Abstract
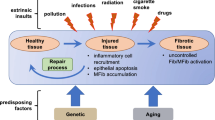
The major distinguishing feature of fibrosis is significant deposition of collagen and other extracellular matrix (ECM) proteins, which can result in scarring if sufficiently excessive. Fibrosis affects many tissue types, and thus contributes to a broad group of diseases which, with few exceptions, continues to lack specific therapy. It has been estimated that nearly 45% of deaths in the developed world are caused by fibroproliferative diseases, which contribute to cardiovascular disease, pulmonary, renal, gut and liver fibrosis, and scleroderma (Bitterman and Henke in Chest 99:81S–84S, [1]). Fibroblasts are the most common stromal cell type of the connective tissues found in the body, and are the primary source of ECM in physiological conditions, i.e. in the absence of disease. The conversion of fibroblasts or similar stromal cells to myofibroblasts is a principal mediator of pathological fibrosis in many tissue types, and frequently occurs in response to ongoing tissue injury and chronic inflammation. While the fibrotic response can occur in response to existing disease, the phenotype conversion of fibroblasts to myofibroblasts due to transient stress or damage may lead to the initiation of long-term fibrotic disease (Bagchi et al. in BMC Biol 14:21, [2]). Inflammation has been found to be a critical inducer of fibrosis, with immune cells generating a variety of growth factors and cytokines that play critical roles in fibroblast activation and subsequent tissue remodelling and fibrosis. A common cellular response to stress stimuli such as inflammation is autophagy, and recent studies have tightly linked the activation or inhibition of autophagy with fibrotic diseases in myriad tissues. Here, we discuss the inter-relationship of these pathways to provide insight into their potential as therapeutic targets in fibrotic disease.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Bitterman PB, Henke CA (1991) Fibroproliferative disorders. Chest 99:81S-84S
Bagchi RA, Roche P, Aroutiounova N, Espira L, Abrenica B, Schweitzer R, Czubryt MP (2016) The transcription factor scleraxis is a critical regulator of cardiac fibroblast phenotype. BMC Biol 14:21
Guenther A, Krauss E, Tello S, Wagner J, Paul B, Kuhn S, Maurer O, Heinemann S, Costabel U, Barbero MAN et al (2018) The European IPF registry (eurIPFreg): baseline characteristics and survival of patients with idiopathic pulmonary fibrosis. Respir Res 19:141
Silvestre JS, Heymes C, Oubenaissa A, Robert V, Aupetit-Faisant B, Carayon A, Swynghedauw B, Delcayre C (1999) Activation of cardiac aldosterone production in rat myocardial infarction: effect of angiotensin II receptor blockade and role in cardiac fibrosis. Circulation 99:2694–2701
Lee JY, Ihm HS, Kim JS, Hwang HS, Jeong KH, Ihm CG (2019) Baseline high blood pressure is associated with clinico-pathologic findings and later renal progression in chronic glomerulonephritis. Electrolyte Blood Press 17:54–61
Bian H, Zhu X, Xia M, Yan H, Chang X, Hu X, Pan B, Guo W, Li X, Gao X (2020) Impact of type 2 diabetes on nonalcoholic steatohepatitis and advanced fibrosis in patients with nonalcoholic fatty liver disease. Endocr Pract
Katzenstein AL, Mukhopadhyay S, Zanardi C, Dexter E (2010) Clinically occult interstitial fibrosis in smokers: classification and significance of a surprisingly common finding in lobectomy specimens. Hum Pathol 41:316–325
