Abstract
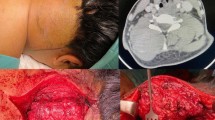
Desmoid tumors are benign soft tissue tumors with an unpredictable natural history. Some desmoid tumors may behave in and indolent fashion or spontaneously regress, while others may behave in a locally aggressive fashion causing significant morbidity or even mortality. Observation, surgery, systemic therapy, and radiation therapy (RT) may all play a role in the optimal management of this disease.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Reitamo JJ, Hayry P, Nykyri E, Saxen E. The desmoid tumor. I. Incidence, sex-, age- and anatomical distribution in the Finnish population. Am J Clin Pathol. 1982;77(6):665–73. https://doi.org/10.1093/ajcp/77.6.665.
Lazar AJF, Tuvin D, Hajibashi S, et al. Specific mutations in the β-catenin gene (CTNNB1) correlate with local recurrence in sporadic desmoid tumors. Am J Pathol. 2008;173(5):1518–27. https://doi.org/10.2353/ajpath.2008.080475.
Aitken SJ, Presneau N, Kalimuthu S, et al. Next-generation sequencing is highly sensitive for the detection of beta-catenin mutations in desmoid-type fibromatoses. Virchows Arch. 2015;467(2):203–10. https://doi.org/10.1007/s00428-015-1765-0.
Nieuwenhuis MH, Lefevre JH, Bülow S, et al. Family history, surgery, and APC mutation are risk factors for desmoid tumors in familial adenomatous polyposis: an international cohort study. Dis Colon Rectum. 2011;54(10):1229–34. https://doi.org/10.1097/DCR.0b013e318227e4e8.
Bertario L, Russo A, Sala P, et al. Genotype and phenotype factors as determinants of desmoid tumors in patients with familial adenomatous polyposis. Int J Cancer. 2001;95(2):102–7. https://doi.org/10.1002/1097-0215(20010320)95:2<102::AID-IJC1018>3.0.CO;2-8.
Gansar GF, Markowitz IP, Cerise EJ. Thirty years of experience with desmoid tumors at Charity Hospital. Am Surg. 1987;53(6):318–9. http://www.ncbi.nlm.nih.gov/pubmed/3579044. Accessed 13 Sep 2019.
De Cian F, Delay E, Rudigoz RC, Ranchère D, Rivoire M. Desmoid tumor arising in a cesarean section scar during pregnancy: monitoring and management. Gynecol Oncol. 1999;75(1):145–8. https://doi.org/10.1006/gyno.1999.5539.
Li C, Bapat B, Alman BA. Adenomatous polyposis coli gene mutation alters proliferation through its β-catenin-regulatory function in aggressive fibromatosis (desmoid tumor). Am J Pathol. 1998;153(3):709–14. https://doi.org/10.1016/S0002-9440(10)65614-3.
Alman BA, Li C, Pajerski ME, Diaz-Cano S, Wolfe HJ. Increased beta-catenin protein and somatic APC mutations in sporadic aggressive fibromatoses (desmoid tumors). Am J Pathol. 1997;151(2):329–34. http://www.ncbi.nlm.nih.gov/pubmed/9250146. Accessed 13 Sep 2019.
Ungerbäck J, Elander N, Grünberg J, Sigvardsson M, Söderkvist P. The Notch-2 gene is regulated by Wnt signaling in cultured colorectal cancer cells. PLoS One. 2011;6(3):e17957. https://doi.org/10.1371/journal.pone.0017957.
Kim H-A, Koo B-K, Cho J-H, et al. Notch1 counteracts WNT/β-catenin signaling through chromatin modification in colorectal cancer. J Clin Invest. 2012;122(9):3248–59. https://doi.org/10.1172/JCI61216.
Shang H, Braggio D, Lee Y-J, et al. Targeting the notch pathway: a potential therapeutic approach for desmoid tumors. Cancer. 2015;121(22):4088–96. https://doi.org/10.1002/cncr.29564.
Timbergen MJM, Smits R, Grünhagen DJ, Verhoef C, Sleijfer S, Wiemer EAC. Activated signaling pathways and targeted therapies in desmoid-type fibromatosis: a literature review. Front. Oncologia. 2019;9 https://doi.org/10.3389/fonc.2019.00397.
Salas S, Chibon F, Noguchi T, et al. Molecular characterization by array comparative genomic hybridization and DNA sequencing of 194 desmoid tumors. Genes Chromosom Cancer. 2010;49(6):560–8. https://doi.org/10.1002/gcc.20766.
