Abstract
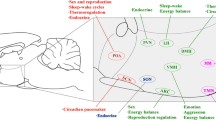
A number of cellular developmental processes occur in an orchestrated fashion to mediate the transition from a simple neural tube to the complexity of the adult brain. A combination of internal programming and external cues provides the molecular specificity needed to direct cells to the correct location, initiate correct gene expression, and connect to the appropriate circuits. The heterogeneous cells of the hypothalamus affect all aspects of physiology through the regulation of the autonomic nervous system, motivated behavior, and endocrine balance. Small disruptions (genetic or environmental) to hypothalamic development can impact adult physiology and contribute to pathology. GABAergic signaling has drawn the attention of developmental neuroendocrinologists as a potential effector system of hypothalamic development. This chapter discusses the role of GABA in directing the development of the hypothalamus, specifically related to the migration of neurons that release gonadotropin-releasing hormone (GnRH), and in the formation of the ventromedial (VMH) and paraventricular (PVN) nuclei of the hypothalamus (Fig. 7.1).
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- AOB:
-
Accessory olfactory bulb
- GABA:
-
Gamma aminobutyric acid
- GAD:
-
Glutamic acid decarboxylase
- GnRH:
-
Gonadotropin releasing hormone
- OB:
-
Olfactory bulb
- OE:
-
Olfactory epithelium
- PVN:
-
Paraventricular nucleus of the hypothalamus
- VMH:
-
Ventromedial nucleus of the hypothalamus
- VNO:
-
Vomeronasal organ
References
Acampora D, Postiglione MP, Avantaggiato V, Di Bonito M, Vaccarino FM, Michaud J, Simeone A (1999) Progressive impairment of developing neuroendocrine cell lineages in the hypothalamus of mice lacking the Orthopedia gene. Genes Dev 13(21):2787–2800
Altman J, Bayer SA (1986) The development of the rat hypothalamus. Advances in anatomy, embryology and cell biology. Springer, Berlin. Monograph
Aujla PK, Bora A, Monahan P, Sweedler JV, Raetzman LT (2011) The Notch effector gene Hes1 regulates migration of hypothalamic neurons, neuropeptide content and axon targeting to the pituitary. Dev Biol 353(1):61–71
Behar TN, Schaffner AE, Scott CA, O’Connell C, Barker JL (1998) Differential response of cortical plate and ventricular zone cells to GABA as a migration stimulus. J Neurosci 18(16):6378–6387
Behar TN, Schaffner AE, Scott CA, Greene CL, Barker JL (2000) GABA receptor antagonists modulate postmitotic cell migration in slice cultures of embryonic rat cortex. Cereb Cortex 10(9):899–909
Bessman SP, Rosen J, Layne EC (1953) Gamma-aminobutyric acid-glutamic acid transamination in brain. J Biol Chem 201(1):385–391
Bless EP, Westaway WA, Schwarting GA, Tobet SA (2000) Effects of gamma-aminobutyric acid(A) receptor manipulation on migrating gonadotropin-releasing hormone neurons through the entire migratory route in vivo and in vitro. Endocrinology 141(3):1254–1262
Bless EP, Walker HJ, Yu KW, Knoll JG, Moenter SM, Schwarting GA, Tobet SA (2005) Live view of gonadotropin-releasing hormone containing neuron migration. Endocrinology 146(1):463–468
Casoni F, Hutchins BI, Donohue D, Fornaro M, Condie BG, Wray S (2012) SDF and GABA interact to regulate axophilic migration of GnRH neurons. J Cell Sci 125(Pt 21):5015–5025
Davis AM, Henion TR, Tobet SA (2002) Gamma-aminobutyric acid B receptors and the development of the ventromedial nucleus of the hypothalamus. J Comp Neurol 449(3):270–280
Davis AM, Seney ML, Stallings NR, Zhao L, Parker KL, Tobet SA (2004) Loss of steroidogenic factor 1 alters cellular topography in the mouse ventromedial nucleus of the hypothalamus. J Neurobiol 60(4):424–436