Tabet E, Genet V, Tiaho F, Lucas-Clerc C, Gelu-Simeon M, Piquet-Pellorce C, Samson M (2016) Chlordecone potentiates hepatic fibrosis in chronic liver injury induced by carbon tetrachloride in mice. Toxicol Lett 255:1–10
Leyva F, Taylor RJ, Foley PW, Umar F, Mulligan LJ, Patel K, Stegemann B, Haddad T, Smith RE, Prasad SK (2012) Left ventricular midwall fibrosis as a predictor of mortality and morbidity after cardiac resynchronization therapy in patients with nonischemic cardiomyopathy. J Am Coll Cardiol 60:1659–1667
Espira L, Czubryt MP (2009) Emerging concepts in cardiac matrix biology. Can J Physiol Pharmacol 87:996–1008
Czubryt MP (2019) Cardiac fibroblast to myofibroblast phenotype conversion-an unexploited therapeutic target. J Cardiovasc Dev Dis 6
Nagalingam RS, Al-Hattab DS, Czubryt MP (2019) What’s in a name? On fibroblast phenotype and nomenclature. Can J Physiol Pharmacol 97:493–497
Czubryt MP (2012) Common threads in cardiac fibrosis, infarct scar formation, and wound healing. Fibrogenesis Tissue Repair 5:19
Ma Y, Iyer RP, Jung M, Czubryt MP, Lindsey ML (2017) Cardiac fibroblast activation post-myocardial infarction: current knowledge gaps. Trends Pharmacol Sci 38:448–458
Blaauboer ME, Boeijen FR, Emson CL, Turner SM, Zandieh-Doulabi B, Hanemaaijer R, Smit TH, Stoop R, Everts V (2014) Extracellular matrix proteins: a positive feedback loop in lung fibrosis? Matrix Biol 34:170–178
Hinz B (2010) The myofibroblast: paradigm for a mechanically active cell. J Biomech 43:146–155
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA (2002) Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3:349–363
Zhao S, Wu H, Xia W, Chen X, Zhu S, Zhang S, Shao Y, Ma W, Yang D, Zhang J (2014) Periostin expression is upregulated and associated with myocardial fibrosis in human failing hearts. J Cardiol 63:373–378
Snider P, Standley KN, Wang J, Azhar M, Doetschman T, Conway SJ (2009) Origin of cardiac fibroblasts and the role of periostin. Circ Res 105:934–947
Kendall RT, Feghali-Bostwick CA (2014) Fibroblasts in fibrosis: novel roles and mediators. Front Pharmacol 5:123
Kanisicak O, Khalil H, Ivey MJ, Karch J, Maliken BD, Correll RN, Brody MJ, SC, JL, Aronow BJ, Tallquist MD et al (2016) Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat Commun 7:12260
Shi-Wen X, Chen Y, Denton CP, Eastwood M, Renzoni EA, Bou-Gharios G, Pearson JD, Dashwood M, du Bois RM, Black CM et al (2004) Endothelin-1 promotes myofibroblast induction through the ETA receptor via a rac/phosphoinositide 3-kinase/Akt-dependent pathway and is essential for the enhanced contractile phenotype of fibrotic fibroblasts. Mol Biol Cell 15:2707–2719
Mori T, Kawara S, Shinozaki M, Hayashi N, Kakinuma T, Igarashi A, Takigawa M, Nakanishi T, Takehara K (1999) Role and interaction of connective tissue growth factor with transforming growth factor-beta in persistent fibrosis: a mouse fibrosis model. J Cell Physiol 181:153–159
Shi-Wen X, Rodriguez-Pascual F, Lamas S, Holmes A, Howat S, Pearson JD, Dashwood MR, du Bois RM, Denton CP, Black CM et al (2006) Constitutive ALK5-independent c-Jun N-terminal kinase activation contributes to endothelin-1 overexpression in pulmonary fibrosis: evidence of an autocrine endothelin loop operating through the endothelin A and B receptors. Mol Cell Biol 26:5518–5527