Kurtz JE, Buy X, Sauleau EA, Toulmonde M, Deschamps F, Honoré C, Bouhamama A, Blay JYGA. CRYODESMO-O1: a French nationwide phase II study on cryoablation in progressing desmoid tumour (DT) patients (pts). Ann Oncol. 2019;30(Suppl 5):v683.
Trautmann M, Rehkämper J, Gevensleben H, et al. Novel pathogenic alterations in pediatric and adult desmoid-type fibromatosis—a systematic analysis of 204 cases. Sci Rep. 2020;10(1):3368. https://doi.org/10.1038/s41598-020-60237-6.
Bonvalot S, Ternes N, Fiore M, et al. Spontaneous regression of primary abdominal wall desmoid tumors: more common than previously thought. Ann Surg Oncol. 2013;20(13):4096–102. https://doi.org/10.1245/s10434-013-3197-x.
Burtenshaw SM, Cannell AJ, McAlister ED, et al. Toward observation as first-line management in abdominal desmoid tumors. Ann Surg Oncol. 2016;23(7):2212–9. https://doi.org/10.1245/s10434-016-5159-6.
de Bruyns A, Li H, MacNeil A, et al. Evolving practice patterns over two decades (1993–2013) in the management of desmoid-type fibromatosis in British Columbia. Clin Oncol. 2019; https://doi.org/10.1016/j.clon.2019.10.005.
Turner B, Alghamdi M, Henning JW, et al. Surgical excision versus observation as initial management of desmoid tumors: a population based study. Eur J Surg Oncol. 2019;45(4):699–703. https://doi.org/10.1016/j.ejso.2018.09.015.
Suit H, Spiro I. Radiation treatment of benign mesenchymal disease. Semin Radiat Oncol. 1999;9(2):171–8. https://doi.org/10.1016/S1053-4296(99)80007-1.
Spear MA, Jennings LC, Mankin HJ, et al. Individualizing management of aggressive fibromatoses. Int J Radiat Oncol Biol Phys. 1998;40(3):637–45. https://doi.org/10.1016/S0360-3016(97)00845-6.
Ballo MT, Zagars GK, Pollack A, Pisters PWT, Pollock RA. Desmoid tumor: prognostic factors and outcome after surgery, radiation therapy, or combined surgery and radiation therapy. J Clin Oncol. 1999;17(1):158–67. https://doi.org/10.1200/jco.1999.17.1.158.
Salas S, Dufresne A, Bui B, et al. Prognostic factors influencing progression-free survival determined from a series of sporadic desmoid tumors: a wait-and-see policy according to tumor presentation. J Clin Oncol. 2011;29(26):3553–8. https://doi.org/10.1200/JCO.2010.33.5489.
Harati K, Jaenisch A, Behr B, et al. Effect of surgical margins on prognosis in aggressive fibromatosis: a single-institutional analysis of 90 patients. Oncol Lett. 2017;14(5):5129–34. https://doi.org/10.3892/ol.2017.6864.
O’Dea FJ, Wunder J, Bell RS, Griffin AM, Catton C, O’Sullivan B. Preoperative radiotherapy is effective in the treatment of fibromatosis. In: Clinical orthopaedics and related research. Lippincott Williams and Wilkins; 2003:19–24. doi:https://doi.org/10.1097/01.blo.0000093892.12372.d4.
Yao X, Corbett T, Gupta AA, et al. A systematic review of active treatment options in patients with Desmoid tumours. Curr Oncol. 2014;21(4):613–29. https://doi.org/10.3747/co.21.1995.
Guadagnolo BA, Zagars GK, Ballo MT. Long-term outcomes for desmoid tumors treated with radiation therapy. Int J Radiat Oncol Biol Phys. 2008;71(2):441–7. https://doi.org/10.1016/j.ijrobp.2007.10.013.
Rüdiger HA, Ngan SYK, Ng M, Powell GJ, Choong PFM. Radiation therapy in the treatment of desmoid tumours reduces surgical indications. Eur J Surg Oncol. 2010;36(1):84–8. https://doi.org/10.1016/j.ejso.2009.07.183.
Santti K, Beule A, Tuomikoski L, et al. Radiotherapy in desmoid tumors treatment response, local control, and analysis of local failures; 2017. https://doi.org/10.1007/s00066-016-1091-8.
Janssen ML, van Broekhoven DLM, Cates JMM, et al. Meta-analysis of the influence of surgical margin and adjuvant radiotherapy on local recurrence after resection of sporadic desmoid-type fibromatosis. Br J Surg. 2017;104(4):347–57. https://doi.org/10.1002/bjs.10477.
Ballo MT, Zagars GK, Pollack A. Radiation therapy in the management of desmoid tumors. Int J Radiat Oncol. 1998;42(5):1007–14. https://doi.org/10.1016/S0360-3016(98)00285-5.