Dellovade TL, Young M, Ross EP, Henderson R, Caron K, Parker K, Tobet SA (2000) Disruption of the gene encoding SF-1 alters the distribution of hypothalamic neuronal phenotypes. J Comp Neurol 423(4):579–589
Dellovade TL, Davis AM, Ferguson C, Sieghart W, Homanics GE, Tobet SA (2001) GABA influences the development of the ventromedial nucleus of the hypothalamus. J Neurobiol 49(4):264–276
Dessens AB, Cohen-Kettenis PT, Mellenbergh GJ, vd Poll N, Koppe JG, Boer K (1999) Prenatal exposure to anticonvulsants and psychosexual development. Arch Sex Behav 28(1):31–44
Frahm KA, Schow MJ, Tobet SA (2012) The vasculature within the paraventricular nucleus of the hypothalamus in mice varies as a function of development, subnuclear location, and GABA signaling. Horm Metab Res 44(8):619–624
Frangaj A, Fan QR (2018) Structural biology of GABAB receptor. Neuropharmacology 136(Pt A):68–79. https://doi.org/10.1016/j.neuropharm.2017.10.011
Fueshko S, Wray S (1994) LHRH cells migrate on peripherin fibers in embryonic olfactory explant cultures: an in vitro model for neurophilic neuronal migration. Dev Biol 166(1):331–348
Herbison AE, Porteous R, Pape JR, Mora JM, Hurst PR (2008) Gonadotropin-releasing hormone neuron requirements for puberty, ovulation, and fertility. Endocrinology 149(2):597–604
Jursky F, Nelson N (1996) Developmental expression of GABA transporters GAT1 and GAT4 suggests involvement in brain maturation. J Neurochem 67(2):857–867
Kim K, Ganesan S, Luo SX, Wu Y, Park E, Huang EJ, Chen L, Ding JB (2015) Aldehyde dehydrogenase 1a1 mediates a GABA synthesis pathway in midbrain dopaminergic neurons. Science 350(6256):102–106
Kusano K, Fueshko S, Gainer H, Wray S (1995) Electrical and synaptic properties of embryonic luteinizing hormone-releasing hormone neurons in explant cultures. Proc Natl Acad Sci U S A 92(9):3918–3922
Laschet J, Grisar T, Bureau M, Guillaume D (1992) Characteristics of putrescine uptake and subsequent GABA formation in primary cultured astrocytes from normal C57BL/6J and epileptic DBA/2J mouse brain cortices. Neuroscience 48:151–157
Le TN, Zhou QP, Cobos I, Zhang S, Zagozewski J, Japoni S, Vriend J, Parkinson T, Du G, Rubenstein JL, Eisenstat DD (2017) GABAergic interneuron differentiation in the basal forebrain is mediated through direct regulation of glutamic acid decarboxylase isoforms by Dlx homeobox transcription factors. J Neurosci 37(36):8816–8829
Lee JM, Tiong J, Maddox DM, Condie BG, Wray S (2008) Temporal migration of gonadotrophin-releasing hormone-1 neurones is modified in GAD67 knockout mice. J Neuroendocrinol 20(1):93–103
Li S, Kumar TP, Joshee S, Kirschstein T, Subburaju S, Khalili JS, Kloepper J, Du C, Elkhal A, Szabó G, Jain RK, Köhling R, Vasudevan A (2018) Endothelial cell-derived GABA signaling modulates neuronal migration and postnatal behavior. Cell Res 28(2):221–248
Lu F, Kar D, Gruenig N, Zhang ZW, Cousins N, Rodgers HM, Swindell EC, Jamrich M, Schuurmans C, Mathers PH, Kurrasch DM (2013) Rax is a selector gene for mediobasal hypothalamic cell types. J Neurosci 33(1):259–272
Luján R, Shigemoto R, López-Bendito G (2005) Glutamate and GABA receptor signalling in the developing brain. Neuroscience 130(3):567–580
McClellan KM, Calver AR, Tobet SA (2008) GABAB receptors role in cell migration and positioning within the ventromedial nucleus of the hypothalamus. Neuroscience 151(4):1119–1131
McClellan KM, Stratton MS, Tobet SA (2010) Roles for gamma-aminobutyric acid in the development of the paraventricular nucleus of the hypothalamus. J Comp Neurol 518(14):2710–2728
Miller MW, Nowakowski RS (1988) Use of bromodeoxyuridine-immunohistochemistry to examine the proliferation, migration and time of origin of cells in the central nervous system. Brain Res 457(1):44–52
