC-independent non-peptide epitopes [51]. They include mucosal-associated invariant T (MAIT) cells and the CD1-restricted natural killer T (NKT) cells and γδ T cells [52, 53]. In vitro, DURTs can directly recognise and kill M.tb infected cells, and they have diverse effector functions and are enriched in the human lung [54,55,56,57]. They could therefore be important in early immune protection, acting as the bridge between the innate and adaptive response. However their protective potential in M.tb is unclear [58, 59]. The MR1 polymorphism rs1052632 is associated with increased susceptibility to TB in a Vietnamese cohort [60], and evidence for protection in mouse and human models for DURTs is contradictory [57, 61,62,63,64,65]. Research into DURTS has been constrained by difficulty in defining these cells and a lack of animal models for some cell types such as group 1 CD1-restricted T cells [54,55,56, 66]. With the advent of tetramer technology, many of these barriers may be overcome.
2.6 Trained Innate Immunity
Traditionally, innate immunity has been viewed as a static non-specific immune system without capacity for memory. However, it is now known that NK cells mature into long-lived effector subclasses upon pathogen exposure [67]. Furthermore, epigenetic reprogramming of bone marrow-derived monocytes, termed training, has been demonstrated following BCG exposure, affecting the subsequent monocyte response to homologous and heterologous pathogens [68]. Trained monocytes have been associated with protection from M.tb challenge following BCG vaccination in mice [69]. Training has also been associated with early clearance in BCG-exposed but uninfected adults [25]. Understanding the plasticity of the innate immune response following M.tb exposure, and its subsequent effect on the adaptive immune system, would enable further targets for vaccine development.
While the evidence for a role of the innate immune system in protection against M.tb is compelling, further research is required to fully elucidate these mechanisms and to understand the impact on any subsequent protective adaptive immune response.
3 Conventional T Cells
3.1 CD4+ T Cells
3.1.1 IFN-γ Producing CD4+ T Cells
A large body of evidence indicates that IFN-γ producing CD4+ T cells are necessary for protection against TB. Mice and non-human primates (NHPs) deficient in CD4+ T cells during acute or chronic M.tb infection have increased bacterial burden and mortality compared with control animals [70,71,72,73]. Furthermore, the adoptive transfer of CD4+ T cells from immunised mice protects non-immunised mice against M.tb challenge [74]. The increased risk of TB disease due to decreased CD4+ T cell number and function associated with HIV infection in humans, and SIV infection in NHPs, provides further evidence of a critical role for this cell type [75,76,77]. Risk of reactivation increases as CD4+ T cell levels decrease, and these patients are more likely to present with disseminated disease [78, 79]. IFN-γ knock-out (KO) mice succumb to rapid and fatal TB disease [80, 81], and partial or complete IFN-γ receptor deficiency in humans leads to disseminated M.tb infection and BCG-osis [82, 83]. However, it should be noted that clinical deterioration in CD4+ T cell-deficient mice cannot be attributed to sustained loss of IFN-γ, as other cell types also produce this cytokine [70, 71]. IFN-γ-independent mechanisms of CD4+ T cell-mediated control of M.tb infection have also been demonstrated [84].
Despite the clear importance of IFN-γ producing CD4+ T cells in the immune response to M.tb, several key findings challenge the idea that this measure represents a correlate of protection. The magnitude of purified protein derivative (PPD)-specific IFN-γ production following BCG vaccination does not correlate with protection against M.tb challenge in mice [85, 86]. Furthermore, studies of TB vaccine candidates have demonstrated the induction of potent antigen-specific IFN-γ producing CD4+ T cell responses, but this did not translate into improved protection over BCG alone [87, 88]. Kagina et al. assessed the frequency and extended cytokine profile of specific T cells in a study of 5662 BCG-vaccinated infants with a 2-year follow-up to identify those who developed TB and those who did not develop TB (divided into 2 groups of protected infants according to household contact status) [89]. There were no differences between groups in CD4+ T cell IFN-γ production or any of the other T cell properties [89]. Interestingly, in a post hoc correlate analysis in the same population, the BCG antigen-specific IFN-γ ELISpot response was associated with reduced risk of TB disease. The main effect appeared to be in the first 6–12 months of follow-up, suggesting an early protective effect in infancy [90]. Such contrasting findings may result from differing time-points of sample collection, the IFN-γ assay used, sample type or case definitions.
In studies comparing patients with ATB and latently M.tb-infected (LTBI) individuals or uninfected household contacts (both considered to have some degree of protection), findings regarding the role of IFN-γ producing CD4+ T cells are conflicting. Some have suggested that M.tb-specific Th1 cells and IFN-γ production are depressed during active TB disease [91, 92], while others report the converse [93, 94]. In such situations it is difficult to disentangle cause from effect, as high levels of IFN-γ may be driven by antigen load in acute infection, and patients with chronic TB may exhibit signs of Th1 inhibition as a secondary process. In a study comparing TB patients of diverse disease severity, antigen-specific IFN-γ CD4+ T cell responses correlated with the activity of M.tb infection but not the severity of TB disease [95, 96].
3.1.2 Polyfunctional CD4+ T Cells
Polyfunctional T cells are defined as those that simultaneously co-produce two or more proinflammatory cytokines. Polyfunctional CD4+ T cells secreting IFN-γ, TNF-α and IL-2 have been shown to correlate with protection from Leishmania and have been associated with slower progression to AIDS in HIV-infected individuals [97, 98], but their role in M.tb infection remains unclear. Individuals with LTBI or ATB patients following therapy have been reported to have higher frequencies of polyfunctional CD4+ T cells than those with ATB, although the converse has also been suggested [99,100,101,102]. As previously noted regarding IFN-γ producing CD4+ T cells, it is not possible to discern whether frequency of this cellular subset plays a causal role in control of M.tb or simply reflects the underlying bacterial burden.
The BCG vaccine and a range of TB vaccine candidates including live mycobacterial vaccines, those using viral vectors and recombinant antigen vaccines have been shown to induce polyfunctional CD4+ T cells [103]. However, the strongest evidence supporting a role for the cellular subset as a correlate of protective immunity comes from studies in which two or more distinct vaccine candidates eliciting a range of protective responses are compared. rBCG-XB has been shown to induce stronger HspX-specific polyfunctional T cell responses than BCG, which was associated with superior protection [104], and delivery of Ag85B:CpG with polypropylene sulphide nanoparticles (NP) induced more polyfunctional Ag85B-specific CD4+ T cells than the same vaccine without NP, which correlated with superior protection in the lung [105]. In a study of BCG and four different TB vaccine candidates, levels of vaccine-induced protection also correlated with the magnitude and quality of polyfunctional CD4+ T cells [106].
Conversely, other preclinical studies do not support such an association [103]. In humans, in the 2-year follow-up study of BCG-vaccinated infants previously described, there was no association between the polyfunctional cytokine profile of induced T cells and protective efficacy [89]. Furthermore, boosting BCG or VPM1002 with MVA85A elicited superior PPD- and Ag85A-specific polyfunctional T cells, but this did not translate into improved protection obtained with either BCG or VPM1002 alone [88, 107]. It is possible that such discrepancies result from differences in vaccination protocols or methods of measuring polyfunctional T cells or that dual-cytokine producing T cells are a better correlate than those producing three cytokines [103]. A comprehensive review by Lewinsohn et al. concluded that polyfunctional CD4+ T cells are not sufficient and may not even be necessary to mediate protection [103].
3.1.3 Th17 Cells
Following exposure to M.tb, innate myeloid cells induce the production of cytokines such as IL-23 and IL-1β which drive the differentiation and polarisation of naïve CD4+ T cells towards Th17 cells. Th17 cells are the primary producers of IL-17 during TB, but they can also produce other cytokines including IL-22, IL-21, TNF-α and GM-CSF. While these cells have an important role in the protective immune response to rapidly growing extracellular bacteria, their contribution to protection against intracellular bacteria such as M.tb is less well-characterised [108]. Memory Th17 cells are present in the blood of people who have been exposed to mycobacteria, and the magnitude of the IL-17 response has been shown to correlate with the clinical outcome of M.tb infection [109, 110]. It appears that Th17 cells are particularly important in the early stages of infection and play a role in granuloma formation and induction of chemokines leading to recruitment of neutrophils and circulating CD4+ T cells [111]. Excessive IL-17 production can lead to tissue damage, and thus Th17 cells have been implicated in TB pathology [112]. During the chronic phase of infection, a balance must be achieved with Th1 and Th17 responses to control bacterial growth but limit immunopathology.
Following BCG vaccination, IL-17 has been shown to drive Th1 responses by downregulating IL-10 and upregulating IL-12 production by DCs [113]. Depletion of IL-17 during M.tb challenge reduces chemokine expression and accumulation of IFN-γ producing CD4+ T cells in the lung [114]. Interestingly, IL-17 KO mice are unable to control infection by the hypervirulent M.tb strain HN878, although they do survive infection with less pathogenic strains [115]. In the 2-year follow-up study of BCG-vaccinated infants by Kagina et al., frequencies of BCG-specific Th17 cells did not correlate with protection against TB [89]. However, polyfunctional Th17 cells were a correlate of local protective immunity following mucosal BCG vaccination in NHPs [116]. Further studies are required to assess whether Th17 cells, or IL-17 production by other cells, represent a correlate of protection.
3.2 CD8+ T Cells
CD8+ T cells are activated by presentation of antigen in association with MHC class I, and M.tb contains several MHC class I restricted immunodominant antigens that are recognised by human populations [117]. Similar to CD4+ T cells, cytokine-producing CD8+ T cells primarily secrete IFN-γ, TNF-α and IL-2 which have critical functions in M.tb infection as described [118]. CD8+ T cells also have cytolytic functions and secrete granzymes, granulysin and perforin. Although their role in protection from TB is less well-defined than CD4+ T cells, antigen-specific CD8+ T cells are induced during M.tb infection and are capable of recognising M.tb-infected macrophages—particularly those that are heavily infected [119, 120]. Studies have also shown that cytotoxic T cells (CTLs) are capable of killing M.tb-infected cells [121]. While some murine studies support a role for CD8+ T cells in the containment of infection, others do not [122,123,124]. The involvement of CD8+ T cells may depend on the phase of infection, and it has been suggested that mice may not be the most appropriate model for evaluating the relevance of this cell type in humans as they lack some important immune features relating directly to CD8+ T cell function and specificity [125]. While CD8+ T cells provide less protection than an equivalent number of CD4+ T cells, adoptive transfer experiments show that CD8+ T cells can mediate protection against TB, even in the absence of CD4+ T cells. In the more closely related NHP, CD8+ T cell depletion leads to a significant decrease in immunity against M.tb in previously infected and treated animals upon reinfection [126].
BCG vaccination has been shown to elicit CD8+ T cell responses, and activation of CD8+ T cells following vaccination can protect against M.tb challenge in mice [127]. Furthermore, depletion of CD8+ T cells compromises BCG vaccine-induced immune control of M.tb replication in NHPs [126]. Several TB vaccine candidates aim to elicit potent CD8+ T cell responses, including VPM1002 for which cross-priming of CD8+ T cells has been proposed as a major mechanism underlying the superior protection conferred over BCG [128] and the RhCMV/TB vaccine which stimulates HLA-E restricted CD8+ T cells and is extremely protective in NHPs [129]. While the induction of CD8+ T cells may prove beneficial, their capacity for mediating protection could be highly dependent on the vaccine antigen(s) selected [130].
4 Humoral Immunity
For most successful prophylactic vaccines, the induction of broadly neutralizing antibodies against exposed and stable immunodominant pathogen epitopes is sufficient to achieve protection [131, 132]. However, for a complex intracellular pathogen such as M.tb, the protective immune response has been traditionally considered to be almost entirely cell-mediated, with humoral immunity largely overlooked [133].
4.1 B Cells
Memory B cells constitute a key element of central immune memory, particularly against pathogens with a long incubation period [132]. The rationale for historically neglecting the biological relevance of humoral immunity to the control of M.tb predominantly derives from early B cell KO mouse models, where the absence of humoral responses did not affect the course of M.tb infection [134,135,136]. Conversely, adoptive transfer of B cells did reverse the increased lung immunopathology in B cell KO M.tb-infected mice, and depletion of CD20+ plasmablasts induced increased bacterial burden in the granulomas of NHPs [137, 138]. Differences in experimental design and implementation (dose, route of delivery, phase of infection and Mycobacterium strain) may be plausible explanations for such inconsistencies [139]. Furthermore, B cell subsets of mice and humans hold considerable phenotypic and functional discrepancies, limiting the ability to translate preclinical studies in this context [140]. Treatment of rheumatoid arthritis patients with the monoclonal anti-CD20 antibody rituximab results in B cell depletion, but this does not affect risk of reactivation or new ATB [141].