Yu X, Xia Y, Zeng L, Zhang X, Chen L, Yan S, Zhang R, Zhao C, Zeng Z, Shu Y et al (2018) A blockade of PI3Kgamma signaling effectively mitigates angiotensin II-induced renal injury and fibrosis in a mouse model. Sci Rep 8:10988
Tanikawa AA, Grotto RM, Silva GF, Ferrasi AC, Sarnighausen VC, Pardini MI (2017) Platelet-derived growth factor A mRNA in platelets is associated with the degree of hepatic fibrosis in chronic hepatitis C. Rev Soc Bras Med Trop 50:113–116
Xu SW, Liu S, Eastwood M, Sonnylal S, Denton CP, Abraham DJ, Leask A (2009) Rac inhibition reverses the phenotype of fibrotic fibroblasts. PloS one 4:e7438
Wojnarowski C, Frischer T, Hofbauer E, Grabner C, Mosgoeller W, Eichler I, Ziesche R (1999) Cytokine expression in bronchial biopsies of cystic fibrosis patients with and without acute exacerbation. Eur Respir J: Off J Eur Soc Clin Respir Physiol 14:1136–1144
Ludwicka A, Ohba T, Trojanowska M, Yamakage A, Strange C, Smith EA, Leroy EC, Sutherland S, Silver RM (1995) Elevated levels of platelet derived growth factor and transforming growth factor-beta 1 in bronchoalveolar lavage fluid from patients with scleroderma. J Rheumatol 22:1876–1883
Rudnicka L, Varga J, Christiano AM, Iozzo RV, Jimenez SA, Uitto J (1994) Elevated expression of type VII collagen in the skin of patients with systemic sclerosis. Regulation by transforming growth factor-beta. J Clin Invest 93:1709–1715
Broekelmann TJ, Limper AH, Colby TV, McDonald JA (1991) Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci U S A 88:6642–6646
Espira L, Lamoureux L, Jones SC, Gerard RD, Dixon IM, Czubryt MP (2009) The basic helix-loop-helix transcription factor scleraxis regulates fibroblast collagen synthesis. J Mol Cell Cardiol 47:188–195
Runyan CE, Schnaper HW, Poncelet AC (2003) Smad3 and PKCdelta mediate TGF-beta1-induced collagen I expression in human mesangial cells. Am J Physiol Renal Physiol 285:F413-422
Gressner AM, Weiskirchen R, Breitkopf K, Dooley S (2002) Roles of TGF-beta in hepatic fibrosis. Front Biosci 7:d793-807
Buscemi L, Ramonet D, Klingberg F, Formey A, Smith-Clerc J, Meister JJ, Hinz B (2011) The single-molecule mechanics of the latent TGF-beta1 complex. Curr Biol 21:2046–2054
Hinz B (2009) Tissue stiffness, latent TGF-beta1 activation, and mechanical signal transduction: implications for the pathogenesis and treatment of fibrosis. Curr Rheumatol Rep 11:120–126
Zeglinski MR, Roche P, Hnatowich M, Jassal DS, Wigle JT, Czubryt MP, Dixon IM (2016) TGFbeta1 regulates Scleraxis expression in primary cardiac myofibroblasts by a Smad-independent mechanism. Am J Physiol Heart Circ Physiol 310:H239-249
Klingberg F, Chow ML, Koehler A, Boo S, Buscemi L, Quinn TM, Costell M, Alman BA, Genot E, Hinz B (2014) Prestress in the extracellular matrix sensitizes latent TGF-beta1 for activation. J Cell Biol 207:283–297
Taipale J, Lohi J, Saarinen J, Kovanen PT, Keski-Oja J (1995) Human mast cell chymase and leukocyte elastase release latent transforming growth factor-beta 1 from the extracellular matrix of cultured human epithelial and endothelial cells. J Biol Chem 270:4689–4696
Chen MM, Lam A, Abraham JA, Schreiner GF, Joly AH (2000) CTGF expression is induced by TGF- beta in cardiac fibroblasts and cardiac myocytes: a potential role in heart fibrosis. J Mol Cell Cardiol 32:1805–1819
Gu J, Liu X, Wang QX, Tan HW, Guo M, Jiang WF, Zhou L (2012) Angiotensin II increases CTGF expression via MAPKs/TGF-beta1/TRAF6 pathway in atrial fibroblasts. Exp Cell Res 318:2105–2115