Okamura H, Abitbol M, Julien JF, Dumas S, Bérod A, Geffard M, Kitahama K, Bobillier P, Mallet J, Wiklund L (1990) Neurons containing messenger RNA encoding glutamate decarboxylase in rat hypothalamus demonstrated by in situ hybridization, with special emphasis on cell groups in medial preoptic area, anterior hypothalamic area and dorsomedial hypothalamic nucleus. Neuroscience 39(3):675–699
Peng L, Schousboe A, Hertz L (1991) Utilization of alpha-ketoglutarate as a precursor for transmitter glutamate in cultured cerebellar granule cells. Neurochem Res 16:29
Puelles L, Rubenstein JL (2015) A new scenario of hypothalamic organization: rationale of new hypotheses introduced in the updated prosomeric model. Front Neuroanat 9:27. https://doi.org/10.3389/fnana.2015.00027
Rakic P (1972) Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol 145(1):61–83
Roberts E, Frankel S (1951) Glutamic acid decarboxylase in brain. J Biol Chem 188:789–795
Salic A, Mitchison TJ (2008) A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci U S A 105(7):2415–2420
Schwanzel-Fukuda M, Pfaff DW (1989) Origin of luteinizing hormone-releasing hormone neurons. Nature 338(6211):161–164
Schwenk J, Pérez-Garci E, Schneider A, Kollewe A, Gauthier-Kemper A, Fritzius T, Raveh A, Dinamarca MC, Hanuschkin A, Bildl W, Klingauf J, Gassmann M, Schulte U, Bettler B, Fakler B (2016) Modular composition and dynamics of native GABAB receptors identified by high-resolution proteomics. Nat Neurosci 19(2):233–242
Simonian SX, Skynner MJ, Sieghart W, Essrich C, Luscher B, Herbison AE (2000) Role of the GABA(A) receptor gamma2 subunit in the development of gonadotropin-releasing hormone neurons in vivo. Eur J Neurosci 12(10):3488–3496
Stratton MS, Searcy BT, Tobet SA (2011) GABA regulates corticotropin releasing hormone levels in the paraventricular nucleus of the hypothalamus in newborn mice. Physiol Behav 104(2):327–333
Stratton MS, Staros M, Budefeld T, Searcy BT, Nash C, Eitel C, Carbone D, Handa RJ, Majdic G, Tobet SA (2014) Embryonic GABA(B) receptor blockade alters cell migration, adult hypothalamic structure, and anxiety- and depression-like behaviors sex specifically in mice. PLoS One 9(8):e106015
Suter KJ, Wuarin JP, Smith BN, Dudek FE, Moenter SM (2000) Whole-cell recordings from preoptic/hypothalamic slices reveal burst firing in gonadotropin-releasing hormone neurons identified with green fluorescent protein in transgenic mice. Endocrinology 141(10):3731–3736
Temple JL, Wray S (2005) Developmental changes in GABA receptor subunit composition within the gonadotrophin-releasing hormone-1 neuronal system. J Neuroendocrinol 17(9):591–599
Tiao J, Bettler B (2007) Characteristics of GABAB receptor mutant mice. In: The GABA receptors, 3rd edn. Springer, Berlin
Tobet SA, Paredes RG, Chickering TW, Baum MJ (1995) Telencephalic and diencephalic origin of radial glial processes in the developing preoptic area/anterior hypothalamus. J Neurobiol 26(1):75–86
Tobet SA, Chickering TW, King JC, Stopa EG, Kim K, Kuo-Leblank V, Schwarting GA (1996a) Expression of gamma-aminobutyric acid and gonadotropin-releasing hormone during neuronal migration through the olfactory system. Endocrinology 137(12):5415–5420
Tobet SA, Hanna IK, Schwarting GA (1996b) Migration of neurons containing gonadotropin releasing hormone (GnRH) in slices from embryonic nasal compartment and forebrain. Brain Res Dev Brain Res 97(2):287–292
Tobet SA, Henderson RG, Whiting PJ, Sieghart W (1999) Special relationship of gamma-aminobutyric acid to the ventromedial nucleus of the hypothalamus during embryonic development. J Comp Neurol 405(1):88–98