The Tyk2 gene of the Janus Kinase (JAK) family is essential for effective IL-23 intracellular signalling and the maintenance of mitochondrial respiration in primary pro-B cells [142, 143]. Interestingly, recent large human cohort studies have found that genetic variants of Tyk2, found in approximately 1 in 600 Europeans, are associated with an increased susceptibility to M.tb infection [142]. Furthermore, IL-23 is required for long-term control of M.tb and B cell follicle formation in the infected lung [144]. Whether Tyk2 variations have a negative impact on the establishment of specific humoral responses against M.tb remains to be determined.
In humans, two studies have reported long-lasting PPD-specific memory B cells that persist for decades after BCG vaccination, but their functionality remains poorly defined [145, 146]. The utilization of memory B cell and plasmablast subsets as a predictive tool for disease stratification and as potential correlates of protection has been previously discussed [147]. However, while the frequency of plasmablasts may correlate with the presence of numerous circulating M.tb antigens, heterogeneous plasmablast dynamics and the inability to detect M.tb can hinder evaluation of which subjects are M.tb resisters, individuals with LTBI, ATB patients or potentially even individuals who have achieved sterilizing immunity [148]. Mucosal vaccination with the attenuated M.tb vaccine candidate MtbΔsigH confers significant protection against a lethal TB challenge, and this is strongly associated with levels of inducible bronchus-associated lymphoid tissue (iBALT) in the lung, suggesting an important role for B cells [149].
4.2 Antibodies
Most efforts to characterize the antibody response to M.tb have relied on non-high-throughput detection methods in serum or plasma against different M.tb antigen mixtures, such as PPD, and lack standardized methods for quantification [150]. This may limit the reliability of results, particularly when comparing data sets from different studies [151]. Furthermore, broad quantification of antigen-specific antibodies of a single isotype might be too simplistic a measure, as antibody titre does not consider the quality of the response based on biochemical and functional properties such as Fc receptor binding, affinity, opsonizing ability and influence on mycobacterial growth restriction. Antibodies could contribute to protection directly through increasing phagocytosis and phagolysosome formation in professional phagocytes, blocking uptake by non-professional phagocytes and/or indirectly enhancing T cell-mediated immunity [133].
Monoclonal antibodies against lipoarabinomannan (LAM) and arabinomannan (AM) have been linked to prolonged survival during M.tb infection in mice, suggesting potential functional relevance for infection control [152, 153]. Intriguingly, Kumagai et al. demonstrated that M.tb infection influenced host protein, including antibody, glycosylation in mice, and the glycosylation patterns of antibodies may affect their function [154, 155]. In humans, individuals with LTBI harbour low-magnitude antibody responses with a distinct constant domain (Fc) glycosylation when compared to ATB patients [156]. In the same study, antibodies from LTBI individuals drove enhanced phagolysosomal maturation, inflammasome activation and macrophage killing of intracellular M.tb [156]. Moreover, healthy individuals highly exposed to M.tb who tested negative by IGRA and TST, so-called ‘resisters’, display enhanced antibody avidity and distinct M.tb-specific IgG Fc profiles [157, 158]. Some antibodies isolated from exposed but uninfected healthcare workers mediated protection against M.tb challenge when transferred to mice [159]. Taken together, the growing body of literature indicates that the relevance of the antibody response in protection against TB may be greater than previously appreciated [160, 161].
However, little is known about the antibody responses induced by BCG or other TB vaccine candidates as few studies have measured this parameter [162]. An early study by de Vallière et al. reported significant induction of LAM-specific IgG following both primary and secondary BCG vaccination in healthy volunteers. Incubation with post-BCG vaccination serum significantly increased BCG internalization by macrophages and mycobacterial growth inhibition in vitro, an effect that was reversible by preabsorption of IgG [163]. Consistent results from an independent study have shown opsonization mediated by BCG vaccine-induced IgG follows a dose-response biological gradient [146]. In addition, in BCG-vaccinated infants, an association has been reported between vaccine-induced Ag85A-specific IgG antibodies and a reduced risk of TB disease [90]. The M72/AS01E vaccine candidate has been shown to be a potent inducer of antibodies which were sustained throughout the 3-year follow-up period, although it remains to be determined whether these contribute to the ~50% efficacy observed against ATB in M.tb-infected individuals [164].
The recent finding that intravenous (IV) BCG confers superior protection from TB compared with other routes of immunisation in NHPs has brought to light the relevance of TB vaccine delivery routes [165]. Among other immune parameters, titres of IgG and IgA against M.tb whole cell lysate were significantly higher in serum and bronchoalveolar lavage (BAL) fluid following BCG administered by the IV route compared with aerosol or intradermal (ID) vaccination [165, 166]. Notably, this response was characterized by robust IgM secretion, which correlated with reduced bacterial burden and prevention of M.tb infection, indicating the relevance of isotype [166]. Secretory IgA may be of particular importance in protecting against pulmonary infection at the mucosal surface by blocking mycobacterial entrance and/or modulating proinflammatory responses [167,168,169,170]. A recent NHP study demonstrated that mucosal BCG vaccination by endobronchial installation prevented M.tb infection and TB disease in NHP, and IgA was a correlate of local protective immunity [116].
Characterization of the biochemical and functional features of M.tb-specific antibodies in preclinical and clinical studies may provide valuable insights regarding their relevance as a potential correlate of in vivo protection against TB (Fig. 6.3).
Potential mechanisms of humoral immunity against M.tb. Circulating frequencies of memory B cells and plasmablasts (a), biochemical characteristics of antibodies such as glycosylation and avidity (b) and antibody effector functions such as ADCP and ADCC (c) may represent potential humoral correlates of protection, although further study is required. ADCP antibody dependent cellular phagocytosis, ADCC antibody dependent cellular cytotoxicity (Created with BioRender.com)
5 Approaches to Identifying Correlates of Protection
There are several methods for characterizing potential correlates of protection from TB, including comparing immune responses between protected and unprotected animals or individuals, conducting human experimental medicine studies and using tractable ex vivo functional assays.
5.1 Preclinical Models
Preclinical studies are pivotal to (a) assess the safety of a vaccine candidate and (b) evaluate vaccine efficacy and identify potential correlates of protection that can then be validated in humans [132]. In addition to expediting TB vaccine development, correlates or surrogates of protection would be valuable from a 3Rs (Replacement, Reduction and Refinement) perspective as they would allow the estimation of vaccine efficacy without the need to infect animals with virulent M.tb—a procedure of ‘Moderate’ severity under EU legislation and UK Animal Scientific Procedures Act (ASPA) licensure [171]. However, surrogates of protection derived from preclinical models have uncertain predictive value, thus hindering candidate prioritization for progression to clinical trials [172]. The typical animal models utilized for TB vaccine evaluation are mice, guinea pigs, cattle and NHPs [173, 174]. Additionally, novel humanized animal models have been introduced as a 3Rs approach [175].
By comparing responses between groups in vaccine studies and by cross-sectional analysis of responses from those animals in which vaccination was successful (protected) compared to animals in which vaccination failed (unprotected), it is possible to identify potential correlates of protection. Preclinical studies have the advantage that one can challenge vaccinated animals with virulent M.tb, which is unethical in humans, and that the exact timing, dose and route of infection can be manipulated. They also allow access to relevant tissue sites that would not be possible in humans. Each model has a unique set of advantages and disadvantages based on its ethical, monetary and logistical costs, susceptibility to M.tb infection and extent to which it can reflect human physiology and the clinical spectrum of disease.
5.1.1 Murine Models
Of all the preclinical models for TB vaccine evaluation, mice are the most extensively utilized due to relatively low cost, short generation time, standardization due to inbreeding and abundance of commercial reagents [175]. However, key features of TB lesions in humans such as necrosis and hypoxia are lacking in the most widely used mouse strains, and the immune system bears discrepancies with humans in both innate and adaptive features [140]. Furthermore, the most commonly utilized mouse strains, BALB/c and C57BL/6, exhibit different sensitivities to M.tb infection underlined by the constitutive expression of IL-10 [176]. There is some inconsistency in how ‘protection’ is defined in murine, and other preclinical, models compared with humans. While in humans vaccine efficacy is based on prevention of M.tb infection or TB disease using clinical endpoints, in preclinical models it is based on an improvement in a disease-related readout such as bacterial load, pathology score or long-term survival. As such, a vaccine may be considered ‘protective’ in preclinical assessment even in the presence of measurable bacteria or pathology or if some animals do not survive [172]. A further limitation is the differences between artificial aerosol M.tb challenge and natural transmission in humans [172]. Nevertheless, murine models have provided critical insights into the immune response to M.tb and allowed the generation of hypotheses about which parameters are associated with protection, as detailed in the preceding sections. Notably, murine studies identified the importance of IFN-γ in the control of M.tb and the potential association between polyfunctional CD4+ T cells and protection from M.tb challenge [81, 106].
5.1.2 Guinea Pigs
Guinea pigs are more susceptible to TB than mice and are generally considered to follow a more representative process of human infection with strong initial immunity which is eventually associated with tissue damage, leading to extensive caseation and tissue necrosis and ultimately death [177]. Due to such parallels with features of human TB and high reproducibility, the guinea pig model is the most commonly used system to further evaluate vaccine candidates which appear promising in the mouse. However, BCG vaccination confers stronger protection in guinea pigs than mice, which may limit ability to detect incremental improvements conferred by vaccine candidates [173]. To overcome the issue of unnatural M.tb challenge models, a natural exposure system has been developed in guinea pigs whereby they breathe the extracted air from a ward of TB patients and thus receive multiple low-dose aerosol exposures to clinical strains [178]. However, use of guinea pigs entails a higher ethical, economic and logistical expense than mice, the commonly available strain is outbred, and 100% of guinea pigs develop active disease compared with ~10% of humans. While a number of studies report safety and efficacy outcomes of candidate TB vaccine studies in guinea pigs, evaluation of immune responses and therefore identification of potential correlates of protection are limited by a lack of reagents. However, the recent emergence of new technologies, reagents and assays provides promise for the utility of this model in the future [179].
5.1.3 Cattle
The adaptive immune system of cattle is similar to that of humans, with several aspects making them more representative of the human response to M.tb infection than rodents including less reliance on antigen-specific IFN-γ activation of macrophages, a more active role for cytotoxic cells and the presence of genes encoding cytokines not found in mice such as IL-26 [180, 181]. Furthermore, cattle are a natural target species of TB infection, and M. bovis infection offers a wide spectrum of TB disease that resembles that found in humans. Antigen-specific expression of IFN-γ and IL-2 following BCG vaccination in calves has been shown to correlate with protection from M. bovis challenge [182], and a study of the viral booster vaccines MVA85A and Ad85A identified antigen-specific IFN-γ memory responses by cultured ELISpot and in vitro IL-17 production as correlates of protection following M. bovis challenge [183].
5.1.4 Non-human Primates (NHPs)
The immune response in humans and NHPs is very similar due to their close evolutionary relationship. Together with susceptibility to pulmonary infection with strains of M.tb that are pathogenic to humans, and similarities in the spectrum of TB disease exhibited, this makes NHPs the most attractive model for preclinical TB vaccine evaluation and the most relevant for identification of immune correlates of protection [175]. However, NHPs incur significant ethical and monetary costs, as well as variability across individuals, which may limit their widespread use [175]. Furthermore, different subspecies of macaques differ in their susceptibility to M.tb infection and response to BCG vaccination [184, 185], although corresponding variations in CD4+ and CD8+ T cell and myeloid DC subsets could bring to light useful immune parameters for the identification of correlates of protection [186].
Importantly, as previously described, recent NHP studies have demonstrated superior protection, and in some cases sterilizing immunity, from TB following BCG vaccination administered by different routes [116, 165, 187]. Pulmonary mucosal BCG vaccination has been shown to prevent infection following repeated limiting-dose M.tb challenge, and polyfunctional Th17 cells, IL-10 and IgA were identified as correlates of local protective immunity [116]. Antigen-specific Th1/Th17 cells in the lung were also associated with the degree of M.tb control in granulomas in a study of M.tb-infected macaques [188]. IV BCG vaccination confers superior protection compared with delivery by ID or aerosol routes or as an intratracheal mucosal boost [165, 187]. Regardless of whether such a strategy could ever be deployable in humans, this finding provides a key opportunity for the identification of immune correlates and mechanisms of vaccine-mediated protection against TB. Superior protection in IV BCG-vaccinated NHPs was associated with greater induction of multifunctional CD4+ T cell producing IFN-γ and TNF-α [187] and with higher numbers of antigen-responsive CD4+ and CD8+ T cells in the blood, spleen, BAL and lung lymph nodes, as well as a higher frequency of antigen-responsive T cells across all lung parenchymal tissues [165]. Further insight into the exact mechanisms of protection will require additional cross-sectional analysis of responses in studies where a greater range of protection is elicited within an intervention group.
