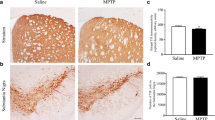
Abstract
MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) is commonly utilized in animal models of Parkinson’s disease. This compound is metabolized in the central nervous system to produce 1-methyl-4-phenylpyridinium (MPP+) which interacts with neuronal mitochondria to suppress cellular energy production, generate reactive oxygen species, increase cytosolic calcium levels, and ultimately promote apoptotic cell death. Dopaminergic neurons are critically affected by this mitochondrial dysfunction. Calcium-activated neutral proteinase (calpain) is also pathologically activated by the increased intracellular calcium levels. Hyperactivated calpain cleaves alpha-synuclein into truncated subunits (prone to aggregation) as found in Lewy bodies. Calpain activation following MPTP administration also plays a role in neuroinflammation with T cell activation, cytokine production, and cell signaling pathways. Dopaminergic neuronal apoptosis is also observed in relation to calpain-mediated Bax/Bcl-2 disruption. In addition to mesencephalic neuronal degeneration, these MPP+-induced calpain-mediated effects have also been identified in extranigral neurons such as spinal motoneurons. Thus, derangements in nigral dopamine production and apoptotic neuronal death in other nervous system sites can contribute to the multiple symptoms experienced by Parkinsonian patients. Moreover, administration of calpain inhibitors and anti-inflammatory agents (e.g., estrogen and melatonin) in these animal models demonstrates significant attenuation of neuronal degeneration and resulting Parkinsonian symptoms.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- 6-OHDA:
-
6-hydroxydopamine
- AIF:
-
Apoptosis inducing factor
- Ca2+:
-
Calcium
- Cdk5:
-
Cyclin-dependent kinase 5
- MPP+:
-
1-Methyl-4-phenylpyridinium
- MPTP:
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NOS:
-
Nitric oxide synthase
- PD:
-
Parkinson’s disease
- ROS:
-
Reactive oxygen species
- SN:
-
Substantia nigra
References
Aguilar Hernández, R., Sánchez De Las Matas, M. J., Arriagada, C., Barcia, C., Caviedes, P., Herrero, M. T., & Segura-Aguilar, J. (2003). MPP(+)-induced degeneration is potentiated by dicoumarol in cultures of the RCSN-3 dopaminergic cell line. Implications of neuromelanin in oxidative metabolism of dopamine neurotoxicity. Neurotoxicity Research, 5, 407–410.
Alvira, D., Tajes, M., Verdaguer, E., Acuña-Castroviejo, D., Folch, J., Camins, A., & Pallas, M. (2006). Inhibition of the cdk5/p25 fragment formation may explain the antiapoptotic effects of melatonin in an experimental model of Parkinson’s disease. Journal of Pineal Research, 40, 251–258.
Arrington, D. D., Van Vleet, T. R., & Schnellmann, R. G. (2006). Calpain 10: A mitochondrial calpain and its role in calcium-induced mitochondrial dysfunction. American Journal of Physiology. Cell Physiology, 291, 1159–1171.
Banerjee, R., Starkov, A. A., Beal, M. F., & Thomas, B. (2009). Mitochondrial dysfunction in the limelight of Parkinson’s disease pathogenesis. Biochimica et Biophysica Acta, 1792, 651–663.
Bano, D., Young, K. W., Guerin, C. J., Lefeuvre, R., Rothwell, N. J., Naldini, L., Rizzuto, R., Carafoli, E., & Nicotera, P. (2005). Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell, 120, 275–285.
Bevers, M. B., & Neumar, R. W. (2008). Mechanistic role of calpains in postischemic neurodegeneration. Journal of Cerebral Blood Flow and Metabolism, 28, 655–673.
Butler, J. T., Samantaray, S., Beeson, C. C., Ray, S. K., & Banik, N. L. (2009). Involvement of Calpain in the process of Jurkat T cell chemotaxis. Journal of Neuroscience Research, 87, 626–635.
Chandra, G., Roy, A., Rangasamy, S. B., & Pahan, K. (2017). Induction of adaptive immunity leads to nigrostriatal disease progression in MPTP mouse model of Parkinson’s disease. Journal of Immunology (Baltimore, MD: 1950), 198, 4312–4326.
