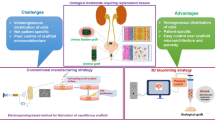
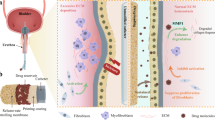
Abstract
Even though urinary stents and catheters have been commonly applied in medicine for several decades and still are constantly being modified and optimized, their structure and performance still requires further improvement. A major drawback of urinary implants is the deposition of organic and non-organic substances on their surface leading to biofilm formation resulting in encrustations, blockages, and infections. Promising research lines are stent coatings with antibodies, enzymes and various bioactive compounds. We will also discuss the possibility of making urinary implants more “tissue friendly” by designing biomimetic surfaces. Finally, in accordance with the paradigm “repair or regrow” we will touch on tissue engineering approaches to replace artificial urinary implants by those generated in vitro or in vivo from homologous tissue. We summarised modern biological approaches to improve the structure, function and performance of urinary stents. Some have been already applied in urinary stent production whilst others have been tested in the field of vascular stents, such as antibody or biomimetic coating. Bioengineering approaches aiming at the generation of complete analogs of damaged urinary tissue from autologous patient-derived cells represent a more futuristic outlook. Nevertheless, we hope that the rapid development of advanced multidisciplinary research platforms in modern biomedicine will make these approaches feasible in the near future.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Urinary implants
- Biofilm
- Encrustation
- Stents
- Coatings
- Enzymes
- Antibodies
- Biomimetic surfaces
- Nanocoating
- Tissue engineering
1 Introduction
Even though urinary stents and catheters have been commonly applied in medicine for several decades and still are constantly being modified and optimized, their structure and performance still requires further improvement. A major drawback of urinary implants is the deposition of organic and non-organic substances on their surface leading to biofilm formation resulting in encrustations, blockages, and infections.
In this chapter, we will present some modern biological research approaches to this problem. Promising research lines are stent coatings with antibodies, enzymes and various bioactive compounds. We will also discuss the possibility for making urinary implants more “tissue friendly” by designing biomimetic surfaces. Finally, in accordance with the paradigm “repair or regrow” we will touch on tissue engineering approaches to replace artificial urinary implants by those generated in vitro or in vivo from homologous tissue.
1.1 Antibody Coating
Despite significant differences between urine and blood as the extracellular environment, designing of urinary implants has always been inspired by research approaches in cardiovascular stent engineering.
Antibody stent coating is a technology that was successfully applied to improve clinical performance of cardiovascular stents and might be considered for further optimization of urinary stents.
Coating of vascular stents with certain types of antibodies (namely: CD34, CD133 and VEGFR2) enhances migration of endothelial progenitor cells (EPCs) circulating in the blood to the site of injury, thus accelerating the healing and regeneration of vascular tissue [1, 2]. At the same time, populating of the stent surface and surrounding tissue with EPCs prevents migration of inflammatory and smooth muscle cells which can cause neo-intimal hyperplasia, and re-stenosis and thrombosis of damaged blood vessels [1,2,3]. Antibody-coated cardiovascular stents showed promising results in preclinical trials [3]. Other antibodies with anti-inflammatory, anti-platelet and anti-proliferative effects also have shown their efficiency in reducing stenosis and neo-intimal hyperplasia in vitro [4, 5].
Despite the success of antibody stent-coating in cardiology, regrettably to our knowledge no preclinical or even experimental researches in this field have been presented for urinary implants to date. Hypothetically, antibodies of implant coatings could attract urinary and cell components that prevent biofilm formation and urothelial hyperplasia on one hand, and promote peri-implant tissue healing on the other hand in cases where such implants are used temporarily after injuries or operations to the urinary tract. As a prerequisite for such a research approach, efforts must be made to identify urinary and cellular components with the desired properties first.
2 Enzyme Coating
Antimicrobial enzymes (AE) have been tested as part of urinary catheter coatings. AE are found in immune systems of living organisms where their task is to attack pathogenic microbes. Hydrolytic AE destroy the structure of their foes, whereas oxidative AE trigger the production of antimicrobial molecules inside them. Quorum quenching AE interfere with bacterial quorum sensing which leads to inhibition of cell aggregation and virulent compounds [6].