Prevention of TB in macaques has also been demonstrated following immunisation with the RhCMV/TB vaccine candidate, which was associated with greater induction and maintenance of high frequencies of highly effector-differentiated circulating and tissue-resident M.tb-specific CD4+ and CD8+ memory T cell responses compared with BCG [129]. Interestingly, there were no significant antibody responses to the nine TB antigens in the RhCMV vector inserts, suggesting that antibodies do not contribute to the protection observed [129]. However, IgG1 was the only subclass tested, and the potential relevance of different isotypes has been highlighted in Sect. 6.4.2 [166].
5.1.5 Novel Humanized Animal Models
While mammals are the most frequently used experimental animal models in TB vaccine development, mycobacterial infection of invertebrates (zebrafish, the fruit fly Drosophila melanogaster and the amoeba Dictyostelium discoideum) has provided novel insights, particularly with respect to elucidating the early events following mycobacterial infection. Such models offer advantages in terms of ethics, resources, costs and technological ease [175]. Zebrafish can develop granulomas after infection with M. marinum with some parallels to human M.tb infection, and the innate and adaptive immune responses share the same primary cellular components with humans [189]. However, a major limitation is that zebrafish do not have lungs, and additional evidence is required to establish the ability to translate these findings to human physiology during M.tb infection [175].
5.2 Clinical Studies
While preclinical studies have undoubtedly been central to advancing our understanding of the immune response to M.tb and generating hypotheses regarding potential correlates of protection, the immunopathogenesis of M.tb in humans is complex, highly heterogeneous between individuals and subject to biological and environmental influences that are impossible to model experimentally in animals. Thus, studies using clinical samples are key. There are two main approaches to identifying potential correlates of risk of, or protection from, TB using clinical samples. The first is to perform studies using samples from TB vaccine efficacy trials, comparing immune responses in individuals who remained healthy (‘controls’) with those who became M.tb infected or developed TB disease (‘cases’). However, few TB vaccine efficacy trials have been conducted to date, which limits opportunities for such analysis. The other strategy is to perform observational studies comparing immune responses in (a) individuals with LTBI, who are considered to have some degree of protection, with ATB patients; (b) LTBI individuals who go on to develop ATB with those who do not; or (c) household contacts of ATB patients who remain uninfected with those who become M.tb infected or develop ATB. An overview of clinical studies and their main findings with respect to potential correlates of protection is summarized in Table 6.1.
5.2.1 Clinical TB Vaccine Trials
As discussed, a study of over 5500 South African infants attempted to identify correlates of protection against childhood TB disease after BCG vaccination at birth. Infants were followed for 2 years and classified as controls if they did not have a household TB contact and did not develop TB disease (community controls, n = 55) or as having a household TB contact in which case they were grouped into those who developed TB disease (cases, n = 29) and those who did not develop TB disease (household contact controls, n = 55) [89]. Unfortunately, no differences were identified between protected and unprotected infants in the frequencies of BCG-specific CD4+ T cells, CD8+ T cells, γδ T cells or the extended cytokine profiles of these cells [89]. A different study in BCG-vaccinated South African infants with a 2-year follow-up compared host responses in blood at 10 weeks of age between infants who developed ATB during the follow-up period and those who remained healthy [190]. Gene expression analysis failed to identify any correlates of protection, but two distinct clusters of infants were evident with different myeloid and lymphoid activation and inflammatory patterns. Cases from each cluster demonstrated distinct patterns of gene expression, suggesting that unique correlates of risk may not be found within clusters. Interestingly, infants with the highest or lowest ratios of monocytes to lymphocytes (ML ratio) were at risk of developing TB disease [190]. This association has also been reported in other studies [29, 191].
Although the candidate TB vaccine MVA85A did not confer statistically significant efficacy in a Phase IIb trial in BCG-vaccinated South African infants [88], the samples collected provide a rich resource for identification of potential correlates of protection. In a case-control correlates of risk analysis using samples from 53 infants who developed TB disease and 205 matched controls, BCG-specific IFN-γ secreting T cells were associated with reduced risk of TB disease [90]. This is inconsistent with the previously described findings of Kagina et al., which may be a result of different methods for measuring IFN-γ and the different time-points post-BCG vaccination at which this was done [89]. Levels of Ag85A-specific IgG were also associated with reduced risk of TB disease, while the frequency of activated HLA-DR+ CD4+ T cells was associated with increased risk as validated in an independent cohort of M.tb-infected adolescents [90]. A follow-up transcriptomics analysis found that cytomegalovirus (CMV) infection was a major driver of CD8+ T cell activation and that a CMV-specific IFN-γ response was associated with increased risk of developing TB disease [192]. In CMV-positive infants, NK cell signatures and cell frequencies were associated with lower risk of TB disease [192]. Interestingly, a potential role for NK cells in protection from TB has also been identified in a multi-cohort study using CyTOF for systems analysis of immune cell frequency combined with transcriptional analysis [193].
Promising results from more recent vaccine trials provide further opportunities for assessing potential correlates of protection. A Phase IIb efficacy trial of the M72/AS01E candidate vaccine reported 54% protection against ATB in M.tb-infected individuals, with an overall efficacy of 49.7% (90% CI, 12.1–71.2; 95% CI, 2.1–74.2) at 3 years [164], while BCG revaccination in South African adolescents reduced the rate of sustained QFT conversion by 45.4% [194]. An international consortium known as the ‘TB Immune Correlate Program’ has been formed to evaluate samples from these trials, although the relatively small number of participants that reached clinical endpoints (39 individuals developed ATB in the M72/AS01E trial, and 57 individuals had sustained QFT conversion in the BCG revaccination trial) may limit statistical power to identify correlates. BCG revaccination has been shown to induce long-lived BCG-reactive NK cell responses in South African adults [195] and to boost adaptive polyfunctional Th1/Th17 responses and innate effector cells in a different cohort of IGRA+ and IGRA− Indian adults [196].
5.2.2 Observational Studies
Observational studies do not include an intervention and are therefore less logistically complex than vaccine trials. Several such studies have taken a systems analysis approach to enable the unbiased simultaneous detection of multiple parameters—particularly through transcriptomics. In a prospective cohort study, Zak et al. defined a 16-gene signature that could predict TB progression a year prior to TB diagnosis. This signature was comparable in whole blood and peripheral blood mononuclear cells, which suggests a minimal role for granulocytes and other whole blood components. The only gene module that was over-represented in the risk signature was the interferon response [197]. Another transcriptomic study reporting a gene signature that could discriminate between LTBI and ATB also demonstrated the importance of the interferon pathway, as it was dominated by a neutrophil-driven IFN-inducible gene profile, consisting of both IFN-γ and Type I IFN-αβ signalling [49].
Additional studies across different countries have validated and refined the signatures for progression to ATB down to four transcripts and again confirm the importance of the Type I IFN pathway in TB disease risk [198, 199]. Scriba et al. showed that progression from LTBI to ATB is defined by sequential inflammatory processes triggered by a rise in Type I IFN and activation of the complement cascade, including a change in ML ratio, a rise in HLA-DR+ CD4+ T cells and a decline in naïve T cells [200]. One challenge to such longitudinal studies of progression to ATB is the relatively low rate of reactivation (5–10%) in LTBI individuals, necessitating large sample sizes and long follow-up periods. Furthermore, it is difficult to draw any inferences, as findings may represent features of subclinical or incipient TB disease rather than being a cause of TB susceptibility. Nevertheless, there is obvious merit in the ability to identify individuals at risk of progression to ATB.
In addition to transcriptomic studies, other high-throughput and unbiased systems approaches such as proteomics, metabolomics and systems serology are being applied to similar cohorts and could shed light on new protective immune pathways and parameters to progress to further study [156, 201].
5.3 Controlled Human Infection Models
Controlled human infection models (CHIMs) are a powerful tool to fast-track vaccine development and to characterise pathophysiology and have led to the licensure of vaccines against influenza, malaria and cholera [202,203,204,205,206]. Given the unclear predictive value of animal models, a Mycobacteria CHIM could expedite large-scale field trials and aid therapeutic development and the identification of correlates of protection. However, infecting humans with virulent M.tb would be unethical. M.tb clearance requires months of treatment with potentially toxic therapeutics, and volunteers may remain infectious during antibiotic therapy, with risk of community transmission [207, 208]. In addition, there is an unclear curative endpoint due to the lack of a definitive marker of infection. This leads to risk of treatment failure and the establishment of latency in volunteers [9, 209]. Instead, PPD and BCG have been used as surrogate models of M.tb infection in CHIMs. Both products are licenced for human use, as PPD is used for TST, while BCG is delivered as a vaccine. This facilitates their ease of procurement and delivery in CHIMs.
5.3.1 PPD CHIMs
While PPD cannot be used to test vaccine efficacy as it is not a live replicating organism and hence quantification of bacterial growth control is not possible, it contains antigens from M.tb not found in BCG and hence could provide adjunct information about immune mechanisms following M.tb antigen exposure.
5.3.2 BCG CHIMs
BCG is a live replicating Mycobacterium, which has been used safely as a licenced ID vaccination against TB in humans for over 100 years [210, 211]. BCG is less virulent than M.tb in humans as it lacks the region of difference 1 (RD1) locus which encodes the virulent ESX-1 secretion system [212, 213]. BCG does not cause disease in immunocompetent people, and therefore a BCG CHIM enables the study of an effective mycobacterial clearing immune response [214, 215]. While BCG elicits a similar CD4+ T cell-mediated immune response to that of M.tb [215,216,217], differences in immune responses between BCG and M.tb have been described in human in vitro and animal in vivo models [13, 218, 219]. In the absence of an M.tb CHIM, the BCG CHIM is a useful surrogate, designed to be used in conjunction with M.tb animal models and human TB epidemiological studies.
The first BCG CHIM utilised the ID route, which enables easy quantification of BCG growth control via skin punch biopsy [215]. Peak BCG growth following 1–4 × 105 colony forming units (CFU) ID injection of BCG Danish 1331 was demonstrated at 1–2 weeks, with 2 weeks providing the least variable endpoint while allowing time for the adaptive immune response to affect growth control. This safe, feasible BCG CHIM model demonstrated superior mycobacterial growth control in historically BCG vaccinated compared with BCG-naïve participants and has been validated against a vaccine effect following virulent challenge in mice, NHPs and cattle [215]. This supports the utility of this model to assess vaccine efficacy in this M.tb-naïve UK adult population, where BCG vaccination is known to be protective [220].
The ID BCG CHIM has also been used to assess the efficacy of MVA85A, demonstrating no added benefit over historic BCG vaccination alone [221]. This finding corresponds to results from the Phase IIb efficacy trial in South African infants, supporting the validity of this model in assessing mycobacterial vaccine efficacy [88]. Importantly, both ID BCG CHIM studies reported an association between the PPD-specific IFN-γ ELISpot response at 2 weeks post-challenge and control of growth of mycobacteria isolated from a skin biopsy of the challenge site [88, 215]. This is consistent with the findings of the correlates of risk analysis using samples from the MVA85A efficacy trial where IFN-γ ELISpot was associated with lower risk of TB disease [90]. Transcriptional analysis of blood samples from human challenge studies also identified IFN-γ and IL-18 transcripts as correlates of in vivo mycobacterial growth control [222].
While ID BCG CHIMs show promise, the natural route of M.tb infection is via the airway. Vaccine efficacy varies depending on route of delivery, and protective immune mechanisms can be localised to the tissue infection site [165, 223]. Hence work is underway to develop an aerosol BCG CHIM. Three studies in the 1960s and 1970s delivered intrapulmonary BCG to healthy volunteers or lung cancer patients and demonstrated good safety profiles [224,225,226]. In 2019, a proof-of-concept safety study was published, which delivered up to 0.5 tuberculin units of PPD and 1 × 104 CFU BCG by local bronchoscopic instillation to mycobacteria-sensitised South African adults without any clinically significant adverse events [227]. However, bronchoscopic instillation is not the natural route of infection. To model naturally acquired M.tb infection, McShane et al. are developing an aerosol CHIM in which BCG is inhaled using an Omron NE-U22 micro air mesh nebuliser. Following aerosol inhalation, BCG is recovered at a defined time-point via bronchoscopic saline lavage (ClinicalTrials.gov NCT02709278) (Fig. 6.4). Work is ongoing to quantify the BCG in the airway, which could then be used as a marker of in vivo mycobacterial growth control, informing vaccine efficacy and providing opportunities for the identification of immune correlates of protection (H McShane, personal communication).
