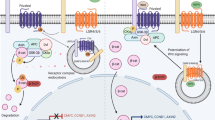
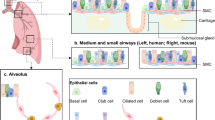
Abstract
The lung needs to maintain its integrity and functionality throughout lifespan since it is critical for survival. At steady-state, lung cell turnover is typically very low, and resident progenitor cells are in a quiescent state. The activation or inhibition of different signaling pathways in epithelial and/or mesenchymal cells of the adult lung tissue is crucial to maintaining the quiescence of progenitor’s niches. Interestingly, growth factors that have been reported as essential for lung development are also key to preserve adult tissue. With this chapter, we aim to describe the current knowledge regarding the molecular players that contribute to maintaining the homeostasis of the adult lung, specifically, SHH, FGF, WNT, Retinoic Acid, TGFβ, BMP, VEGF, and PDGF.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- ABCA3:
-
ATP Binding Cassette Subfamily A Member 3
- AEC:
-
Alveolar epithelial cells
- AM:
-
Alveolar macrophages
- ASMC:
-
Airway smooth muscle cells
- BC:
-
Basal cells
- BMP:
-
Bone Morphogenetic Protein
- CYP26A1:
-
Cytochrome P450 26A1
- DHH:
-
Desert Hedgehog
- FGF:
-
Fibroblast Growth Factor
- FGFR:
-
Fibroblast Growth Factor Receptor
- GLI:
-
Glioma-associated oncogene
- GREM2:
-
Gremlin 2
- IHH:
-
Indian Hedgehog
- LIF:
-
Lipofibroblast
- LRP:
-
Lipoprotein receptor-related protein
- NRP:
-
Neuropilin
- PDGF:
-
Platelet Derived Growth Factor
- PDGFR:
-
Platelet Derived Growth Factor Receptor
- PTCH:
-
Patched
- RA:
-
Retinoic Acid
- RALDH1:
-
Retinaldehyde dehydrogenase 1
- RAR:
-
Retinoic Acid Receptor
- SFRP1:
-
Secreted frizzled-related protein 1
- SHH:
-
Sonic Hedgehog
- SMO:
-
Smoothened
- SOX2:
-
SRY (sex determining region Y)-box 2
- SP:
-
Surfactant protein
- SPRY2:
-
Sprouty homolog 2
- TGFβ:
-
Transforming Growth Factor β
- VEGF:
-
Vascular Endothelial Growth Factor
- VEGFR:
-
Vascular Endothelial Growth Factor Receptor
- WNT:
-
Wingless-related Integration Site
References
Das S, Stewart P (2016) The influence of lung surfactant liquid crystalline nanostructures on respiratory drug delivery. Int J Pharm 514(2):465–474
Patwa A, Shah A (2015) Anatomy and physiology of respiratory system relevant to anaesthesia. Indian J Anaesth 59(9):533–541
Suki B, Stamenović D (2011) Lung Parenchymal Mechanics. Compr Physiol 1(3):1317–1351
Morrisey E, Hogan B (2010) Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell 18(1):8–23
Tomashefski JF, Farver CF (2008) Anatomy and histology of the lung. In: Tomashefski JF, Cagle PT, Farver CF, Fraire AE (eds) Dail and Hammar’s pulmonary pathology. Springer, New York, NY, pp 20–48
Herriges M, Morrisey E (2014) Lung development: orchestrating the generation and regeneration of a complex organ. Development 141(3):502–513
Mullassery D, Smith N (2015) Lung development. Semin Pediatr Surg 24(4):152–155
Ma J, Rubin B, Voynow J (2018) Mucins, mucus, and goblet cells. Chest 154(1):169–176
Evans MJ, Van Winkle LS, Fanucchi MV et al (2001) Cellular and molecular characteristics of basal cells in airway epithelium. Exp Lung Res 27(5):401–415
Cutz E (2015) Hyperplasia of pulmonary neuroendocrine cells in infancy and childhood. Semin Diagn Pathol 32(6):420–437
Sonar S, Ehmke M, Marsh L et al (2011) Clara cells drive eosinophil accumulation in allergic asthma. Eur Respir J 39(2):429–438
Yang J, Hernandez B, Martinez Alanis D et al (2015) The development and plasticity of alveolar type 1 cells. Development 143(1):54–65
Hussell T, Bell T (2014) Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol 14(2):81–93
Petrova R, Joyner A (2014) Roles for Hedgehog signaling in adult organ homeostasis and repair. Development 141(18):3445–3457
Lee R, Zhao Z, Ingham P (2016) Hedgehog signalling. Development 143(3):367–372