AE can be attached to surfaces of medical devices, either permanently or ready to be released. Integrated methods for a controlled release include chelation or metal binding, disulfide [7], physical and ionic bonds [8].
Permanent or irreversible binding has however the advantage of better stability and decreased leaching. This can be achieved by crosslinking with linker molecules, entrapment, microencapsulation and covalent bonding [6].
For instance, cellobiose dehydrogenase (CDH), using cello-oligosaccharides as electron donors to produce H2O2, inhibited different urinary microbes including MRSA in the presence of either cellobiose or extracellular polysaccharides (EPS). The latter are essential in biofilm formation. Therefore, CDH could act as an antimicrobial agent “on demand” whenever beginning biofilm formation is triggering the reaction [9].
Oxalobacter formigenes is part of our gut microbiome and has been linked to non-infectious stone formation. It can degrade oxalate with the help of oxalate-degrading enzymes. These enzymes have been attached to stent coatings resulting in a 53% reduction in encrustation [10, 11].
When compared with current antibiotics or other antimicrobial agents used as active parts of urinary implant coatings, AE have certain advantages. They are highly specific targeting only a particular bacterium without disturbing the natural microbiome. Bacterial resistance to enzymes is very rare. Care must be taken not to overdose the enzymes though in order to prevent such resistance development. Enzymes are safe, natural, non-reactive and non-toxic to other than their target organisms.
Currently, AE are expensive to produce which puts them at a disadvantage to cheaper alternatives like silver and antibiotic coatings. And it has to be borne in mind that they are proteins. That means they can get denatured during i.e. sterilization, storage and transport [6].
3 Biomimetic Stents
Natural surfaces have been the envy of many researchers. However, they are difficult to mimic and usually outperform their artificial copies. If it comes to internal and external surfaces, researchers try to get as close to the properties of natural surfaces as possible. Natural surfaces are made to repel or let seep through whatever is physiologically required by their environment. Blood and blood vessels form such an environment. With cardiology leading the way for a long time in stent research, it is no surprise that the attempt to create biomimetic surfaces gets its push from cardiology as well.
Although stents have been used extensively in cardiology, they do have inherent problems such as inflammatory responses, thrombosis, endothelial hyperproliferation, delayed re-endothelialization, and ultimately stenosis and thrombotic obstruction [12]. An endothelium-like stent could alleviate many of these problems. A metal (copper)–catechol–(amine) (MCA) coated stent has thus been shown to facilitate a fast regeneration of a functional endothelium [12].
Earlier, a phosphorylcholine coating was effective in resisting platelet adhesion and prolong plasma recalcification time significantly. Contact angle measurements showed that the surface rearranged to become more hydrophilic at the polymer/water interface [13].
In blood vessels, biomimetic surfaces may create an enhanced anti-thrombogenic, anti-inflammatory and anti-proliferative micro-environment. In addition, such coatings could be made drug eluting [14]. Biomimetic stents will improve biocompatibility in the future [12]. Whether lessons learned from cardiology can be applied to a urinary environment will be the subject of future research. In theory, biomimetic surfaces have the ability to resist bacterial adhesion and consecutive biofilm formation.
4 Bioactive Nanocoating
As soon as urinary implants are inserted into a recipient, different biological materials start to accumulate on their surfaces. These substances, especially extracellular matrix (ECM) proteins, attract cells which migrate from surrounding tissue and form a biofilm thus affecting shape, mechanical properties and functionality of the implant. Application of nanomaterials [15, 16] can mimic cell—ECM interaction and, depending on what is required, can either suppress or induce cell migration.
To date, optimization of the structural and functional performance of the implants was mostly achieved by surface coating with different bioactive molecular substances such as growth factors and immunosuppressive and anti-inflammatory drugs.
Interaction between cells, tissues and implanted mechanical objects strongly depend on the surface to volume ratio and, consequently, varies significantly when the objects are represented at macro-, micro- or nano-scale size ranges. In particular, this concerns particles < 10–15 nm and irregular in size distribution which affects greatly such material properties as roughness, friction and surface dislocation [17].