Chera, B., Schaecher, K. E., Rocchini, A., Imam, S. Z., Ray, S. K., Ali, S. F., & Banik, N. L. (2002). Calpain upregulation and neuron death in spinal cord of MPTP-induced parkinsonism in mice. Annals of the New York Academy of Sciences, 965, 274–280.
Choi, W. S., Lee, E. H., Chung, C. W., Jung, Y. K., Jin, B. K., Kim, S. U., Oh, T. H., Saido, T. C., & Oh, Y. J. (2001). Cleavage of Bax is mediated by caspase-dependent or -independent calpain activation in dopaminergic neuronal cells: Protective role of Bcl-2. Journal of Neurochemistry, 77, 1531–1541.
Choi, W. S., Lee, E., Lim, J., & Oh, Y. J. (2008). Calbindin-D28K prevents drug-induced dopaminergic neuronal death by inhibiting caspase and calpain activity. Biochemical and Biophysical Research Communications, 371, 127–131.
Chu, Y., Morfini, G. A., Langhamer, L. B., He, Y., Brady, S. T., & Kordower, J. H. (2012). Alterations in axonal transport motor proteins in sporadic and experimental Parkinson’s disease. Brain, 135, 2058–2073.
Cleeter, M. W. J., Cooper, J. M., & Schapira, A. H. V. (1992). Irreversible inhibition of mitochondrial complex I by 1-methyl-4-phenylpyridinium: Evidence for free radical involvement. Journal of Neurochemistry, 58, 786–789.
Domingues, A. F., Esteves, A. R., Swerdlow, R. H., Oliveira, C. R., & Cardoso, S. M. (2008). Calpain-mediated MPP+ toxicity in mitochondrial DNA depleted cells. Neurotoxicity Research, 13, 31–38.
Dufty, B. M., Warner, L. R., Hou, S. T., Jiang, S. X., Gomez-Isla, T., Leenhouts, K. M., Oxford, J. T., Feany, M. B., Masliah, E., & Rohn, T. T. (2007). Calpain-cleavage of alpha-synuclein: Connecting proteolytic processing to disease-linked aggregation. The American Journal of Pathology, 170, 1725–1738.
Gamerdinger, M., Manthey, D., & Behl, C. (2006). Oestrogen receptor subtype-specific repression of calpain expression and calpain enzymatic activity in neuronal cells – Implications for neuroprotection against Ca-mediated excitotoxicity. Journal of Neurochemistry, 97, 57–68.
Goll, D. E., Thompson, V. F., Li, H., Wei, W., & Cong, J. (2003). The calpain system. Physiological Reviews, 83, 731–801.
González, H., Francisco, C., Carolina, P., Daniela, E., Dafne, F., & Sebastián, B. (2013). Rodrigo Pacheco Dopamine receptor D3 expressed on CD4+ T cells favors neurodegeneration of dopaminergic neurons during Parkinson’s disease. J Immunol, 190(10), 5048–5056.
Guerrero, J. M., & Reiter, R. J. (2002). Melatonin-immune system relationships. Current Topics in Medicinal Chemistry, 2, 167–179.
Haque, A., Samantaray, S., Knaryan, V. H., Capone, M., Hossain, A., Matzelle, D., Chandran, R., Shields, D. C., Farrand, A. Q., Boger, H. A., & Banik, N. L. (2020). Calpain mediated expansion of CD4+ cytotoxic T cells in rodent models of Parkinson’s disease. Experimental Neurology, 330, 113315.
Harbison, R. A., Ryan, K. R., Wilkins, H. M., Schroeder, E. K., Loucks, F. A., Bouchard, R. J., & Linseman, D. A. (2011). Calpain plays a central role in 1-methyl-4-phenylpyridinium (MPP+)-induced neurotoxicity in cerebellar granule neurons. Neurotoxicity Research, 19, 374–388.
Hatcher, J. M., Pennell, K. D., & Miller, G. W. (2008). Parkinson’s disease and pesticides: A toxicological perspective. Trends in Pharmacological Sciences, 29, 322–329.
Hof, P. R., Glezer, I. I., Conde, F., Flagg, R. A., Rubin, M. B., Nimchinsky, E. A., & Vogt Weisenhorn, D. M. (1999). Cellular distribution of the calcium-binding proteins parvalbumin, calbindin, and calretinin in the neocortex of mammals: Phylogenetic and developmental patterns. Journal of Chemical Neuroanatomy, 16, 77–116.