Inhaled BCG controlled human infection model (CHIM). Healthy M.tb-naïve subjects receive BCG by the aerosol route using a nebuliser. BCG is recovered via bronchoscopic saline lavage at a defined time-point. AE aerosol. (Created with BioRender.com)
5.3.3 Other CHIM Models
One major drawback of the BCG CHIM is the inability to test efficacy of vaccine candidates based on the RD1 antigens, many of which are currently in the vaccine development pipeline [228, 229]. Furthermore, an M.tb CHIM would enable full elucidation of immune mechanisms, some of which may not occur following exposure to the less virulent BCG. Candidates for an M.tb CHIM could include the attenuated vaccine candidate MTBVAC [230]. More preferable would be a genetically modified but virulent strain that can be definitely cleared, such as the M.tb strain being developed by a consortium of bacterial geneticists with support from the Gates Foundation, which only survives in the presence of continuous antibiotic or other therapy (S Fortune, personal communication).
In the absence of an ethical virulent M.tb strain available for CHIM studies, the BCG CHIM promises to be a useful tool to fast-track vaccine candidate selection and to further our understanding of protective immune mechanisms.
5.4 Mycobacterial Growth Inhibition Assays
An alternative approach to identifying correlates of protection is the use of functional mycobacterial growth inhibition assays (MGIAs). Rather than measuring predefined individual immune parameters of unknown relevance, MGIAs are unbiased and take into account a range of immune mechanisms and their complex interactions in an ex vivo environment; as such they may represent a surrogate of protective efficacy in themselves [231]. They also provide a tractable model in which effector functions may be studied through such techniques as cell depletion or concentration or in vitro ‘adoptive transfer’. MGIAs offer further advantages of being high-throughput, inexpensive and a 3Rs refinement to preclinical vaccine efficacy and correlates of protection studies, as they avoid the need for in vivo M.tb challenge [232].
While simple in principle—blood or cells are collected pre- and post-vaccination, and ability to inhibit growth of mycobacteria is measured following in vitro inoculation—these assays are notoriously technically challenging and have historically suffered from a lack of reproducibility and transferability [233]. Several approaches have been described with varying degrees of success, including the addition of stimulated lymphocytes to infected monocytes [234], the co-culture of bone marrow-derived macrophages and splenocytes [235] and the use of luciferase-expressing reporter strains of mycobacteria [236]. These and other MGIAs have been comprehensively reviewed elsewhere [231]. More recently, a significant effort has been made by Tanner et al. to develop a simplified cross-species assay known as the ‘direct MGIA’. This assay, adapted from a method originally developed for TB drug evaluation by Wallis et al. [237], has now been optimized and harmonized for use in humans, mice and NHPs [238,239,240]. Mycobacterial growth in the MGIA has been associated with TB disease state and treatment status [241], and ability to detect a BCG vaccine effect using this assay has been demonstrated across multiple studies [238, 239, 242,243,244,245]. Some degree of biological validation has been achieved through correlation with protection from in vivo mycobacterial challenge [239, 245] or protection from vaccine candidates of varying efficacy [246, 247].
Various MGIA studies have indicated a detrimental effect of depletion, or enhancing effect of enrichment, of CD4+ T cells and/or CD8+ T cells on control of mycobacterial growth [248,249,250,251]. A role for γδ T cells has also been proposed [249]. Notably, most published MGIA studies report no correlation between mycobacterial growth inhibition and IFN-γ responses, despite both being significantly enhanced following BCG vaccination [25, 236, 238, 242, 252]. However, in an in vivo challenge study in UK adults, control of mycobacterial growth at baseline in historically BCG-vaccinated volunteers was associated with the IFN-γ ELISpot response measured at 2 weeks post-challenge [245]. The authors suggest that this disparity is likely because the challenge study focused on associations with post-infection in vivo responses, which permits consideration of re-stimulated memory responses in historically vaccinated volunteers [245]. Since the MGIA models an infection, immune parameters induced by in vivo challenge and contributing to control of mycobacterial replication in vivo may reveal those driving control of mycobacterial growth in the MGIA. Interestingly, there was an association between MGIA control and frequencies of several subsets of polyfunctional CD4+ T cells including IFN-γ, TNF-α and IL-2 triple-positive cells [245], which was consistent with findings by Smith et al. [244]. However, others have reported no correlation between MGIA response and polyfunctional T cells, which may be due to the measurement of effector responses soon after vaccination [25, 247].
In an MGIA study comparing healthy volunteers, individuals with LTBI and patients with active TB disease, several immune parameters were associated with differential mycobacterial control including distinct monocyte subsets, B cell subsets and IgG1 responses [241]. IgG responses post-challenge have also been weakly associated with control of mycobacterial growth in BCG-vaccinated individuals [245]. Hierarchical cluster analysis of serum cytokine responses revealed correlations between high analyte levels and enhanced mycobacterial control for several cytokines including CXCL-10 and PDGF-BB [241]. Interestingly, Joosten et al. noted an association between control of mycobacterial growth and the presence of a CXCL10-producing CD14dim monocyte population, which was dependent on the presence of T cells. Thus, trained innate immunity may contribute to the superior control of mycobacterial growth they observed in individuals with recent exposure to M.tb and some BCG vaccinated individuals [25]. Finally, distinct transcriptomic profiles have been associated with good vs. poor mycobacterial control in the MGIA, with good controllers showing enrichment for gene sets associated with antigen processing/presentation and the IL-23 pathway and poor controllers showing enrichment for hypoxia-related pathways [245]. Such insights may be hypothesis-generating with respect to potential correlates of protection that can be taken forward to in vivo studies.
6 Conclusion
The lack of validated immune correlates of protection remains a major barrier to the development of new TB vaccines. While IFN-γ secreting CD4+ T cells are clearly a cornerstone of the immune response to TB, the fact that boosting with MVA85A failed to confer enhanced protection over BCG alone, despite generating a higher level of these cells, suggests that we need to look beyond this paradigm. Progress is now being made in understanding the role of previously under-explored immune parameters including unconventional T cells, Th17 cells, B cells and antibodies, although their exact contribution to protective immunity and potential as correlates of protection remain ill-defined. For such a complex pathogen, it is possible that a ‘biosignature’ comprising a combination of immune measures rather than any given single parameter will be necessary. Compartmentalization of immune responses may also be important to consider, particularly as local responses in the lung may correlate better with protection from initial infection than those in the periphery. Recent advances in the TB field are providing key opportunities for correlate of protection analysis. Notably, evidence of sterilizing immunity in preclinical studies, positive efficacy results in proof-of-concept clinical trials, advances in systems technologies and the development of in vivo CHIMs and in vitro MGIAs will provide the samples and tools to identify new correlates, with the aim of facilitating and expediting the design, optimization, prioritization and clinical assessment of candidate TB vaccines.
References
Plotkin SA, Gilbert PB. Nomenclature for immune correlates of protection after vaccination. Clin Infect Dis. 2012;54(11):1615–7.
Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol. 2009;27:393–422.
Simmons JD, Stein CM, Seshadri C, Campo M, Alter G, Fortune S, et al. Immunological mechanisms of human resistance to persistent Mycobacterium tuberculosis infection. Nat Rev Immunol. 2018;18(9):575–89.
Fox GJ, Barry SE, Britton WJ, Marks GB. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2013;41(1):140.
Mahomed H, Hawkridge T, Verver S, Abrahams D, Geiter L, Hatherill M, et al. The tuberculin skin test versus QuantiFERON TB Gold® in predicting tuberculosis disease in an adolescent cohort study in South Africa. PLoS One. 2011;6(3):e17984.
Andrews JR, Hatherill M, Mahomed H, Hanekom WA, Campo M, Hawn TR, et al. The dynamics of QuantiFERON-TB gold in-tube conversion and reversion in a cohort of South African adolescents. Am J Respir Crit Care Med. 2015;191(5):584–91.
Verrall AJ, Schneider M, Alisjahbana B, Apriani L, van Laarhoven A, Koeken VACM, et al. Early clearance of Mycobacterium tuberculosis is associated with increased innate immune responses. J Infect Dis. 2019;221(8):1342–50.
Ewer K, Millington KA, Deeks JJ, Alvarez L, Bryant G, Lalvani A. Dynamic antigen-specific T-cell responses after point-source exposure to Mycobacterium tuberculosis. Am J Respir Crit Care Med. 2006;174(7):831–9.
Ringshausen FC, Nienhaus A, Schablon A, Schlösser S, Schultze-Werninghaus G, Rohde G. Predictors of persistently positive Mycobacterium-tuberculosis-specific interferon-gamma responses in the serial testing of health care workers. BMC Infect Dis. 2010;10(1):220.
Howard WL, Klopfenstein MD, Steininger WJ, Woodruff CE. The loss of tuberculin sensitivity in certain patients with active pulmonary tuberculosis. Chest. 1970;57(6):530–4.
Pai M, Lewinsohn DM. Interferon-γ assays for tuberculosis. Am J Respir Crit Care Med. 2005;172(5):519–21.
Pai M, O’Brien R. Serial testing for tuberculosis: can we make sense of T cell assay conversions and reversions? PLoS Med. 2007;4(6):e208.
Dwivedi VP, Bhattacharya D, Chatterjee S, Prasad DVR, Chattopadhyay D, Van Kaer L, et al. Mycobacterium tuberculosis directs T helper 2 cell differentiation by inducing interleukin-1β production in dendritic cells. J Biol Chem. 2012;287(40):33656–63.
Sia JK, Georgieva M, Rengarajan J. Innate immune defenses in human tuberculosis: an overview of the interactions between Mycobacterium tuberculosis and innate immune cells. J Immunol Res. 2015;2015:747543.
Cohen SB, Gern BH, Delahaye JL, Adams KN, Plumlee CR, Winkler JK, et al. Alveolar macrophages provide an early Mycobacterium tuberculosis niche and initiate dissemination. Cell Host Microbe. 2018;24(3):439–46.e4.
Taylor PR, Gordon S. Monocyte heterogeneity and innate immunity. Immunity. 2003;19(1):2–4.
Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol. 2014;14(8):571–8.
Hambleton S, Salem S, Bustamante J, Bigley V, Boisson-Dupuis S, Azevedo J, et al. IRF8 mutations and human dendritic-cell immunodeficiency. N Engl J Med. 2011;365(2):127–38.
Granot T, Senda T, Carpenter DJ, Matsuoka N, Weiner J, Gordon CL, et al. Dendritic cells display subset and tissue-specific maturation dynamics over human life. Immunity. 2017;46(3):504–15.
de Chastellier C. The many niches and strategies used by pathogenic mycobacteria for survival within host macrophages. Immunobiology. 2009;214(7):526–42.
Wolf AJ, Desvignes L, Linas B, Banaiee N, Tamura T, Takatsu K, et al. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J Exp Med. 2007;205(1):105–15.
Wolf AJ, Linas B, Trevejo-Nuñez GJ, Kincaid E, Tamura T, Takatsu K, et al. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J Immunol. 2007;179(4):2509–19.
Chackerian AA, Alt JM, Perera TV, Dascher CC, Behar SM. Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infect Immunity. 2002;70(8):4501–9.
Tian T, Woodworth J, Sköld M, Behar SM. In vivo depletion of CD11c+ cells delays the CD4+ T cell response to Mycobacterium tuberculosis and exacerbates the outcome of infection. J Immunol. 2005;175(5):3268–72.
Joosten SA, van Meijgaarden KE, Arend SM, Prins C, Oftung F, Korsvold GE, et al. Mycobacterial growth inhibition is associated with trained innate immunity. J Clin Invest. 2018;128(5):1837–51.
Tornack J, Reece ST, Bauer WM, Vogelzang A, Bandermann S, Zedler U, et al. Human and mouse hematopoietic stem cells are a depot for dormant Mycobacterium tuberculosis. PLoS One. 2017;12(1):e0169119.
de Jong R, Altare F, Haagen IA, Elferink DG, Boer T, van Breda Vriesman PJ, et al. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science. 1998;280(5368):1435–8.
Fortin A, Abel L, Casanova JL, Gros P. Host genetics of mycobacterial diseases in mice and men: forward genetic studies of BCG-osis and tuberculosis. Annu Rev Genomics Hum Genet. 2007;8(1):163–92.