Materials with a particular 3D nanostructure can be generated either by coating of the surface with nanoparticles or modification of the surface by chemical and physical treatments. Combining these methods is even more effective. A controllable release of nanoparticles to the surface of the implant creates an additional modality for surface coating which helps to reduce potential toxicity.
Different materials could be proposed for nanocoating of urinary implants with each of them possessing certain advantages and disadvantages as to their applicability and functionality. Polymer-based and lipid nanoparticles (i.e. liposomes) are usually biocompatible and can be easily biodegradable. They are suitable for transportation of biologically active compounds to the cells due to their similarity to natural nanoparticles—extracellular vesicles, which take part in cell-cell communication [18].
Carbon nanotubes and fullerenes likewise possess a high biocompatibility since carbon is one of the main components of living tissues. They are smaller in size and have a high surface-to-volume ratio. Since carbon nanoparticles can form complex three-dimensional structures, they are especially useful for targeted delivery of particular bioactive substances.
Metal-based nanoparticles produced usually from transition metals like zinc, gold, silver and copper, are described broadly in the scientific literature. However, despite demonstrated good antimicrobial properties their antifungal and anti-viral efficiencies have not been established.
Toxicity of nanocoating particles has not been a problem so far [19]. However, a deep understanding is required due to the wide range of nanomaterials proposed for medical application and the great variety of their physico-chemical properties and ensuing potential effects on biological tissues.
5 Tissue Engineering and Regenerative Medicine as Future Research Lines
This research line has preliminarily looked at urinary stents in the lower urinary tract. Stents were available for the treatment of lower urinary tract obstruction from the late 1980s. Despite initial enthusiasm, further studies questioned their usefulness as a primary treatment for urethral stricture disease [20]. Fibrotic tissue ingrowth is the main complication in permanently implanted stents but it can occur even in temporarily indwelling stents.
An ideal urinary stent should promote tissue healing, reduce fibrosis and scar formation, and maintain the physiological functions of the lower urinary tract. In addition, it would be desirable if they would be biodegradable and could stimulate their gradual replacement by regenerated autologous tissue.
Even though this is yet wishful thinking, some progress has been made in the field of vascular and coronary artery stenting where implantation of complex tissue composites rather than a simple stent is required. How can this be translated into urology, and more specifically into the treatment of lower urinary tract stricture disease?
There are few experimental trials on the designing of urethral stents using tissue engineering approaches [21, 22]. To our knowledge though, these clinical and preclinical applications have not proceeded beyond phase two trials.
The main problem of tissue engineered grafts or stents is the lack of appropriate acceptance by the host leading to fibrosis or rejection. If the wound bed consists of intact and viable tissue, transplantation of epithelium only might be sufficient [23]. In cases of fibrosis or developmental defects, vascularized grafts should be generated, e.g. by populating scaffolds with endothelial cells of endothelial progenitors [24,25,26].
In contrast to vascular stents, where the cells required for tissue regeneration can migrate from the blood, in urinary stents the recruitment of the cells from urine is rather unlikely though not impossible. If the urethral graft is well vascularized, blood flow might deliver important cells and cell progenitors to the sites of tissue regeneration. To make this approach clinically relevant, comprehensive studies on the role of inflammatory cells in tissue regeneration (which must be induced) and fibrosis (which must be prevented) are required.
In addition to in situ approaches where biodegradable urinary stents could be gradually replaced by regenerated tissue, we must consider cases where the tissue damage caused by trauma or disease is beyond the regenerative potential of the organism. In these cases, reconstruction of parts of the urinary tract must be done completely in vitro, using synthetic or naturally derived scaffolds [27, 28], manufactured by 3D printing [29] and electrostatic spinning [30], and populated by different types of cells (including autologous cells and progenitors) [31, 32]. Application of endothelial cell-attracting extracellular matrix into a graft scaffold and pre-vascularization of a transplant on vasculature-rich tissues (such as omentum) [31], as well as engineering of perfusable blood vessels of the transplant in vitro [33, 34] could be used for vascularization of the grafts. Combination of a “cell sheet technology” with bioprinting of cellularized scaffolds has a great potential for future perspectives of engineering of urinary stents.