Fernandes-Silva H, Correia-Pinto J, Moura RS (2017) Canonical sonic hedgehog signaling in early lung development. J Dev Biol 5(1):3
Peng T, Frank D, Kadzik R et al (2015) Hedgehog actively maintains adult lung quiescence and regulates repair and regeneration. Nature 526(7574):578–582
Liu L, Kugler M, Loomis C et al (2013) Hedgehog signaling in neonatal and adult lung. Am J Respir Cell Mol Biol 48(6):703–710
Krause A, Xu Y, Joh J et al (2010) Overexpression of sonic hedgehog in the lung mimics the effect of lung injury and compensatory lung growth on pulmonary Sca-1 and CD34 positive cells. Mol Ther 18(2):404–412
Ornitz D, Itoh N (2015) The fibroblast growth factor signaling pathway. Wiley Interdiscip Rev Dev Biol 4(3):215–266
Fon Tacer K, Bookout A, Ding X et al (2010) Research resource: comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol Endocrinol 24(10):2050–2064
Zepp J, Zacharias W, Frank D et al (2017) Distinct mesenchymal lineages and niches promote epithelial self-renewal and myofibrogenesis in the lung. Cell 170(6):1134–1148
El Agha E, Herold S, Alam D et al (2014) Fgf10-positive cells represent a progenitor cell population during lung development and postnatally. Development 141(2):296–306
McQualter J, McCarty R, Van der Velden J et al (2013) TGF-β signaling in stromal cells acts upstream of FGF-10 to regulate epithelial stem cell growth in the adult lung. Stem Cell Res 11(3):1222–1233
Volckaert T, Yuan T, Chao C et al (2017) Fgf10-hippo epithelial-mesenchymal crosstalk maintains and recruits lung basal stem cells. Dev Cell 43(1):48–59
Balasooriya G, Johnson J, Basson M et al (2016) An FGFR1-SPRY2 signaling axis limits basal cell proliferation in the steady-state airway epithelium. Dev Cell 37(1):85–97
Balasooriya G, Goschorska M, Piddini E et al (2017) FGFR2 is required for airway basal cell self-renewal and terminal differentiation. Development 144(9):1600–1606
Gammons M, Bienz M (2018) Multiprotein complexes governing Wnt signal transduction. Curr Opin Cell Biol 51:42–49
Königshoff M, Eickelberg O (2010) WNT signaling in lung disease. Am J Respir Cell Mol Biol 42(1):21–31
Mucenski M, Nation J, Thitoff A, Besnard et al (2005) β-Catenin regulates differentiation of respiratory epithelial cells in vivo. Am J Physiol Lung Cell Mol Physiol 289(6):L971–L979
Reynolds S, Zemke A, Giangreco A et al (2008) Conditional stabilization of β-Catenin expands the pool of lung stem cells. Stem Cells 26(5):1337–1346
Nabhan A, Brownfield D, Harbury P et al (2018) Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science 359(6380):1118–1123
Zacharias W, Frank D, Zepp J et al (2018) Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature 555(7695):251–255
Kedishvili N (2016) Retinoic acid synthesis and degradation. Subcell Biochem 81:127–161
Schünemann HJ, Grant BJ, Freudenheim JL et al (2001) The relation of serum levels of antioxidant Vitamins C and E, retinol and carotenoids with pulmonary function in the general population. Am J Respir Crit Care Med 163(5):1246–1255
Collins S, Lucas J, Inskip H et al (2013) HHIP, HDAC4, NCR3 and RARB polymorphisms affect fetal, childhood and adult lung function. Eur Respir J 41(3):756–757
Ng-Blichfeldt J, Alçada J, Montero M et al (2017) Deficient retinoid-driven angiogenesis may contribute to failure of adult human lung regeneration in emphysema. Thorax 72(6):510–521
Ng-Blichfeldt J, Schrik A, Kortekaas R et al (2018) Retinoic acid signaling balances adult distal lung epithelial progenitor cell growth and differentiation. EBioMedicine 36:461–474
Chen F, Shao F, Hinds A et al (2018) Retinoic acid signaling is essential for airway smooth muscle homeostasis. JCI Insight 3(16):e120398