Naranbhai V, Kim S, Fletcher H, Cotton MF, Violari A, Mitchell C, et al. The association between the ratio of monocytes:lymphocytes at age 3 months and risk of tuberculosis (TB) in the first two years of life. BMC Med. 2014;12(1):120.
Rakotosamimanana N, Richard V, Raharimanga V, Gicquel B, Doherty TM, Zumla A, et al. Biomarkers for risk of developing active tuberculosis in contacts of TB patients: a prospective cohort study. Eur Respir J. 2015;46(4):1095–103.
Sutherland JS, Jeffries DJ, Donkor S, Walther B, Hill PC, Adetifa IMO, et al. High granulocyte/lymphocyte ratio and paucity of NKT cells defines TB disease in a TB-endemic setting. Tuberculosis. 2009;89(6):398–404.
Rogers PM. A study of the blood monocytes in children with tuberculosis. N Engl J Med. 1928;198(14):740–9.
Sánchez MD, García Y, Montes C, París SC, Rojas M, Barrera LF, et al. Functional and phenotypic changes in monocytes from patients with tuberculosis are reversed with treatment. Microbes Infect. 2006;8(9):2492–500.
Kaufmann SHE, Brill KJ, Li Q, Larkin R, Canaday DH, Kaplan DR, et al. Human natural killer cells mediate killing of intracellular Mycobacterium tuberculosis H37Rv via granule-independent mechanisms. Infect Immunity. 2001;69(3):1755–65.
Molloy A, Meyn PA, Smith KD, Kaplan G. Recognition and destruction of Bacillus Calmette-Guerin-infected human monocytes. J Exp Med. 1993;177(6):1691–8.
Harris LD, Khayumbi J, Ongalo J, Sasser LE, Tonui J, Campbell A, et al. Distinct human NK cell phenotypes and functional responses to Mycobacterium tuberculosis in adults from TB endemic and non-endemic regions. Front Cellular Infect Microbiol. 2020;10:120.
Prabowo SA, Zelmer A, Stockdale L, Ojha U, Smith SG, Seifert K, et al. Historical BCG vaccination combined with drug treatment enhances inhibition of mycobacterial growth ex vivo in human peripheral blood cells. Sci Rep. 2019;9(1):4842.
Prabowo SA, Smith SG, Seifert K, Fletcher HA. Impact of individual-level factors on Ex vivo mycobacterial growth inhibition: associations of immune cell phenotype, cytomegalovirus-specific response and sex with immunity following BCG vaccination in humans. Tuberculosis. 2019;119:101876.
Junqueira-Kipnis AP, Kipnis A, Jamieson A, Juarrero MG, Diefenbach A, Raulet DH, et al. NK cells respond to pulmonary infection with Mycobacterium tuberculosis, but play a minimal role in protection. J Immunol. 2003;171(11):6039.
Lyadova IV. Neutrophils in tuberculosis: heterogeneity shapes the way? Mediators Inflamm. 2017;2017:8619307.
Lacy P. Mechanisms of degranulation in neutrophils. Allergy Asthma Clin Immunol. 2006;2(3):98.
Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2004;23(1):197–223.
Martineau AR, Newton SM, Wilkinson KA, Kampmann B, Hall BM, Nawroly N, et al. Neutrophil-mediated innate immune resistance to mycobacteria. J Clin Invest. 2007;117(7):1988–94.
Fulton SA, Reba SM, Martin TD, Boom WH. Neutrophil-mediated mycobacteriocidal immunity in the lung during Mycobacterium bovis BCG infection in C57BL/6 mice. Infect Immunity. 2002;70(9):5322–7.
Bickett TE, McLean J, Creissen E, Izzo L, Hagan C, Izzo AJ, et al. Characterizing the BCG induced macrophage and neutrophil mechanisms for defense against Mycobacterium tuberculosis. Front Immunol. 2020;11:1202.
Nouailles G, Dorhoi A, Koch M, Zerrahn J, Weiner J III, Faé KC, et al. CXCL5-secreting pulmonary epithelial cells drive destructive neutrophilic inflammation in tuberculosis. J Clin Invest. 2014;124(3):1268–82.
Gopal R, Monin L, Torres D, Slight S, Mehra S, McKenna KC, et al. S100A8/A9 proteins mediate neutrophilic inflammation and lung pathology during tuberculosis. Am J Respir Crit Care Med. 2013;188(9):1137–46.
Scott NR, Swanson RV, Al-Hammadi N, Domingo-Gonzalez R, Rangel-Moreno J, Kriel BA, et al. S100A8/A9 regulates CD11b expression and neutrophil recruitment during chronic tuberculosis. J Clin Invest. 2020;130(6):3098–112.
Berry MPR, Graham CM, McNab FW, Xu Z, Bloch SAA, Oni T, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466(7309):973–7.
Eum S-Y, Kong J-H, Hong M-S, Lee Y-J, Kim J-H, Hwang S-H, et al. Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest. 2010;137(1):122–8.
Siddiqui S, Visvabharathy L, Wang C-R. Role of group 1 CD1-restricted T cells in infectious disease. Front Immunol. 2015;6:337.
Howson LJ, Salio M, Cerundolo V. MR1-restricted mucosal-associated invariant T cells and their activation during infectious diseases. Front Immunol. 2015;6:303.
Provine NM, Klenerman P. MAIT cells in health and disease. Annu Rev Immunol. 2020;38(1):203–28.
Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol. 2013;13(2):101–17.
Crosby CM, Kronenberg M. Tissue-specific functions of invariant natural killer T cells. Nat Rev Immunol. 2018;18(9):559–74.
Montoya CJ, Pollard D, Martinson J, Kumari K, Wasserfall C, Mulder CB, et al. Characterization of human invariant natural killer T subsets in health and disease using a novel invariant natural killer T cell-clonotypic monoclonal antibody, 6B11. Immunology. 2007;122(1):1–14.
Vorkas CK, Wipperman MF, Li K, Bean J, Bhattarai SK, Adamow M, et al. Mucosal-associated invariant and γδ T cell subsets respond to initial Mycobacterium tuberculosis infection. JCI Insight. 2018;3(19):e121899.
Sakala IG, Kjer-Nielsen L, Eickhoff CS, Wang X, Blazevic A, Liu L, et al. Functional heterogeneity and anti-mycobacterial effects of mouse mucosal associated invariant T (MAIT) cells specific for riboflavin metabolites. J Immunol. 2015;195(2):587–601.
Gold MC, Cerri S, Smyk-Pearson S, Cansler ME, Vogt TM, Delepine J, et al. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 2010;8(6):e1000407.
Seshadri C, Thuong NTT, Mai NTH, Bang ND, Chau TTH, Lewinsohn DM, et al. A polymorphism in human MR1 is associated with mRNA expression and susceptibility to tuberculosis. Genes Immun. 2017;18(1):8–14.
Coulter F, Parrish A, Manning D, Kampmann B, Mendy J, Garand M, et al. IL-17 production from T helper 17, mucosal-associated invariant T, and γδ cells in tuberculosis infection and disease. Front Immunol. 2017;8:1252.
Sakai S, Kauffman KD, Oh S, Nelson CE, Barry CE, Barber DL. MAIT cell-directed therapy of Mycobacterium tuberculosis infection. Mucosal Immunol. 2021;14(1):199–208.
Chancellor A, White A, Tocheva AS, Fenn JR, Dennis M, Tezera L, et al. Quantitative and qualitative iNKT repertoire associations with disease susceptibility and outcome in macaque tuberculosis infection. Tuberculosis. 2017;105:86–95.
Chackerian A, Alt J, Perera V, Behar SM. Activation of NKT cells protects mice from tuberculosis. Infect Immunity. 2002;70(11):6302–9.
Sada-Ovalle I, Chiba A, Gonzales A, Brenner MB, Behar SM. Innate invariant NKT cells recognize Mycobacterium tuberculosis–infected macrophages, produce interferon-γ, and kill intracellular bacteria. PLoS Pathog. 2008;4(12):e1000239.
Hiromatsu K, Dascher CC, LeClair KP, Sugita M, Furlong ST, Brenner MB, et al. Induction of CD1-restricted immune responses in guinea pigs by immunization with mycobacterial lipid antigens. J Immunol. 2002;169(1):330.
Freud AG, Mundy-Bosse BL, Yu J, Caligiuri MA. The broad spectrum of human natural killer cell diversity. Immunity. 2017;47(5):820–33.
Kleinnijenhuis J, Quintin J, Preijers F, Joosten LAB, Jacobs C, Xavier RJ, et al. BCG-induced trained immunity in NK cells: role for non-specific protection to infection. Clin Immunol. 2014;155(2):213–9.
Kaufmann E, Sanz J, Dunn JL, Khan N, Mendonça LE, Pacis A, et al. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell. 2018;172(1–2):176–90.e19.
Caruso AM, Serbina N, Klein E, Triebold K, Bloom BR, Flynn JL. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J Immunol. 1999;162(9):5407–16.
Scanga CA, Mohan VP, Yu K, Joseph H, Tanaka K, Chan J, et al. Depletion of CD4(+) T cells causes reactivation of murine persistent tuberculosis despite continued expression of interferon gamma and nitric oxide synthase 2. J Exp Med. 2000;192(3):347–58.
Mogues T, Goodrich ME, Ryan L, LaCourse R, North RJ. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J Exp Med. 2001;193(3):271–80.
Lin PL, Rutledge T, Green AM, Bigbee M, Fuhrman C, Klein E, et al. CD4 T cell depletion exacerbates acute Mycobacterium tuberculosis while reactivation of latent infection is dependent on severity of tissue depletion in cynomolgus macaques. AIDS Res Hum Retroviruses. 2012;28(12):1693–702.
Orme IM. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987;138(1):293–8.
Diedrich CR, Mattila JT, Klein E, Janssen C, Phuah J, Sturgeon TJ, et al. Reactivation of latent tuberculosis in cynomolgus macaques infected with SIV is associated with early peripheral T cell depletion and not virus load. PLoS One. 2010;5(3):e9611.
Geldmacher C, Zumla A, Hoelscher M. Interaction between HIV and Mycobacterium tuberculosis: HIV-1-induced CD4 T-cell depletion and the development of active tuberculosis. Curr Opin HIV AIDS. 2012;7(3):268–75.
Jones BE, Young SM, Antoniskis D, Davidson PT, Kramer F, Barnes PF. Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. Am Rev Respir Dis. 1993;148(5):1292–7.
Lawn SD, Myer L, Edwards D, Bekker LG, Wood R. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. Aids. 2009;23(13):1717–25.
Barnes PF, Lakey DL, Burman WJ. Tuberculosis in patients with HIV infection. Infect Dis Clin North Am. 2002;16(1):107–26.
Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178(6):2249–54.
Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178(6):2243–7.
Seneviratne SL, Doffinger R, Macfarlane J, Ceron-Gutierrez L, Amel Kashipaz MR, Robbins A, et al. Disseminated Mycobacterium tuberculosis infection due to interferon gamma deficiency. Response to replacement therapy. Thorax. 2007;62(1):97–9.
Jouanguy E, Altare F, Lamhamedi S, Revy P, Emile JF, Newport M, et al. Interferon-gamma-receptor deficiency in an infant with fatal bacille Calmette-Guérin infection. N Engl J Med. 1996;335(26):1956–61.
Gallegos AM, van Heijst JW, Samstein M, Su X, Pamer EG, Glickman MS. A gamma interferon independent mechanism of CD4 T cell mediated control of M. tuberculosis infection in vivo. PLoS Pathog. 2011;7(5):e1002052.
Elias D, Akuffo H, Britton S. PPD induced in vitro interferon gamma production is not a reliable correlate of protection against Mycobacterium tuberculosis. Trans R Soc Trop Med Hyg. 2005;99(5):363–8.
Mittrücker HW, Steinhoff U, Köhler A, Krause M, Lazar D, Mex P, et al. Poor correlation between BCG vaccination-induced T cell responses and protection against tuberculosis. Proc Natl Acad Sci U S A. 2007;104(30):12434–9.
Majlessi L, Simsova M, Jarvis Z, Brodin P, Rojas MJ, Bauche C, et al. An increase in antimycobacterial Th1-cell responses by prime-boost protocols of immunization does not enhance protection against tuberculosis. Infect Immun. 2006;74(4):2128–37.
Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet. 2013;381(9871):1021–8.
Kagina BM, Abel B, Scriba TJ, Hughes EJ, Keyser A, Soares A, et al. Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis after bacillus Calmette-Guérin vaccination of newborns. Am J Respir Crit Care Med. 2010;182(8):1073–9.
Fletcher HA, Snowden MA, Landry B, Rida W, Satti I, Harris SA, et al. T-cell activation is an immune correlate of risk in BCG vaccinated infants. Nat Commun. 2016;7:11290.