Another completely different approach based on a “let mother nature do the job” paradigm can also be considered for designing urinary stents. The fundamental discovery of iPS cells [35] and the not yet that well-known tissue engineering technology of “blastocyst complementation” [36, 37] cumulatively might open the possibility to grow patient-specific organs (including different components of the lower urinary tract) in humanized animals. With this technique, certain gene modifications in the host organism prevents formation of the target organ during embryogenesis. Patient-derived iPS cells delivered to the blastocysts of immunocompromised animals will rescue the developmental program and form the target organ which will consist of human cells. After maturation in the animal host, the complete tissue/organ or its parts can be transplanted to the patient. As an alternative to the “blastocyst complementation” assay, based on the use of “humanized animals”, it is also proposed to transplant specific human progenitor cells to actual sites of organogenesis thus leading to formation of target organs that consist of patient-derived cells [38]. These futuristic approaches, however, have not been tested in preclinical trials and can only be considered as a proof of concept to date.
6 Conclusions
In this chapter we have summarised modern biological approaches to improve the structure, function and performance of urinary stents. Some have been already applied in urinary stent production whilst others have been tested in the field of vascular stents, such as antibody or biomimetic coating. Bioengineering approaches aiming at the generation of complete analogs of damaged urinary tissue from autologous patient-derived cells represent a more futuristic outlook. Nevertheless, we hope that the rapid development of advanced multidisciplinary research platforms in modern biomedicine will make these approaches feasible in the near future.
References
Wawrzyńska M, Duda M, Wysokińska E, Strządała L, Biały D, Ulatowska-Jarża A, Kałas W, Kraszewski S, Pasławski R, Biernat P, Pasławska U, Zielonka A, Podbielska H, Kopaczyńska M. Functionalized CD133 antibody coated stent surface simultaneously promotes EPCs adhesion and inhibits smooth muscle cell proliferation—a novel approach to prevent in-stent restenosis. Colloids Surf B Biointerfaces. 2019;174(1):587–97.
Wawrzyńska M, Kraskiewicz H, Paprocka M, Krawczenko A, Bielawska-Pohl A, Biały D, Roleder T, Wojakowski W, O'Connor IB, Duda M, Michal R, Wasyluk Ł, Plesch G, Podbielska H, Kopaczyńska M, Wall JG. Functionalization with a VEGFR2-binding antibody fragment leads to enhanced endothelialization of a cardiovascular stent in vitro and in vivo. J Biomed Mater Res B Appl Biomater. 2020;108(1):213–24.
de Winter RJ, Chandrasekhar J, Kalkman DN, Aquino MB, Woudstra P, Beijk MA, Sartori S, Baber U, Tijssen JG, Koch KT, Dangas GD, Colombo A, Mehran R, MASCOT; REMEDEE Registry Investigators. 1-Year clinical outcomes of all-comer patients treated with the dual-therapy COMBO stent: primary results of the COMBO Collaboration. JACC Cardiovasc Interv. 2018;11(19):1969–78.
Cui S, Liu JH, Song XT, Ma GL, Du BJ, Lv SZ, Meng LJ, Gao QS, Li K. A novel stent coated with antibodies to endoglin inhibits neointimal formation of porcine coronary arteries. Biomed Res Int. 2014;2014:428619.
Lim KS, Jeong MH, Bae IH, Park JK, Park DS, Kim JM, Kim JH, Kim HS, Kim YS, Jeong HY, Song SJ, Yang EJ, Cho DL, Sim DS, Park KH, Hong YJ, Ahn Y. Effect of polymer-free TiO2 stent coated with abciximab or alpha lipoic acid in porcine coronary restenosis model. J Cardiol. 2014;64(5):409–18.
Singha P, Locklin J, Handa H. A review of the recent advances in antimicrobial coatings for urinary catheters. Acta Biomater. 2017;50:20–40.