Yang F, Chung AC, Huang XR, Lan HY (2009) Angiotensin II induces connective tissue growth factor and collagen I expression via transforming growth factor-beta-dependent and -independent Smad pathways: the role of Smad3. Hypertension 54:877–884
Campbell SE, Katwa LC (1997) Angiotensin II stimulated expression of transforming growth factor-beta1 in cardiac fibroblasts and myofibroblasts. J Mol Cell Cardiol 29:1947–1958
Leask A (2010) Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res 106:1675–1680
Ma L, Hua J, He L, Li Q, Zhou J, Yu J (2012) Anti-fibrotic effect of Aliskiren in rats with deoxycorticosterone induced myocardial fibrosis and its potential mechanism. Bosn J Basic Med Sci/Udruzenje basicnih mediciniskih znanosti = Assoc Basic Med Sci 12:69–73
Dobaczewski M, Bujak M, Li N, Gonzalez-Quesada C, Mendoza LH, Wang XF, Frangogiannis NG (2010) Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res 107:418–428
Borthwick LA, Wynn TA, Fisher AJ (2013) Cytokine mediated tissue fibrosis. Biochim Biophys Acta 1832:1049–1060
Spellberg B, Edwards JE Jr (2001) Type 1/type 2 immunity in infectious diseases. Clin Infect Dis 32:76–102
Sandler NG, Mentink-Kane MM, Cheever AW, Wynn TA (2003) Global gene expression profiles during acute pathogen-induced pulmonary inflammation reveal divergent roles for Th1 and Th2 responses in tissue repair. J Immunol 171:3655–3667
Hoffmann KF, McCarty TC, Segal DH, Chiaramonte M, Hesse M, Davis EM, Cheever AW, Meltzer PS, Morse HC 3rd, Wynn TA (2001) Disease fingerprinting with cDNA microarrays reveals distinct gene expression profiles in lethal type 1 and type 2 cytokine-mediated inflammatory reactions. FASEB J 15:2545–2547
Lei L, Zhao C, Qin F, He ZY, Wang X, Zhong XN (2016) Th17 cells and IL-17 promote the skin and lung inflammation and fibrosis process in a bleomycin-induced murine model of systemic sclerosis. Clin Exp Rheumatol 34(Suppl 100):14–22
Conti P, Caraffa A, Mastrangelo F, Tettamanti L, Ronconi G, Frydas I, Kritas SK, Theoharides TC (2018) Critical role of inflammatory mast cell in fibrosis: Potential therapeutic effect of IL-37. Cell Prolif 51:e12475
Hugle T (2014) Beyond allergy: the role of mast cells in fibrosis. Swiss Med Wkly 144:w13999
Eggli PS, Graber W (1993) Cytochemical localization of hyaluronan in rat and human skin mast cell granules. J Invest Dermatol 100:121–125
Veerappan A, O’Connor NJ, Brazin J, Reid AC, Jung A, McGee D, Summers B, Branch-Elliman D, Stiles B, Worgall S et al (2013) Mast cells: a pivotal role in pulmonary fibrosis. DNA Cell Biol 32:206–218
Dona A, Abera M, Alemu T, Hawaria D (2018) Timely initiation of postpartum contraceptive utilization and associated factors among women of child bearing age in Aroressa District, Southern Ethiopia: a community based cross-sectional study. BMC Public Health 18:1100
Pechkovsky DV, Prasse A, Kollert F, Engel KM, Dentler J, Luttmann W, Friedrich K, Muller-Quernheim J, Zissel G (2010) Alternatively activated alveolar macrophages in pulmonary fibrosis-mediator production and intracellular signal transduction. Clin Immunol 137:89–101
Gieseck RL 3rd, Wilson MS, Wynn TA (2018) Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol 18:62–76
Zhu Z, Ding J, Ma Z, Iwashina T, Tredget EE (2017) Alternatively activated macrophages derived from THP-1 cells promote the fibrogenic activities of human dermal fibroblasts. Wound Repair Regen 25:377–388
Misharin AV, Morales-Nebreda L, Reyfman PA, Cuda CM, Walter JM, McQuattie-Pimentel AC, Chen CI, Anekalla KR, Joshi N, Williams KJN et al (2017) Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J Exp Med 214:2387–2404
Wang S, Meng XM, Ng YY, Ma FY, Zhou S, Zhang Y, Yang C, Huang XR, Xiao J, Wang YY et al (2016) TGF-beta/Smad3 signalling regulates the transition of bone marrow-derived macrophages into myofibroblasts during tissue fibrosis. Oncotarget 7:8809–8822
Suetomi T, Willeford A, Brand CS, Cho Y, Ross RS, Miyamoto S, Brown JH (2018) Inflammation and NLRP3 Inflammasome Activation Initiated in Response to Pressure Overload by Ca(2+)/Calmodulin-Dependent Protein Kinase II delta Signaling in Cardiomyocytes Are Essential for Adverse Cardiac Remodeling. Circulation 138:2530–2544
Groslambert M, Py BF (2018) Spotlight on the NLRP3 inflammasome pathway. J Inflamm Res 11:359–374
Artlett CM (2018) The IL-1 family of cytokines. Do they have a role in scleroderma fibrosis? Immunol Lett 195:30–37
Lonnemann G, Shapiro L, Engler-Blum G, Muller GA, Koch KM, Dinarello CA (1995) Cytokines in human renal interstitial fibrosis. I. Interleukin-1 is a paracrine growth factor for cultured fibrosis-derived kidney fibroblasts. Kidney Int 47:837–844
Tan Z, Liu Q, Jiang R, Lv L, Shoto SS, Maillet I, Quesniaux V, Tang J, Zhang W, Sun B et al (2018) Interleukin-33 drives hepatic fibrosis through activation of hepatic stellate cells. Cell Mol Immunol 15:388–398
Sullivan DE, Ferris M, Nguyen H, Abboud E, Brody AR (2009) TNF-alpha induces TGF-beta1 expression in lung fibroblasts at the transcriptional level via AP-1 activation. J Cell Mol Med 13:1866–1876
Doucet C, Brouty-Boye D, Pottin-Clemenceau C, Canonica GW, Jasmin C, Azzarone B (1998) Interleukin (IL) 4 and IL-13 act on human lung fibroblasts. Implication in asthma. J Clin Invest 101:2129–2139
Gharaee-Kermani M, Phan SH (1997) Lung interleukin-5 expression in murine bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 16:438–447
Takemura N, Kurashima Y, Mori Y, Okada K, Ogino T, Osawa H, Matsuno H, Aayam L, Kaneto S, Park EJ et al (2018) Eosinophil depletion suppresses radiation-induced small intestinal fibrosis. Sci Transl Med 10
Li Y, Gao Q, Xu K, Peng X, Yuan X, Jiang W, Li M (2018) Interleukin-37 attenuates bleomycin-induced pulmonary inflammation and fibrosis in mice. Inflammation 41:1772–1779
Feng XX, Chi G, Wang H, Gao Y, Chen Q, Ru YX, Luo ZL, Yan W, Li PY, Liu M et al (2019) IL-37 suppresses the sustained hepatic IFN-gamma/TNF-alpha production and T cell-dependent liver injury. Int Immunopharmacol 69:184–193
Kim MS, Baek AR, Lee JH, Jang AS, Kim DJ, Chin SS, Park SW (2019) IL-37 Attenuates lung fibrosis by inducing autophagy and regulating TGF-beta1 production in mice. J Immunol 203:2265–2275
Kurosaki F, Uchibori R, Sehara Y, Saga Y, Urabe M, Mizukami H, Hagiwara K, Kume A (2018) AAV6-mediated IL-10 expression in the lung Ameliorates bleomycin-induced pulmonary fibrosis in mice. Hum Gene Ther 29:1242–1251
Balaji S, Wang X, King A, Le LD, Bhattacharya SS, Moles CM, Butte MJ, de Jesus Perez VA, Liechty KW, Wight TN et al (2017) Interleukin-10-mediated regenerative postnatal tissue repair is dependent on regulation of hyaluronan metabolism via fibroblast-specific STAT3 signaling. FASEB J 31:868–881
Khawar MB, Azam F, Sheikh N, Abdul Mujeeb K (2016) How does interleukin-22 mediate liver regeneration and prevent injury and fibrosis? J Immunol Res 2016:2148129
Ghavami S, Cunnington RH, Gupta S, Yeganeh B, Filomeno KL, Freed DH, Chen S, Klonisch T, Halayko AJ, Ambrose E et al (2015) Autophagy is a regulator of TGF-beta1-induced fibrogenesis in primary human atrial myofibroblasts. Cell Death Dis 6:e1696
Gupta SS, Zeglinski MR, Rattan SG, Landry NM, Ghavami S, Wigle JT, Klonisch T, Halayko AJ, Dixon IM (2016) Inhibition of autophagy inhibits the conversion of cardiac fibroblasts to cardiac myofibroblasts. Oncotarget 7:78516–78531
Zeglinski MR, Davies JJ, Ghavami S, Rattan SG, Halayko AJ, Dixon IM (2016) Chronic expression of Ski induces apoptosis and represses autophagy in cardiac myofibroblasts. Biochim Biophys Acta 1863:1261–1268
Ghavami S, Yeganeh B, Zeki AA, Shojaei S, Kenyon NJ, Ott S, Samali A, Patterson J, Alizadeh J, Moghadam AR et al (2018) Autophagy and the unfolded protein response promote profibrotic effects of TGF-beta1 in human lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 314:L493–L504
Kim SI, Na HJ, Ding Y, Wang Z, Lee SJ, Choi ME (2012) Autophagy promotes intracellular degradation of type I collagen induced by transforming growth factor (TGF)-beta1. J Biol Chem 287:11677–11688
Nho RS, Hergert P (2014) IPF fibroblasts are desensitized to type I collagen matrix-induced cell death by suppressing low autophagy via aberrant Akt/mTOR kinases. PloS one 9:e94616
Alvarez D, Cardenes N, Sellares J, Bueno M, Corey C, Hanumanthu VS, Peng Y, D’Cunha H, Sembrat J, Nouraie M et al (2017) IPF lung fibroblasts have a senescent phenotype. Am J Physiol Lung Cell Mol Physiol 313:L1164–L1173
Kiyono K, Suzuki HI, Matsuyama H, Morishita Y, Komuro A, Kano MR, Sugimoto K, Miyazono K (2009) Autophagy is activated by TGF-beta and potentiates TGF-beta-mediated growth inhibition in human hepatocellular carcinoma cells. Cancer Res 69:8844–8852
Korah J, Canaff L, Lebrun JJ (2016) The retinoblastoma tumor suppressor protein (pRb)/E2 promoter binding factor 1 (E2F1) pathway as a novel mediator of TGFbeta-induced autophagy. J Biol Chem 291:2043–2054
Chitra P, Saiprasad G, Manikandan R, Sudhandiran G (2015) Berberine inhibits Smad and non-Smad signaling cascades and enhances autophagy against pulmonary fibrosis. J Mol Med (Berl) 93:1015–1031
Al Hattab D, Czubryt MP (2017) A primer on current progress in cardiac fibrosis. Can J Physiol Pharmacol 95:1091–1099
Araya J, Kojima J, Takasaka N, Ito S, Fujii S, Hara H, Yanagisawa H, Kobayashi K, Tsurushige C, Kawaishi M et al (2013) Insufficient autophagy in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 304:L56-69
Cosin-Roger J, Canet F, Macias-Ceja DC, Gisbert-Ferrandiz L, Ortiz-Masia D, Esplugues JV, Alos R, Navarro F, Barrachina MD, Calatayud S (2019) Autophagy stimulation as a potential strategy against intestinal fibrosis. Cells 8
Acknowledgements
This work was supported by a Project Grant from the Canadian Institutes of Health Research (PJT-162422 to MPC).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Chattopadhyaya, S., Czubryt, M.P. (2022). Fibroblasts, Fibrosis and Autophagy. In: Kirshenbaum, L.A. (eds) Biochemistry of Apoptosis and Autophagy. Advances in Biochemistry in Health and Disease, vol 18. Springer, Cham. https://doi.org/10.1007/978-3-030-78799-8_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-78799-8_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-78798-1
Online ISBN: 978-3-030-78799-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)