Sahiratmadja E, Alisjahbana B, de Boer T, Adnan I, Maya A, Danusantoso H, et al. Dynamic changes in pro- and anti-inflammatory cytokine profiles and gamma interferon receptor signaling integrity correlate with tuberculosis disease activity and response to curative treatment. Infect Immunity. 2007;75(2):820–9.
Hirsch CS, Toossi Z, Othieno C, Johnson JL, Schwander SK, Robertson S, et al. Depressed T-cell interferon-gamma responses in pulmonary tuberculosis: analysis of underlying mechanisms and modulation with therapy. J Infect Dis. 1999;180(6):2069–73.
Morosini M, Meloni F, Marone Bianco A, Paschetto E, Uccelli M, Pozzi E, et al. The assessment of IFN-gamma and its regulatory cytokines in the plasma and bronchoalveolar lavage fluid of patients with active pulmonary tuberculosis. Int J Tuberc Lung Dis. 2003;7(10):994–1000.
Verbon A, Juffermans N, Van Deventer SJ, Speelman P, Van Deutekom H, Van Der Poll T. Serum concentrations of cytokines in patients with active tuberculosis (TB) and after treatment. Clin Exp Immunol. 1999;115(1):110–3.
Panteleev AV, Nikitina IY, Burmistrova IA, Kosmiadi GA, Radaeva TV, Amansahedov RB, et al. Severe tuberculosis in humans correlates best with neutrophil abundance and lymphocyte deficiency and does not correlate with antigen-specific CD4 T-cell response. Front Immunol. 2017;8:963.
Nikitina IY, Panteleev AV, Sosunova EV, Karpina NL, Bagdasarian TR, Burmistrova IA, et al. Antigen-specific IFN-γ responses correlate with the activity of M. tuberculosis infection but are not associated with the severity of tuberculosis disease. J Immunol Res. 2016;2016:7249369.
Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13(7):843–50.
Boaz MJ, Waters A, Murad S, Easterbrook PJ, Vyakarnam A. Presence of HIV-1 Gag-specific IFN-gamma+IL-2+ and CD28+IL-2+ CD4 T cell responses is associated with nonprogression in HIV-1 infection. J Immunol. 2002;169(11):6376–85.
Pollock KM, Whitworth HS, Montamat-Sicotte DJ, Grass L, Cooke GS, Kapembwa MS, et al. T-cell immunophenotyping distinguishes active from latent tuberculosis. J Infect Dis. 2013;208(6):952–68.
Petruccioli E, Petrone L, Vanini V, Sampaolesi A, Gualano G, Girardi E, et al. IFNγ/TNFα specific-cells and effector memory phenotype associate with active tuberculosis. J Infect. 2013;66(6):475–86.
Sutherland JS, Adetifa IM, Hill PC, Adegbola RA, Ota MO. Pattern and diversity of cytokine production differentiates between Mycobacterium tuberculosis infection and disease. Eur J Immunol. 2009;39(3):723–9.
Qiu Z, Zhang M, Zhu Y, Zheng F, Lu P, Liu H, et al. Multifunctional CD4 T cell responses in patients with active tuberculosis. Sci Rep. 2012;2:216.
Lewinsohn DA, Lewinsohn DM, Scriba TJ. Polyfunctional CD4(+) T cells as targets for tuberculosis vaccination. Front Immunol. 2017;8:1262.
Yuan X, Teng X, Jing Y, Ma J, Tian M, Yu Q, et al. A live attenuated BCG vaccine overexpressing multistage antigens Ag85B and HspX provides superior protection against Mycobacterium tuberculosis infection. Appl Microbiol Biotechnol. 2015;99(24):10587–95.
Ballester M, Nembrini C, Dhar N, De Titta A, De Piano C, Pasquier M, et al. Nanoparticle conjugation and pulmonary delivery enhance the protective efficacy of Ag85B and CpG against tuberculosis. Vaccine. 2011;29(40):6959–66.
Derrick SC, Yabe IM, Yang A, Morris SL. Vaccine-induced anti-tuberculosis protective immunity in mice correlates with the magnitude and quality of multifunctional CD4 T cells. Vaccine. 2011;29(16):2902–9.
Tchilian EZ, Desel C, Forbes EK, Bandermann S, Sander CR, Hill AVS, et al. Immunogenicity and protective efficacy of prime-boost regimens with recombinant Δ ureC hly+ Mycobacterium bovis BCG and modified vaccinia virus Ankara expressing M. tuberculosis antigen 85A against murine tuberculosis. Infect Immunity. 2009;77(2):622–31.
Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, et al. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol. 2001;25(3):335–40.
Scriba TJ, Kalsdorf B, Abrahams D-A, Isaacs F, Hofmeister J, Black G, et al. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J Immunol. 2008;180(3):1962.
Chen X, Zhang M, Liao M, Graner MW, Wu C, Yang Q, et al. Reduced Th17 response in patients with tuberculosis correlates with IL-6R expression on CD4+ T Cells. Am J Respir Crit Care Med. 2010;181(7):734–42.
Okamoto Yoshida Y, Umemura M, Yahagi A, O’Brien RL, Ikuta K, Kishihara K, et al. Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. J Immunol. 2010;184(8):4414–22.
Torrado E, Cooper AM. IL-17 and Th17 cells in tuberculosis. Cytokine Growth Factor Rev. 2010;21(6):455–62.
Gopal R, Lin Y, Obermajer N, Slight S, Nuthalapati N, Ahmed M, et al. IL‐23‐dependent IL‐17 drives Th1‐cell responses following Mycobacterium bovis BCG vaccination. Eur J Immunol. 2012;42(2):364–73.
Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369.
Gopal R, Monin L, Slight S, Uche U, Blanchard E, Fallert Junecko BA, et al. Unexpected role for IL-17 in protective immunity against hypervirulent Mycobacterium tuberculosis HN878 infection. PLoS Pathog. 2014;10(5):e1004099.
Dijkman K, Sombroek CC, Vervenne RAW, Hofman SO, Boot C, Remarque EJ, et al. Prevention of tuberculosis infection and disease by local BCG in repeatedly exposed rhesus macaques. Nat Med. 2019;25(2):255–62.
Lewinsohn DA, Swarbrick GM, Park B, Cansler ME, Null MD, Toren KG, et al. Comprehensive definition of human immunodominant CD8 antigens in tuberculosis. NPJ Vaccines. 2017;2(1):8.
Stenger S, Modlin RL. T cell mediated immunity to Mycobacterium tuberculosis. Curr Opin Microbiol. 1999;2(1):89–93.
Lewinsohn DA, Heinzel AS, Gardner JM, Zhu L, Alderson MR, Lewinsohn DM. Mycobacterium tuberculosis–specific CD8+ T cells preferentially recognize heavily infected cells. Am J Respir Crit Care Med. 2003;168(11):1346–52.
Kamath AB, Woodworth J, Xiong X, Taylor C, Weng Y, Behar SM. Cytolytic CD8+ T cells recognizing CFP10 are recruited to the lung after Mycobacterium tuberculosis infection. J Exp Med. 2004;200(11):1479–89.
Serbina NV, Liu CC, Scanga CA, Flynn JL. CD8+ CTL from lungs of Mycobacterium tuberculosis-infected mice express perforin in vivo and lyse infected macrophages. J Immunol. 2000;165(1):353–63.
Flynn JL, Goldstein MM, Triebold KJ, Koller B, Bloom BR. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci U S A. 1992;89(24):12013–7.
Sousa AO, Mazzaccaro RJ, Russell RG, Lee FK, Turner OC, Hong S, et al. Relative contributions of distinct MHC class I-dependent cell populations in protection to tuberculosis infection in mice. Proc Natl Acad Sci U S A. 2000;97(8):4204–8.
Urdahl KB, Liggitt D, Bevan MJ. CD8+ T cells accumulate in the lungs of Mycobacterium tuberculosis-infected Kb-/-Db-/- mice, but provide minimal protection. J Immunol. 2003;170(4):1987–94.
Stenger S, Rosat JP, Bloom BR, Krensky AM, Modlin RL. Granulysin: a lethal weapon of cytolytic T cells. Immunol Today. 1999;20(9):390–4.
Chen CY, Huang D, Wang RC, Shen L, Zeng G, Yao S, et al. A critical role for CD8 T cells in a nonhuman primate model of tuberculosis. PLoS Pathog. 2009;5(4):e1000392.
Wang J, Santosuosso M, Ngai P, Zganiacz A, Xing Z. Activation of CD8 T cells by mycobacterial vaccination protects against pulmonary tuberculosis in the absence of CD4 T cells. J Immunol. 2004;173(7):4590.
Farinacci M, Weber S, Kaufmann SH. The recombinant tuberculosis vaccine rBCG ΔureC::hly(+) induces apoptotic vesicles for improved priming of CD4(+) and CD8(+) T cells. Vaccine. 2012;30(52):7608–14.
Hansen SG, Zak DE, Xu G, Ford JC, Marshall EE, Malouli D, et al. Prevention of tuberculosis in rhesus macaques by a cytomegalovirus-based vaccine. Nat Med. 2018;24(2):130–43.
Yang JD, Mott D, Sutiwisesak R, Lu YJ, Raso F, Stowell B, et al. Mycobacterium tuberculosis-specific CD4+ and CD8+ T cells differ in their capacity to recognize infected macrophages. PLoS Pathog. 2018;14(5):e1007060.
Sok D, Moldt B, Burton DR. SnapShot: broadly neutralizing antibodies. Cell. 2013;155(3):728–728.e1.
Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17(7):1055–65.
Li H, Javid B. Antibodies and tuberculosis: finally coming of age? Nat Rev Immunol. 2018;18(9):591–6.
Vordermeier HM, Venkataprasad N, Harris DP, Ivanyi J. Increase of tuberculous infection in the organs of B cell-deficient mice. Clin Exp Immunol. 1996;106(2):312–6.
Johnson CM, Cooper AM, Frank AA, Bonorino CB, Wysoki LJ, Orme IM. Mycobacterium tuberculosis aerogenic rechallenge infections in B cell-deficient mice. Tuber Lung Dis. 1997;78(5–6):257–61.
Turner J, Frank AA, Brooks JV, Gonzalez-Juarrero M, Orme IM. The progression of chronic tuberculosis in the mouse does not require the participation of B lymphocytes or interleukin-4. Exp Gerontol. 2001;36(3):537–45.
Maglione PJ, Xu J, Chan J. B cells moderate inflammatory progression and enhance bacterial containment upon pulmonary challenge with Mycobacterium tuberculosis. J Immunol. 2007;178(11):7222–34.
Phuah J, Wong EA, Gideon HP, Maiello P, Coleman MT, Hendricks MR, et al. Effects of B cell depletion on early mycobacterium tuberculosis infection in cynomolgus macaques. Infect Immun. 2016;84(5):1301–11.
Rijnink WF, Ottenhoff THM, Joosten SA. B-cells and antibodies as contributors to effector immune responses in tuberculosis. Front Immunol. 2021;12:640168.
Mestas J, Hughes CCW. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172(5):2731–8.
Gottenberg JE, Ravaud P, Bardin T, Cacoub P, Cantagrel A, Combe B, et al. Risk factors for severe infections in patients with rheumatoid arthritis treated with rituximab in the autoimmunity and rituximab registry. Arthritis Rheum. 2010;62(9):2625–32.
Boisson-Dupuis S, Ramirez-Alejo N, Li Z, Patin E, Rao G, Kerner G, et al. Tuberculosis and impaired IL-23-dependent IFN-γ immunity in humans homozygous for a common TYK2 missense variant. Sci Immunol. 2018;3(30):eaau8714.
Potla R, Koeck T, Wegrzyn J, Cherukuri S, Shimoda K, Baker DP, et al. Tyk2 tyrosine kinase expression is required for the maintenance of mitochondrial respiration in primary Pro-B lymphocytes. Mol Cell Biol. 2006;26(22):8562–71.
Khader SA, Guglani L, Rangel-Moreno J, Gopal R, Junecko BAF, Fountain JJ, et al. IL-23 is required for long-term control of Mycobacterium tuberculosis and B cell follicle formation in the infected lung. J Immunol. 2011;187(10):5402–7.
Sebina I, Cliff JM, Smith SG, Nogaro S, Webb EL, Riley EM, et al. Long-lived memory B-cell responses following BCG vaccination. PLoS One. 2012;7(12):e51381.
Bitencourt J, Wilkie M, Alvarez MPP, Jacobs A, Wright D, Harris SA, et al. Induction of specific antibodies, IgG-secreting plasmablasts and memory B cells following BCG vaccination. bioRxiv. 2021:2021.02.18.431837.