Cabral J, Novais J, Kennedy J. Immobilization studies of whole microbial cells on transition metal activated inorganic supports. Appl Microbiol Biotechnol. 1986;23(3–4):157–62.
Roig M. Immobilised cells and enzymes—a practical approach: Edited by J Woodward. Oxford: IRL Press; 1985. p. 177. ISBN-947946-21-7.
Thallinger B, Argirova M, Lesseva M, Ludwig R, Sygmund C, Schlick A, Nyanhongo GS, Guebitz GM. Preventing microbial colonisation of catheters: antimicrobial and antibiofilm activities of cellobiose dehydrogenase. Int J Antimicrob Agents. 2014;44(5):402–8.
Malpass CA, Millsap KW, Sidhu H, Gower LB. Immobilization of an oxalate-degrading enzyme on silicone elastomer. J Biomed Mater Res. 2002;63(6):822–9.
Watterson JD, Cadieux PA, Beiko DT, Cook AJ, Burton JP, Harbottle RR, Lee C, Rowe E, Sidhu H, Reid G, Denstedt JD. Oxalate-degrading enzymes from Oxalobacter formigenes: a novel device coating to reduce urinary tract biomaterial-related encrustation. J Endourol. 2003;17(5):269–74.
Yang Y, Gao P, Wang J, Tu Q, Bai L, Xiong K, Qiu H, Zhao X, Maitz MF, Wang H, Li X, Zhao Q, Xiao Y, Huang N, Yang Z. Endothelium-mimicking multifunctional coating modified cardiovascular stents via a stepwise metal-catechol-(amine) surface engineering strategy. AAAS Res. 2020;2020:9203906.
Fan D, Jia Z, Yan X, Liu X, Dong W, Sun F, Ji J, Xu J, Ren K, Chen W, Shen J, Qiu H, Gao R. Pilot study of a cell membrane like biomimetic drug-eluting coronary stent. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2007;24(3):599–602.
Elsawy MM, de Mel A. Biofabrication and biomaterials for urinary tract reconstruction. Res Rep Urol. 2017;9:79–92.
Webster TJ, Ahn ES. Nanostructured biomaterials for tissue engineering bone. In: Lee K, Kaplan D (eds) Tissue engineering II: basics of tissue engineering and tissue applications. 2007, Berlin: Springer. p. 275–308.
Brackman G, Coenye T. Quorum sensing inhibitors as anti-biofilm agents. Curr Pharm Des. 2015;21:5–11.
Rane GK, Welzel U, Meka SR, Mittemeijer EJ. Non-monotonic lattice parameter variation with crystallite size in nanocrystalline solids. Acta Mater. 2013;61(12):4524–33.
Jaggessar A, Shahali H, Mathew A, Yarlagadda PKDV. Bio-mimicking nano and micro-structured surface fabrication for antibacterial properties in medical implants. J Nanobiotechnol. 2017;15(1):64.
Raja IS, Song SJ, Kang MS, et al. Toxicity of zero- and one-dimensional carbon nanomaterials. Nanomaterials (Basel). 2019;9(9):1214.
Djordjevic ML. Treatment of urethral stricture disease by internal urethrotomy, dilatation, or stenting. Eur Urol Suppl. 2016;15(1):7–12.
de Kemp V, de Graaf P, Fledderus JO, Ruud Bosch JL, de Kort LM. Tissue engineering for human urethral reconstruction: systematic review of recent literature. PLoS One. 2015;10(2):e0118653.
Versteegden LRM, de Jonge PKJD, IntHout J, van Kuppevelt TH, Oosterwijk E, Feitz WFJ, de Vries RBM, Daamen WF. Tissue engineering of the urethra: a systematic review and meta-analysis of preclinical and clinical studies. Eur Urol. 2017;72(4):594–606.