Goy BW, Lee SP, Eilber F, et al. The role of adjuvant radiotherapy in the treatment of resectable desmoid tumors. Int J Radiat Oncol. 1997;39(3):659–65. https://doi.org/10.1016/S0360-3016(97)00334-9.
Azzarelli A, Gronchi A, Bertulli R, et al. Low-dose chemotherapy with methotrexate and vinblastine for patients with advanced aggressive fibromatosis. Cancer. 2001;92(5):1259–64. https://doi.org/10.1002/1097-0142(20010901)92:5<1259::AID-CNCR1446>3.0.CO;2-Y.
Patel SR, Evans HL, Benjamin RS. Combination chemotherapy in adult desmoid tumors. Cancer. 1993;72(11):3244–7. https://doi.org/10.1002/1097-0142(19931201)72:11<3244::AID-CNCR2820721118>3.0.CO;2-D.
Gega M, Yanagi H, Yoshikawa R, et al. Successful chemotherapeutic modality of doxorubicin plus dacarbazine for the treatment of desmoid tumors in association with familial adenomatous polyposis. J Clin Oncol. 2006;24(1):102–5. https://doi.org/10.1200/JCO.2005.02.1923.
Bocale D, Rotelli MT, Cavallini A, Altomare DF. Anti-oestrogen therapy in the treatment of desmoid tumours: a systematic review. Color Dis. 2011;13(12):e388–95. https://doi.org/10.1111/j.1463-1318.2011.02758.x.
Janinis J, Patriki M, Vini L, Aravantinos G, Whelan JS. The pharmacological treatment of aggressive fibromatosis: a systematic review. Ann Oncol. 2003;14:181–90. https://doi.org/10.1093/annonc/mdg064.
Garbay D, Le Cesne A, Penel N, et al. Chemotherapy in patients with desmoid tumors: a study from the French Sarcoma Group (FSG) original articles Annals of Oncology. Ann Oncol. 2012;23:182–6. https://doi.org/10.1093/annonc/mdr051.
Chugh R, Wathen JK, Patel SR, et al. Efficacy of imatinib in aggressive fibromatosis: results of a phase II multicenter sarcoma Alliance for research through collaboration (SARC) trial. Clin Cancer Res. 2010;16(19):4884–91. https://doi.org/10.1158/1078-0432.CCR-10-1177.
Penel N, Le Cesne A, Bui BN, et al. Imatinib for progressive and recurrent aggressive fibromatosis (desmoid tumors): an FNCLCC/French sarcoma group phase II trial with a long-term follow-up. Ann Oncol. 2011;22:452–7. https://doi.org/10.1093/annonc/mdq341.
Kasper B, Gruenwald V, Reichardt P, Bauer S, Hohenberger P, Haller F. Correlation of CTNNB1 mutation status with progression arrest rate in RECIST progressive desmoid-type fibromatosis treated with Imatinib: translational research results from a phase 2 study of the German Interdisciplinary Sarcoma Group (GISG-01). Ann Surg Oncol. 2016;23(6):1924–7. https://doi.org/10.1245/s10434-016-5132-4.
Kasper B, Gruenwald V, Reichardt P, et al. Imatinib induces sustained progression arrest in RECIST progressive desmoid tumours: final results of a phase II study of the German Interdisciplinary Sarcoma Group (GISG). Eur J Cancer. 2017;76:60–7. https://doi.org/10.1016/j.ejca.2017.02.001.
Gounder MM, Mahoney MR, Van Tine B, et al. Sorafenib for advanced and refractory desmoid tumors. NEJM. 2018;379:2417–28.
Acker JC, Bossen EH, Halperin EC. The management of desmoid tumors. Int J Radiat Oncol Biol Phys. 1993;26(5):851–8. https://doi.org/10.1016/0360-3016(93)90501-L.
Crago AM, Denton B, Salas S, et al. A prognostic nomogram for prediction of recurrence in desmoid fibromatosis. Ann Surg. 2013;258(2):347–53. https://doi.org/10.1097/SLA.0b013e31828c8a30.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Cho, CK.J., Kim, E. (2022). Desmoid Tumors. In: Kim, E., Parvathaneni, U., Welliver, M.X. (eds) Radiation Therapy for Sarcomas and Skin Cancers. Practical Guides in Radiation Oncology. Springer, Cham. https://doi.org/10.1007/978-3-031-06706-8_8
Download citation
DOI: https://doi.org/10.1007/978-3-031-06706-8_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-06705-1
Online ISBN: 978-3-031-06706-8
eBook Packages: MedicineMedicine (R0)