Choreño-Parra JA, Weinstein LI, Yunis EJ, Zúñiga J, Hernández-Pando R. Thinking outside the box: innate- and B cell-memory responses as novel protective mechanisms against tuberculosis. Front Immunol. 2020;11:226.
Boom WH, Schaible UE, Achkar JM. The knowns and unknowns of latent Mycobacterium tuberculosis infection. J Clin Invest. 2021;131(3):e136222.
Kaushal D, Foreman TW, Gautam US, Alvarez X, Adekambi T, Rangel-Moreno J, et al. Mucosal vaccination with attenuated Mycobacterium tuberculosis induces strong central memory responses and protects against tuberculosis. Nat Commun. 2015;6:8533.
Jacobs AJ, Mongkolsapaya J, Screaton GR, McShane H, Wilkinson RJ. Antibodies and tuberculosis. Tuberculosis (Edinb). 2016;101:102–13.
Miura K, Orcutt AC, Muratova OV, Miller LH, Saul A, Long CA. Development and characterization of a standardized ELISA including a reference serum on each plate to detect antibodies induced by experimental malaria vaccines. Vaccine. 2008;26(2):193–200.
Hamasur B, Haile M, Pawlowski A, Schroder U, Kallenius G, Svenson SB. A mycobacterial lipoarabinomannan specific monoclonal antibody and its F(ab′) fragment prolong survival of mice infected with Mycobacterium tuberculosis. Clin Exp Immunol. 2004;138(1):30–8.
Teitelbaum R, Glatman-Freedman A, Chen B, Robbins JB, Unanue E, Casadevall A, et al. A mAb recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc Natl Acad Sci U S A. 1998;95(26):15688–93.
Kumagai T, Palacios A, Casadevall A, García MJ, Toro C, Tiemeyer M, et al. Serum IgM glycosylation associated with tuberculosis infection in mice. mSphere. 2019;4(2):e00684-18.
Irvine EB, Alter G. Understanding the role of antibody glycosylation through the lens of severe viral and bacterial diseases. Glycobiology. 2020;30(4):241–53.
Lu LL, Chung AW, Rosebrock TR, Ghebremichael M, Yu WH, Grace PS, et al. A functional role for antibodies in tuberculosis. Cell. 2016;167(2):433–43.e14.
Lu LL, Smith MT, Yu KKQ, Luedemann C, Suscovich TJ, Grace PS, et al. IFN-γ-independent immune markers of Mycobacterium tuberculosis exposure. Nat Med. 2019;25(6):977–87.
Li H, Wang X-X, Wang B, Fu L, Liu G, Lu Y, et al. Latently and uninfected healthcare workers exposed to TB make protective antibodies against Mycobacterium tuberculosis. Proc Natl Acad Sci. 2017;114(19):5023–8.
Li H, Wang XX, Wang B, Fu L, Liu G, Lu Y, et al. Latently and uninfected healthcare workers exposed to TB make protective antibodies against Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2017;114(19):5023–8.
Glatman-Freedman A, Casadevall A. Serum therapy for tuberculosis revisited: reappraisal of the role of antibody-mediated immunity against Mycobacterium tuberculosis. Clin Microbiol Rev. 1998;11(3):514–32.
Achkar JM, Casadevall A. Antibody-mediated immunity against tuberculosis: implications for vaccine development. Cell Host Microbe. 2013;13(3):250–62.
Tanner R, Villarreal-Ramos B, Vordermeier HM, McShane H. The humoral immune response to BCG vaccination. Front Immunol. 2019;10:1317.
de Vallière S, Abate G, Blazevic A, Heuertz RM, Hoft DF. Enhancement of innate and cell-mediated immunity by antimycobacterial antibodies. Infect Immun. 2005;73(10):6711–20.
Tait DR, Hatherill M, Van Der Meeren O, Ginsberg AM, Van Brakel E, Salaun B, et al. Final analysis of a trial of M72/AS01E vaccine to prevent tuberculosis. N Engl J Med. 2019;381(25):2429–39.
Darrah PA, Zeppa JJ, Maiello P, Hackney JA, Wadsworth MH II, Hughes TK, et al. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature. 2020;577(7788):95–102.
Irvine EB, O’Neil A, Darrah PA, Shin S, Choudhary A, Li W, et al. Robust IgM responses following vaccination are associated with prevention of Mycobacterium tuberculosis infection in macaques. bioRxiv. 2021. 2021.05.06.442979.
Reljic R, Williams A, Ivanyi J. Mucosal immunotherapy of tuberculosis: is there a value in passive IgA? Tuberculosis (Edinb). 2006;86(3–4):179–90.
Reljic R, Clark SO, Williams A, Falero-Diaz G, Singh M, Challacombe S, et al. Intranasal IFNgamma extends passive IgA antibody protection of mice against Mycobacterium tuberculosis lung infection. Clin Exp Immunol. 2006;143(3):467–73.
Rodríguez A, Tjärnlund A, Ivanji J, Singh M, García I, Williams A, et al. Role of IgA in the defense against respiratory infections IgA deficient mice exhibited increased susceptibility to intranasal infection with Mycobacterium bovis BCG. Vaccine. 2005;23(20):2565–72.
Williams A, Reljic R, Naylor I, Clark SO, Falero-Diaz G, Singh M, et al. Passive protection with immunoglobulin A antibodies against tuberculous early infection of the lungs. Immunology. 2004;111(3):328–33.
Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Official Journal of the European Union L276/33; 2010.
McShane H, Williams A. A review of preclinical animal models utilised for TB vaccine evaluation in the context of recent human efficacy data. Tuberculosis (Edinb). 2014;94(2):105–10.
Cardona P-J, Williams A. Experimental animal modelling for TB vaccine development. Int J Infect Dis. 2017;56:268–73.
Satti I, McShane H. Current approaches toward identifying a correlate of immune protection from tuberculosis. Expert Rev Vaccines. 2019;18(1):43–59.
Gong W, Liang Y, Wu X. Animal models of tuberculosis vaccine research: an important component in the fight against tuberculosis. Biomed Res Int. 2020;2020:4263079.
Roque S, Nobrega C, Appelberg R, Correia-Neves M. IL-10 underlies distinct susceptibility of BALB/c and C57BL/6 mice to Mycobacterium avium infection and influences efficacy of antibiotic therapy. J Immunol. 2007;178(12):8028.
Baldwin Susan L, D’Souza C, Roberts Alan D, Kelly Brian P, Frank Anthony A, Lui Margaret A, et al. Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Infect Immun. 1998;66(6):2951–9.
Dharmadhikari AS, Basaraba RJ, Van Der Walt ML, Weyer K, Mphahlele M, Venter K, et al. Natural infection of guinea pigs exposed to patients with highly drug-resistant tuberculosis. Tuberculosis (Edinb). 2011;91(4):329–38.
Williams A, Hall Y, Orme IM. Evaluation of new vaccines for tuberculosis in the guinea pig model. Tuberculosis (Edinb). 2009;89(6):389–97.
Goddeeris BM. Immunology of cattle. In: Handbook of vertebrate immunology. Amsterdam: Elsevier; 1998. p. 439–84.
Waters WR, Palmer MV, Thacker TC, Davis WC, Sreevatsan S, Coussens P, et al. Tuberculosis immunity: opportunities from studies with cattle. Clin Dev Immunol. 2011;2011:768542.
Buddle BM, Pollock JM, Skinner MA, Wedlock DN. Development of vaccines to control bovine tuberculosis in cattle and relationship to vaccine development for other intracellular pathogens. Int J Parasitol. 2003;33(5):555–66.
Vordermeier HM, Villarreal-Ramos B, Cockle Paul J, McAulay M, Rhodes Shelley G, Thacker T, et al. Viral booster vaccines improve Mycobacterium bovis BCG-induced protection against bovine tuberculosis. Infect Immun. 2009;77(8):3364–73.
Maiello P, DiFazio RM, Cadena AM, Rodgers MA, Lin PL, Scanga CA, et al. Rhesus Macaques are more susceptible to progressive tuberculosis than Cynomolgus Macaques: a quantitative comparison. Infect Immun. 2018;86(2):e00505–17.
Sharpe S, White A, Gleeson F, McIntyre A, Smyth D, Clark S, et al. Ultra low dose aerosol challenge with Mycobacterium tuberculosis leads to divergent outcomes in rhesus and cynomolgus macaques. Tuberculosis. 2016;96:1–12.
Sibley L, Daykin-Pont O, Sarfas C, Pascoe J, White AD, Sharpe S. Differences in host immune populations between rhesus macaques and cynomolgus macaque subspecies in relation to susceptibility to Mycobacterium tuberculosis infection. Sci Rep. 2021;11(1):8810.
Sharpe S, White A, Sarfas C, Sibley L, Gleeson F, McIntyre A, et al. Alternative BCG delivery strategies improve protection against Mycobacterium tuberculosis in non-human primates: protection associated with mycobacterial antigen-specific CD4 effector memory T-cell populations. Tuberculosis (Edinb). 2016;101:174–90.
Gideon HP, Phuah J, Myers AJ, Bryson BD, Rodgers MA, Coleman MT, et al. Variability in tuberculosis granuloma T cell responses exists, but a balance of pro- and anti-inflammatory cytokines is associated with sterilization. PLoS Pathog. 2015;11(1):e1004603.
Traver D, Herbomel P, Patton EE, Murphey RD, Yoder JA, Litman GW, et al. The zebrafish as a model organism to study development of the immune system. Adv Immunol. 2003;81:253–330.
Fletcher HA, Filali-Mouhim A, Nemes E, Hawkridge A, Keyser A, Njikan S, et al. Human newborn bacille Calmette-Guérin vaccination and risk of tuberculosis disease: a case-control study. BMC Med. 2016;14:76.
Naranbhai V, Hill AV, Abdool Karim SS, Naidoo K, Abdool Karim Q, Warimwe GM, et al. Ratio of monocytes to lymphocytes in peripheral blood identifies adults at risk of incident tuberculosis among HIV-infected adults initiating antiretroviral therapy. J Infect Dis. 2014;209(4):500–9.
Muller J, Tanner R, Matsumiya M, Snowden MA, Landry B, Satti I, et al. Cytomegalovirus infection is a risk factor for TB disease in Infants. bioRxiv. 2019:222646.
Chowdhury RR, Vallania F, Yang Q, Angel CJL, Darboe F, Penn-Nicholson A, et al. A multi-cohort study of the immune factors associated with M. tuberculosis infection outcomes. Nature. 2018;560(7720):644–8.
Nemes E, Geldenhuys H, Rozot V, Rutkowski KT, Ratangee F, Bilek N, et al. Prevention of M. tuberculosis infection with H4:IC31 vaccine or BCG revaccination. N Engl J Med. 2018;379(2):138–49.
Suliman S, Geldenhuys H, Johnson JL, Hughes JE, Smit E, Murphy M, et al. Bacillus Calmette-Guérin (BCG) revaccination of adults with latent Mycobacterium tuberculosis infection induces long-lived BCG-reactive NK cell responses. J Immunol. 2016;197(4):1100–10.
Rakshit S, Ahmed A, Adiga V, Sundararaj BK, Sahoo PN, Kenneth J, et al. BCG revaccination boosts adaptive polyfunctional Th1/Th17 and innate effectors in IGRA+ and IGRA– Indian adults. JCI Insight. 2019;4(24):e130540.
Zak DE, Penn-Nicholson A, Scriba TJ, Thompson E, Suliman S, Amon LM, et al. A blood RNA signature for tuberculosis disease risk: a prospective cohort study. Lancet. 2016;387(10035):2312–22.
Suliman S, Thompson E, Sutherland J, Weiner RJ, Ota MOC, Shankar S, et al. Four-gene Pan-African blood signature predicts progression to tuberculosis. Am J Respir Crit Care Med. 2018;197(9):1198–208.
Penn-Nicholson A, Mbandi SK, Thompson E, Mendelsohn SC, Suliman S, Chegou NN, et al. RISK6, a 6-gene transcriptomic signature of TB disease risk, diagnosis and treatment response. Sci Rep. 2020;10(1):8629.
Scriba TJ, Penn-Nicholson A, Shankar S, Hraha T, Thompson EG, Sterling D, et al. Sequential inflammatory processes define human progression from M. tuberculosis infection to tuberculosis disease. PLoS Pathog. 2017;13(11):e1006687.
Fletcher HA. Systems approaches to correlates of protection and progression to TB disease. Semin Immunol. 2018;39:81–7.