Hsieh, Y. C., Mounsey, R. B., & Teismann, P. (2011). MPP(+)-induced toxicity in the presence of dopamine is mediated by COX-2 through oxidative stress. Naunyn-Schmiedeberg’s Archives of Pharmacology, 384, 157–167.
Hwang, J. Y., Lee, J., Oh, C. K., Kang, H. W., Hwang, I. Y., Um, J. W., Park, H. C., Kim, S., Shin, J. H., Park, W. Y., Darnell, R. B., Um, H. D., Chung, K. C., Kim, K., & Oh, Y. J. (2016). Proteolytic degradation and potential role of onconeural protein cdr2 in neurodegeneration. Cell Death & Disease, 7, e2240.
Ji, J., Su, L., & Liu, Z. (2016). Critical role of calpain in inflammation. Biomedical Reports, 5, 647–652.
Jourdi, H., Hamo, L., Oka, T., Seegan, A., & Baudry, M. (2009). BDNF mediates the neuroprotective effects of positive AMPA receptor modulators against MPP+-induced toxicity in cultured hippocampal and mesencephalic slices. Neuropharmacology, 56, 876–885.
Jung, S., Chung, Y., & Oh, Y. J. (2018). Breaking down autophagy and the ubiquitin proteasome system. Parkinsonism & Related Disorders, 46(Suppl 1), S97–S100.
Kanagaraj, N., Beiping, H., Dheen, S. T., & Tay, S. S. (2014). Downregulation of miR-124 in MPTP-treated mouse model of Parkinson’s disease and MPP iodide-treated MN9D cells modulates the expression of the calpain/cdk5 pathway proteins. Neuroscience, 272, 167–179.
Kar, P., Samanta, K., Shaikh, S., Chowdhury, A., Chakraborti, T., & Chakraborti, S. (2010). Mitochondrial calpain system: An overview. Archives of Biochemistry and Biophysics, 495, 1–7.
Kass, G. E., Wright, J. M., Nicotera, P., & Orrenius, S. (1988). The mechanism of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity: Role of intracellular calcium. Archives of Biochemistry and Biophysics, 260, 789–797.
Knaryan, V. H., Samantaray, S., Park, S., Azuma, M., Inoue, J., & Banik, N. L. (2014). SNJ-1945, a calpain inhibitor, protects SH-SY5Y cells against MPP(+) and rotenone. Journal of Neurochemistry, 130, 280–290.
Kopil, C. M., Siebert, A. P., Foskett, J. K., & Neumar, R. W. (2012). Calpain-cleaved type 1 inositol 1,4,5-trisphosphate receptor impairs ER Ca(2+) buffering and causes neurodegeneration in primary cortical neurons. Journal of Neurochemistry, 123, 147–158.
Lehtonen, Š., Jaronen, M., Vehviläinen, P., Lakso, M., Rudgalvyte, M., Keksa-Goldsteine, V., Wong, G., Courtney, M. J., Koistinaho, J., & Goldsteins, G. (2016). Inhibition of excessive oxidative protein folding is protective in MPP(+) toxicity-induced Parkinson’s disease models. Antioxidants & Redox Signaling, 25, 485–497.
Leist, M., Volbracht, C., Fava, E., & Nicotera, P. (1998). 1-Methyl-4-phenylpyridinium induces autocrine excitotoxicity, protease activation, and neuronal apoptosis. Molecular Pharmacology, 54, 789–801.
Levesque, S., Wilson, B., Gregoria, V., Thorpe, L. B., Dallas, S., Polikov, V. S., Hong, J. S., & Block, M. L. (2010). Reactive microgliosis: Extracellular micro-calpain and microglia-mediated dopaminergic neurotoxicity. Brain, 133, 808–821.
Li, J., Chen, H., Wu, S., Cheng, Y., Li, Q., Wang, J., & Zhu, G. (2017). MPP+ inhibits mGluR1/5-mediated long-term depression in mouse hippocampus by calpain activation. European Journal of Pharmacology, 795, 22–27.
Liou, A. K., Zhou, Z., Pei, W., Lim, T. M., Yin, X. M., & Chen, J. (2005). BimEL up-regulation potentiates AIF translocation and cell death in response to MPTP. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 19, 1350–1352.