Ram-Liebig G, Barbagli G, Heidenreich A, Fahlenkamp D, Romano G, Rebmann U, Standhaft D, van Ahlen H, Schakaki S, Balsmeyer U, Spiegler M, Knispel H. Results of use of tissue-engineered autologous oral mucosa graft for urethral reconstruction: a multicenter, prospective, observational trial. EBioMedicine. 2017;23:185–92.
de Graaf P, Ramadan R, Linssen EC, Staller NA, Hendrickx APA, Pigot GLS, Meuleman EJH, Bouman M, Özer M, Bosch JLHR, de Kort LMO. The multilayered structure of the human corpus spongiosum. Histol Histopathol. 2018;33(12):1335–45.
van Velthoven MJJ, Ramadan R, Zügel FS, Klotz BJ, Gawlitta D, Costa PF, Malda J, Castilho MD, de Kort LMO, de Graaf P. Gel casting as an approach for tissue engineering of multilayered tubular structures. Tissue Eng Part C Methods. 2020;26(3):190–8.
Zhang K, Fu Q, Yoo J, Chen X, Chandra P, Mo X, Song L, Atala A, Zhao W. 3D bioprinting of urethra with PCL/PLCL blend and dual autologous cells in fibrin hydrogel: an in vitro evaluation of biomimetic mechanical property and cell growth environment. Acta Biomater. 2017;50:154–64.
Wissing TB, Bonito V, Bouten CVC, Smits AIPM. Biomaterial-driven in situ cardiovascular tissue engineering—a multi-disciplinary perspective. NPJ Regen Med. 2017;16(2):18. https://doi.org/10.1038/s41536-017-0023-2.
Setayeshmehr M, Esfandiari E, Rafieinia M, Hashemibeni B, Taheri-Kafrani A, Samadikuchaksaraei A, Kaplan DL, Moroni L, Joghataei MT. Hybrid and composite scaffolds based on extracellular matrices for cartilage tissue engineering. Tissue Eng Part B Rev. 2019;25(3):202–24.
Kolesky DB, Truby RL, Gladman AS, Busbee TA, Homan KA, Lewis JA. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater. 2014;26(19):3124–30.
Fu WJ, Zhang X, Zhang BH, Zhang P, Hong BF, Gao JP, Meng B, Kun H, Cui FZ. Biodegradable urethral stents seeded with autologous urethral epithelial cells in the treatment of post-traumatic urethral stricture: a feasibility study in a rabbit model. BJU Int. 2009;104(2):263–8.
Zhao Z, Liu D, Chen Y, Kong Q, Li D, Zhang Q, Liu C, Tian Y, Fan C, Meng L, Zhu H, Yu H. Ureter tissue engineering with vessel extracellular matrix and differentiated urine-derived stem cells. Acta Biomater. 2019;88:266–79.
Chapple C. Tissue engineering of the urethra: where are we in 2019? World J Urol. 2020;38(9):2101–5.
Sakaguchi K, Shimizu T, Horaguchi S, Sekine H, Yamato M, Umezu M, Okano T. In vitro engineering of vascularized tissue surrogates. Sci Rep. 2013;3:1316.
Sekiya S, Shimizu T. Introduction of vasculature in engineered three-dimensional tissue. Inflamm Regen. 2017;37:25.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76.
Stanger BZ, Tanaka AJ, Melton DA. Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature. 2007;445(7130):886–91.
Yokoo T. Kidney regeneration with stem cells: an overview. Nephron Exp Nephrol. 2014;126(2):54.
Fujimoto T, Yamanaka S, Tajiri S, Takamura T, Saito Y, Matsumoto K, Takase K, Fukunaga S, Okano HJ, Yokoo T. In vivo regeneration of interspecies chimeric kidneys using a nephron progenitor cell replacement system. Sci Rep. 2019;9(1):6965.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this chapter
Cite this chapter
Buchholz, N. et al. (2022). Preventing Biofilm Formation and Encrustation on Urinary Implants: (Bio)coatings and Tissue Engineering. In: Soria, F., Rako, D., de Graaf, P. (eds) Urinary Stents. Springer, Cham. https://doi.org/10.1007/978-3-031-04484-7_33
Download citation
DOI: https://doi.org/10.1007/978-3-031-04484-7_33
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-04483-0
Online ISBN: 978-3-031-04484-7
eBook Packages: MedicineMedicine (R0)