Dunachie S, Hill AVS, Fletcher HA. Profiling the host response to malaria vaccination and malaria challenge. Vaccine. 2015;33(40):5316–20.
Jin C, Gibani MM, Moore M, Juel HB, Jones E, Meiring J, et al. Efficacy and immunogenicity of a Vi-tetanus toxoid conjugate vaccine in the prevention of typhoid fever using a controlled human infection model of Salmonella Typhi: a randomised controlled, phase 2b trial. Lancet. 2017;390(10111):2472–80.
Chen WH, Cohen MB, Kirkpatrick BD, Brady RC, Galloway D, Gurwith M, et al. Single-dose live oral cholera vaccine CVD 103-HgR protects against human experimental infection with Vibrio cholerae O1 El Tor. Clin Infect Dis. 2016;62(11):1329–35.
Kester KE, Cummings JF, Ofori-Anyinam O, Ockenhouse CF, Krzych U, Moris P, et al. Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J Infect Dis. 2009;200(3):337–46.
Clements ML, Betts RF, Murphy BR. Advantage of live attenuated cold-adapted influenza A virus over inactivated vaccine for A/Washington/80 (H3N2) wild-type virus infection. Lancet. 1984;1(8379):705–8.
Hernández-Garduño E, Cook V, Kunimoto D, Elwood RK, Black WA, FitzGerald JM. Transmission of tuberculosis from smear negative patients: a molecular epidemiology study. Thorax. 2004;59(4):286–90.
Tweed CD, Crook AM, Amukoye EI, Dawson R, Diacon AH, Hanekom M, et al. Toxicity associated with tuberculosis chemotherapy in the REMoxTB study. BMC Infect Dis. 2018;18(1):317.
Nemes E, Rozot V, Geldenhuys H, Bilek N, Mabwe S, Abrahams D, et al. Optimization and interpretation of serial QuantiFERON testing to measure acquisition of Mycobacterium tuberculosis infection. Am J Respir Crit Care Med. 2017;196(5):638–48.
Yamazaki-Nakashimada MA, Unzueta A, Berenise Gámez-González L, González-Saldaña N, Sorensen RU. BCG: a vaccine with multiple faces. Hum Vaccin Immunother. 2020;16(8):1841–50.
Lange C, Aaby P, Behr MA, Donald PR, Kaufmann SHE, Netea MG, et al. 100 years of Mycobacterium bovis bacille Calmette-Guérin. Lancet Infect Dis. 2022;22(1):e2–e12.
Pym AS, Brodin P, Brosch R, Huerre M, Cole ST. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol Microbiol. 2002;46(3):709–17.
Brosch R, Gordon SV, Garnier T, Eiglmeier K, Frigui W, Valenti P, et al. Genome plasticity of BCG and impact on vaccine efficacy. Proc Natl Acad Sci. 2007;104(13):5596–601.
Chard AN, Gacic-Dobo M, Diallo MS, Sodha SV, Wallace AS. Routine vaccination coverage - worldwide, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(45):1706–10.
Minassian AM, Satti I, Poulton ID, Meyer J, Hill AV, McShane H. A human challenge model for Mycobacterium tuberculosis using Mycobacterium bovis bacille Calmette-Guerin. J Infect Dis. 2012;205(7):1035–42.
Connor LM, Harvie MC, Rich FJ, Quinn KM, Brinkmann V, Gros GL, et al. A key role for lung-resident memory lymphocytes in protective immune responses after BCG vaccination. Eur J Immunol. 2010;40(9):2482–92.
Moliva JI, Turner J, Torrelles JB. Immune responses to Bacillus Calmette-Guerin vaccination: why do they fail to protect against Mycobacterium tuberculosis? Front Immunol. 2017;8:407.
Romagnoli A, Etna MP, Giacomini E, Pardini M, Remoli ME, Corazzari M, et al. ESX-1 dependent impairment of autophagic flux by Mycobacterium tuberculosis in human dendritic cells. Autophagy. 2012;8(9):1357–70.
Wassermann R, Gulen Muhammet F, Sala C, Perin Sonia G, Lou Y, Rybniker J, et al. Mycobacterium tuberculosis differentially activates cGAS- and inflammasome-dependent intracellular immune responses through ESX-1. Cell Host Microbe. 2015;17(6):799–810.
Hart PD, Sutherland I. BCG and vole bacillus vaccines in the prevention of tuberculosis in adolescence and early adult life. Br Med J. 1977;2(6082):293–5.
Harris SA, Meyer J, Satti I, Marsay L, Poulton ID, Tanner R, et al. Evaluation of a human BCG challenge model to assess antimycobacterial immunity induced by BCG and a candidate tuberculosis vaccine, MVA85A, alone and in combination. J Infect Dis. 2014;209(8):1259–68.
Matsumiya M, Satti I, Chomka A, Harris SA, Stockdale L, Meyer J, et al. Gene expression and cytokine profile correlate with mycobacterial growth in a human BCG challenge model. J Infect Dis. 2015;211(9):1499–509.
Morrison H, McShane H. Local pulmonary immunological biomarkers in tuberculosis. Front Immunol. 2021;12:640916.
Rosenthal SR, McEnery JT, Raisys N. Aerogenic BCG vaccination against tuberculosis in animal and human subjects. J Asthma Res. 1968;5(4):309–23.
Cusumano CL, Jernigan JA, Waldman RH. Aerosolized BCG (Tice strain) treatment of bronchogenic carcinoma: phase I study. J Natl Cancer Inst. 1975;55(2):275–9.
Garner FB, Meyer CA, White DS, Lipton A. Aerosol BCG treatment of carcinoma metastatic to the lung: a phase I study. Cancer. 1975;35(4):1088–94.
Davids M, Pooran A, Hermann C, Mottay L, Thompson F, Cardenas J, et al. A human lung challenge model to evaluate the safety and immunogenicity of PPD and live Bacillus Calmette-Guérin. Am J Respir Crit Care Med. 2020;201(10):1277–91.
Hoang T, Aagaard C, Dietrich J, Cassidy JP, Dolganov G, Schoolnik GK, et al. ESAT-6 (EsxA) and TB10.4 (EsxH) based vaccines for pre- and post-exposure tuberculosis vaccination. PLoS One. 2013;8(12):e80579.
Pym AS, Brodin P, Majlessi L, Brosch R, Demangel C, Williams A, et al. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat Med. 2003;9(5):533–9.
Arbues A, Aguilo JI, Gonzalo-Asensio J, Marinova D, Uranga S, Puentes E, et al. Construction, characterization and preclinical evaluation of MTBVAC, the first live-attenuated M. tuberculosis-based vaccine to enter clinical trials. Vaccine. 2013;31(42):4867–73.
Tanner R, O’Shea MK, Fletcher HA, McShane H. In vitro mycobacterial growth inhibition assays: a tool for the assessment of protective immunity and evaluation of tuberculosis vaccine efficacy. Vaccine. 2016;34(39):4656–65.
Tanner R, McShane H. Replacing, reducing and refining the use of animals in tuberculosis vaccine research. ALTEX. 2017;34(1):157–66.
Brennan MJ, Tanner R, Morris S, Scriba TJ, Achkar JM, Zelmer A, et al. The cross-species mycobacterial growth inhibition assay (MGIA) project, 2010-2014. Clin Vaccine Immunol. 2017;24(9):e00142–17.
Worku S, Hoft DF. In vitro measurement of protective mycobacterial immunity: antigen-specific expansion of T cells capable of inhibiting intracellular growth of bacille Calmette-Guérin. Clin Infect Dis. 2000;30(Suppl 3):S257–61.
Parra M, Yang AL, Lim J, Kolibab K, Derrick S, Cadieux N, et al. Development of a murine mycobacterial growth inhibition assay for evaluating vaccines against Mycobacterium tuberculosis. Clin Vaccine Immunol. 2009;16(7):1025–32.
Kampmann B, Tena GN, Mzazi S, Eley B, Young DB, Levin M. Novel human in vitro system for evaluating antimycobacterial vaccines. Infect Immun. 2004;72(11):6401–7.
Wallis RS, Palaci M, Vinhas S, Hise AG, Ribeiro FC, Landen K, et al. A whole blood bactericidal assay for tuberculosis. J Infect Dis. 2001;183(8):1300–3.
Tanner R, Smith SG, van Meijgaarden KE, Giannoni F, Wilkie M, Gabriele L, et al. Optimisation, harmonisation and standardisation of the direct mycobacterial growth inhibition assay using cryopreserved human peripheral blood mononuclear cells. J Immunol Methods. 2019;469:1–10.
Tanner R, White AD, Boot C, Sombroek CC, O’Shea MK, Wright D, et al. A non-human primate in vitro functional assay for the early evaluation of TB vaccine candidates. NPJ Vaccines. 2021;6(1):3.
Zelmer A, Tanner R, Stylianou E, Damelang T, Morris S, Izzo A, et al. A new tool for tuberculosis vaccine screening: ex vivo mycobacterial growth inhibition assay indicates BCG-mediated protection in a murine model of tuberculosis. BMC Infect Dis. 2016;16:412.
O’Shea MK, Tanner R, Müller J, Harris SA, Wright D, Stockdale L, et al. Immunological correlates of mycobacterial growth inhibition describe a spectrum of tuberculosis infection. Sci Rep. 2018;8(1):14480.
Fletcher HA, Tanner R, Wallis RS, Meyer J, Manjaly ZR, Harris S, et al. Inhibition of mycobacterial growth in vitro following primary but not secondary vaccination with Mycobacterium bovis BCG. Clin Vaccine Immunol. 2013;20(11):1683–9.
Marsay L, Matsumiya M, Tanner R, Poyntz H, Griffiths KL, Stylianou E, et al. Mycobacterial growth inhibition in murine splenocytes as a surrogate for protection against Mycobacterium tuberculosis (M. tb). Tuberculosis (Edinb). 2013;93(5):551–7.
Smith SG, Zelmer A, Blitz R, Fletcher HA, Dockrell HM. Polyfunctional CD4 T-cells correlate with in vitro mycobacterial growth inhibition following Mycobacterium bovis BCG-vaccination of infants. Vaccine. 2016;34(44):5298–305.
Tanner R, Satti I, Harris SA, O’Shea MK, Cizmeci D, O’Connor D, et al. Tools for assessing the protective efficacy of TB vaccines in humans: in vitro mycobacterial growth inhibition predicts outcome of in vivo mycobacterial infection. Front Immunol. 2020;10:2983.
Yang AL, Schmidt TE, Stibitz S, Derrick SC, Morris SL, Parra M. A simplified mycobacterial growth inhibition assay (MGIA) using direct infection of mouse splenocytes and the MGIT system. J Microbiol Methods. 2016;131:7–9.
Jensen C, Lindebo Holm L, Svensson E, Aagaard C, Ruhwald M. Optimisation of a murine splenocyte mycobacterial growth inhibition assay using virulent Mycobacterium tuberculosis. Sci Rep. 2017;7(1):2830.
Silver RF, Li Q, Boom WH, Ellner JJ. Lymphocyte-dependent inhibition of growth of virulent Mycobacterium tuberculosis H37Rv within human monocytes: requirement for CD4+ T cells in purified protein derivative-positive, but not in purified protein derivative-negative subjects. J Immunol. 1998;160(5):2408–17.
Worku S, Hoft DF. Differential effects of control and antigen-specific T cells on intracellular mycobacterial growth. Infect Immun. 2003;71(4):1763–73.
Cheon SH, Kampmann B, Hise AG, Phillips M, Song HY, Landen K, et al. Bactericidal activity in whole blood as a potential surrogate marker of immunity after vaccination against tuberculosis. Clin Diagn Lab Immunol. 2002;9(4):901–7.
Cowley SC, Elkins KL. CD4+ T cells mediate IFN-gamma-independent control of Mycobacterium tuberculosis infection both in vitro and in vivo. J Immunol. 2003;171(9):4689–99.
Hoft DF, Worku S, Kampmann B, Whalen CC, Ellner JJ, Hirsch CS, et al. Investigation of the relationships between immune-mediated inhibition of mycobacterial growth and other potential surrogate markers of protective Mycobacterium tuberculosis immunity. J Infect Dis. 2002;186(10):1448–57.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Peralta Alvarez, M.P., Marshall, J.L., Tanner, R. (2023). Correlates of Protection from Tuberculosis. In: Christodoulides, M. (eds) Vaccines for Neglected Pathogens: Strategies, Achievements and Challenges . Springer, Cham. https://doi.org/10.1007/978-3-031-24355-4_6
Download citation
DOI: https://doi.org/10.1007/978-3-031-24355-4_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-24354-7
Online ISBN: 978-3-031-24355-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)