Mandavilli, B. S., Ali, S. F., & Van Houten, B. (2000). DNA damage in brain mitochondria caused by aging and MPTP treatment. Brain Research, 885, 45–52.
Mizuno, Y., Sone, N., & Saitoh, T. (1987). Effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and 1-methyl-4-phenylpyridinium ion on activities of the enzymes in the electron transport system in mouse brain. Journal of Neurochemistry, 48, 1787–1793.
Nicholls, D. G., Vesce, S., Kirk, L., & Chalmers, S. (2003). Interactions between mitochondrial bioenergetics and cytoplasmic calcium in cultured cerebellar granule cells. Cell Calcium, 34, 407–424.
Nicklas, W. J., Youngster, S. K., Kindt, M. V., & Heikkila, R. E. (1987). MPTP, MPP+ and mitochondrial function. Life Sciences, 40, 721–729.
Ozaki, T., Tomita, H., Tamai, M., & Ishiguro, S. (2007). Characteristics of mitochondrial calpains. Journal of Biochemistry, 142, 365–376.
Pánico, P., Salazar, A. M., Burns, A. L., & Ostrosky-Wegman, P. (2014). Role of calpain-10 in the development of diabetes mellitus and its complications. Archives of Medical Research, 45, 103–115.
Parker, W. D., Jr., Parks, J. K., & Swerdlow, R. H. (2008). Complex I deficiency in Parkinson’s disease frontal cortex. Brain Research, 1189, 215–218.
Periquet, M., Fulga, T., Myllykangas, L., Schlossmacher, M. G., & Feany, M. B. (2007). Aggregated alpha-synuclein mediates dopaminergic neurotoxicity in vivo. The Journal of Neuroscience, 27, 3338–3346.
Przedborski, S., Jackson-Lewis, V., Djaldetti, R., et al. (2000). The parkinsonian toxin MPTP: Action and mechanism. Restorative Neurology and Neuroscience, 16, 135–142.
Qu, D., Rashidian, J., Mount, M. P., Aleyasin, H., Parsanejad, M., Lira, A., Haque, E., Zhang, Y., Callaghan, S., Daigle, M., Rousseaux, M. W., Slack, R. S., Albert, P. R., Vincent, I., Woulfe, J. M., & Park, D. S. (2007). Role of Cdk5-mediated phosphorylation of Prx2 in MPTP toxicity and Parkinson’s disease. Neuron, 55, 37–52.
Ray, S. K., Wilford, G. G., Ali, S. F., & Banik, N. L. (2000). Calpain upregulation in spinal cords of mice with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced Parkinson’s disease. Annals of the New York Academy of Sciences, 914, 275–283.
Samantaray, S., Butler, J. T., Ray, S. K., & Banik, N. L. (2008). Extranigral neurodegeneration in Parkinson’s disease. Annals of the New York Academy of Sciences, 1139, 331–336.
Samantaray, S., Knaryan, V. H., Le Gal, C., Ray, S. K., & Banik, N. L. (2011). Calpain inhibition protected spinal cord motoneurons against 1-methyl-4-phenylpyridinium ion and rotenone. Neuroscience, 192, 263–274.
Samantaray, S., Knaryan, V. H., Shields, D. C., Cox, A. A., Haque, A., & Banik, N. L. (2015). Inhibition of calpain activation protects MPTP-induced nigral and spinal cord neurodegeneration, reduces inflammation, and improves gait dynamics in mice. Molecular Neurobiology, 52, 1054–1066.
Samantaray, S., Knaryan, V. H., M Del Re, A., Woodward, J. J., Shields, D. C., Azuma, M., Inoue, J., Ray, S. K., & Banik, N. L. (2020). Cell-permeable calpain inhibitor SJA6017 provides functional protection to spinal motoneurons exposed to MPP. Neurotoxicity Research, 38, 640–649.
Schapira, A. H. V., Cooper, J. M., Dexter, D., Clark, J. B., Jenner, P., & Marsden, C. D. (1990). Mitochondrial complex I deficiency in Parkinson’s disease. Journal of Neurochemistry, 54, 823–827.
Sherer, T. B., Betarbet, R., & Greenamyre, J. T. (2002). Environment, mitochondria, and Parkinson’s disease. The Neuroscientist, 8, 192–197.