Tobet SA, Bless EP, Schwarting GA (2001) Developmental aspect of the gonadotropin-releasing hormone system. Mol Cell Endocrinol 185(1–2):173–184
Tobet SA, Walker HJ, Seney ML, Yu KW (2003) Viewing cell movements in the developing neuroendocrine brain. Integr Comp Biol 43(6):794–801
Tobet SA, Handa RJ, Goldstein JM (2013) Sex-dependent pathophysiology as predictors of comorbidity of major depressive disorder and cardiovascular disease. Pflugers Arch 465(5):585–594
Vastagh C, Schwirtlich M, Kwakowsky A, Erdélyi F, Margolis FL, Yanagawa Y, Katarova Z, Szabó G (2015) The spatiotemporal segregation of GAD forms defines distinct GABA signaling functions in the developing mouse olfactory system and provides novel insights into the origin and migration of GnRH neurons. Dev Neurobiol 75(3):249–270
Wierman ME, Kiseljak-Vassiliades K, Tobet S (2011) Gonadotropin-releasing hormone (GnRH) neuron migration: initiation, maintenance and cessation as critical steps to ensure normal reproductive function. Front Neuroendocrinol 32(1):43–52
Wray S, Nieburgs A, Elkabes S (1989) Spatiotemporal cell expression of luteinizing hormone-releasing hormone in the prenatal mouse: evidence for an embryonic origin in the olfactory placode. Brain Res Dev Brain Res 46(2):309–318
Wu C, Sun D (2015) GABA receptors in brain development, function, and injury. Metab Brain Dis 30(2):367–379
Yoon BE, Lee CJ (2014) GABA as a rising gliotransmitter. Front Neural Circuits 8:141
Acknowledgments
We would like to thank Dr. Kristy McClellan for helpful discussion, Mr. Luke Schwerdtfeger for expert assistance in preparing figures, and Dr. Deborah Kurrasch for providing an original digital image for use in Fig. 7.4. MS was supported by 1K01AG056848.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Key References
Key References
-
Altman and Bayer (1986). This is a classic description of the birth of cells in the diencephalon.
-
Frahm et al. (2012). First paper to indicate a role for regulated development of hypothalamic vasculature.
-
Herbison et al. (2008). This paper is notable for being the first to directly address the redundancy in the GnRH population needed for fertility.
-
Miller and Nowakowski (1988). This paper introduced the BrdU method to the neuroscience community and kicked off a plethora of studies of cell proliferation in the nervous system due to the relative ease compared to the use of tritiated thymidine.
-
Okamura et al. (1990). This paper was the first to note the special relationship between cell bodies for GABA synthesis based on the localization of glutamic acid decarboxylases and how they lie outside of major hypothalamic cell groups.
-
Puelles and Rubenstein (2015). This paper updates history and approaches to parcellation of the hypothalamus on the basis of large anatomical assumptions to smaller units informed by molecular data.
-
Rakic (1972). This paper defined the classic view of radial glia providing migratory guidance for neuroblasts in cerebral cortex.
-
Salic and Mitchison (2008). This paper provides an impetus to switch from BrdU to EdU as a marker for DNA synthesis and thereby (indirectly) proliferation.
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Stratton, M., Tobet, S. (2020). Hypothalamic Development: Role of GABA. In: Wray, S., Blackshaw, S. (eds) Developmental Neuroendocrinology. Masterclass in Neuroendocrinology, vol 9. Springer, Cham. https://doi.org/10.1007/978-3-030-40002-6_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-40002-6_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-40001-9
Online ISBN: 978-3-030-40002-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)