Derynck R, Budi E (2019) Specificity, versatility, and control of TGF-β family signaling. Sci Signal 12(570):eaav5183
Magnan A, Frachon I, Rain B et al (1994) Transforming growth factor beta in normal human lung: preferential location in bronchial epithelial cells. Thorax 49(8):789–792
Coker R, Laurent G, Shahzeidi S et al (1996) Diverse cellular TGF-beta 1 and TGF-beta 3 gene expression in normal human and murine lung. Eur Respir J 9(12):2501–2507
Ryan R, Mineo-Kuhn M, Kramer C et al (1994) Growth factors alter neonatal type II alveolar epithelial cell proliferation. Am J Physiol Lung Cell Mol Physiol 266(1):L17–L22
Zhao L, Yee M, O’Reilly M (2013) Transdifferentiation of alveolar epithelial type II to type I cells is controlled by opposing TGF-β and BMP signaling. Am J Physiol Lung Cell Mol Physiol 305(6):L409–L418
Correll K, Edeen K, Zemans R et al (2018) TGF beta inhibits expression of SP-A, SP-B, SP-C, but not SP-D in human alveolar type II cells. Biochem Biophys Res Commun 499(4):843–848
Lynn T, Molloy E, Masterson J et al (2016) SMAD signaling in the airways of healthy rhesus macaques versus rhesus macaques with asthma highlights a relationship between inflammation and bone morphogenetic proteins. Am J Respir Cell Mol Biol 54(4):562–573
Tadokoro T, Gao X, Hong C et al (2016) BMP signaling and cellular dynamics during regeneration of airway epithelium from basal progenitors. Development 143(5):764–773
Chung M, Bujnis M, Barkauskas C et al (2018) Niche-mediated BMP/SMAD signaling regulates lung alveolar stem cell proliferation and differentiation. Development 145(9):dev163014
Koch S, Claesson-Welsh L (2012) Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb Perspect Med 2(7):a006502
Kaner R, Crystal R (2001) Compartmentalization of vascular endothelial growth factor to the epithelial surface of the human lung. Mol Med 7(4):240–246
Fehrenbach H, Haase M, Kasper M et al (1999) Alterations in the immunohistochemical distribution patterns of vascular endothelial growth factor receptors Flk1 and Flt1 in bleomycin-induced rat lung fibrosis. Virchows Arch 435(1):20–31
Kasahara Y, Tuder R, Taraseviciene-Stewart L et al (2000) Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest 106(11):1311–1319
Roberts J, Perkins G, Fujisawa T et al (2007) Vascular endothelial growth factor promotes physical wound repair and is anti-apoptotic in primary distal lung epithelial and A549 cells. Crit Care Med 35(9):2164–2170
Kearns M, Dalal S, Horstmann S et al (2012) Vascular endothelial growth factor enhances macrophage clearance of apoptotic cells. Am J Physiol Lung Cell Mol Physiol 302(7):L711–L718
Kazlauskas A (2017) PDGFs and their receptors. Gene 614:1–7
Barkauskas C, Cronce M, Rackley C et al (2013) Type 2 alveolar cells are stem cells in adult lung. J Clin Invest 123(7):3025–3036
Acknowledgments
This work has been funded by FEDER through the Competitiveness Factors Operational Programme (COMPETE), by National funds through the Foundation for Science and Technology (FCT) under the scope of the project UID/Multi/50026/2019; and by the project NORTE-01-0145-FEDER-000013, supported by the Northern Portugal Regional Operational Programme (NORTE 2020), under the Portugal 2020 Partnership Agreement, through the European Regional Development Fund (FEDER).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Araújo-Silva, H., Correia-Pinto, J., Moura, R.S. (2020). Growth Factor Signaling in the Maintenance of Adult Lung Homeostasis. In: Silva, J.V., Freitas, M.J., Fardilha, M. (eds) Tissue-Specific Cell Signaling. Springer, Cham. https://doi.org/10.1007/978-3-030-44436-5_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-44436-5_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-44435-8
Online ISBN: 978-3-030-44436-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)