Shields, D. C., Schaecher, K. E., Saido, T. C., & Banik, N. L. (1999). A putative mechanism of demyelination in multiple sclerosis by a proteolytic enzyme, calpain. Proceedings of the National Academy of Sciences of the United States of America, 96, 11486–11491.
Singh, A., Verma, P., Raju, A., & Mohanakumar, K. P. (2019). Nimodipine attenuates the parkinsonian neurotoxin, MPTP-induced changes in the calcium binding proteins, calpain and calbindin. Journal of Chemical Neuroanatomy, 95, 89–94.
Smith, M. A., & Schnellmann, R. G. (2012). Calpains, mitochondria, and apoptosis. Cardiovascular Research, 96, 32–37.
Sun, Y., Sukumaran, P., Selvaraj, S., Cilz, N. I., Schaar, A., Lei, S., & Singh, B. B. (2018). TRPM2 promotes neurotoxin MPP+/MPTP-induced cell death. Molecular Neurobiology, 55, 409–420.
Takeshige, K., & Minakami, S. (1979). NADH- and NADPH-dependent formation of superoxide anions by bovine heart submitochondrial particles and NADH-ubiquinone reductase preparation. Biochemical Journal, 180, 129–135.
Tanner, C. M. (2003). Is the cause of Parkinson’s disease environmental or hereditary? Evidence from twin studies. Advances in Neurology, 91, 133–142.
Teismann, P., Tieu, K., Choi, D. K., Wu, D. C., Naini, A., Hunot, S., Vila, M., Jackson-Lewis, V., & Przedborski, S. (2003). Cyclooxygenase-2 is instrumental in Parkinson’s disease neurodegeneration. Proceedings of the National Academy of Sciences of the United States of America, 100, 5473–5478.
Thadathil, N., Xiao, J., Hori, R., Always, S. E., & Khan, M. M. (2020). Brain selective estrogen treatment protects dopaminergic neurons and preserves behavioral function in MPTP-induced mouse model of Parkinson’s disease. Journal of Neuroimmune Pharmacology. https://doi.org/10.1007/s11481-020-09972-1
Venderova, K., & Park, D. S. (2012). Programmed cell death in Parkinson’s disease. Cold Spring Harbor Perspectives in Medicine, 2, a009365.
Vosler, P. S., Brennan, C. S., & Chen, J. (2008). Calpain-mediated signaling mechanisms in neuronal injury and neurodegeneration. Molecular Neurobiology, 38, 78–100.
Wales, S. Q., Laing, J. M., Chen, L., & Aurelian, L. (2008). ICP10PK inhibits calpain-dependent release of apoptosis-inducing factor and programmed cell death in response to the toxin MPP+. Gene Therapy, 15, 1397–1409.
Warner, T. T., & Schapira, A. H. (2003). Genetic and environmental factors in the cause of Parkinson’s disease. Annals of Neurology, 53, S16–S23.
Yildiz-Unal, A., Korulu, S., & Karabay, A. (2015). Neuroprotective strategies against calpain-mediated neurodegeneration. Neuropsychiatric Disease and Treatment, 11, 297–310.
Zhang, H., Chang, L., Zhang, H., Nie, J., Zhang, Z., Yang, X., Vuong, A. M., Wang, Z., Chen, A., & Niu, Q. (2017). Calpain-2/p35-p25/Cdk5 pathway is involved in the neuronal apoptosis induced by polybrominated diphenyl ether-153. Toxicology Letters, 277, 41–53.
Zhu, G., Li, J., He, L., Wang, X., & Hong, X. (2015). MPTP-induced changes in hippocampal synaptic plasticity and memory are prevented by memantine through the BDNF-TrkB pathway. British Journal of Pharmacology, 172, 2354–2368.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this entry
Cite this entry
Shields, D.C., Haque, A., Banik, N.L. (2022). Neurotoxicity: Calpain and 1-Methyl-4-phenylpyridinium (MPP+). In: Kostrzewa, R.M. (eds) Handbook of Neurotoxicity. Springer, Cham. https://doi.org/10.1007/978-3-031-15080-7_188
Download citation
DOI: https://doi.org/10.1007/978-3-031-15080-7_188
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-15079-1
Online ISBN: 978-3-031-15080-7
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences