Abstract
This chapter is divided into three parts. The first part deals with the interrelation of tonicity, osmotic pressure, osmolarity, osmosis, passive transmembrane diffusion, and drug absorption and distribution. The second part discusses the preparation of isotonic solutions. The third part describes the tonicity adjustment of hypotonic and hypertonic solutions. A thermodynamic approach is used to rationalize the preparation of isotonic solutions and conversion of hypotonic or hypertonic solutions to isotonic. The application of the formulas is demonstrated step-by-step through many examples extracted from physiology and pharmaceutics. An extensive justification of the importance of isotonic solutions in pharmacy is provided to motivate you to read the chapter carefully and solve all the practice problems. The equations are not just cited in the book, but they are rather derived and explained creatively. In addition, concepts like osmolarity and osmotic pressure are explained from the biophysical and physiological point of view, thus offering to you a better understanding of the phenomenon. Many of the problems are unique in the sense that you learn to calculate osmolarity of a drug solution solely from the chemical structure of the compound. Lastly, adjusting the osmolarity of hypertonic solutions is a new concept presented in this book.
This is a preview of subscription content, log in via an institution.
Buying options
Tax calculation will be finalised at checkout
Purchases are for personal use only
Learn about institutional subscriptionsAuthor information
Authors and Affiliations
7.1 Electronic Supplementary Material
(MP4 687872 kb)
(MP4 973351 kb)
(MP4 747519 kb)
(MP4 554776 kb)
Appendices
Exercises
-
7.1.
A molecule has shown unparallel biological activity when tested in vitro in cell cultures. Does the molecule have potential to become a drug or is it already a drug?
-
7.2.
Which of the following substances have the potential to passively diffuse through plasma membrane?
Substance
MW
Characteristic property
A
160
Nonionic, hydrophobic
B
55
Nonionic, amphiphilic
C
3000
Nonionic, hydrophobic
D
76
Ionic
E
1250
Nonionic, hydrophilic
-
7.3.
Choose the statement that best expresses the concept of tonicity.
-
(a)
Tonicity refers to the osmolarity of a solution in relation to the osmolarity of intracellular fluid.
-
(b)
Tonicity refers to the hydrostatic pressure of cells.
-
(c)
Tonicity refers to the osmolarity of a solution.
-
(d)
Tonicity refers to the diffusion of solutes through plasma membranes by osmosis.
-
(e)
The relative solute concentration of two solutions.
-
(a)
-
7.4.
Which of the following solutions is hypertonic to intracellular fluid? Pick all that apply.
-
(a)
1% w/v glucose
-
(b)
6% w/v glucose
-
(c)
5% w/v NaCl
-
(d)
0.95% w/v dextrose
-
(e)
0.9% w/v NaCl
-
(a)
-
7.5.
The sketch below shows a conical flask containing a solution (C2) embedded within a beaker also containing a solution (C1).
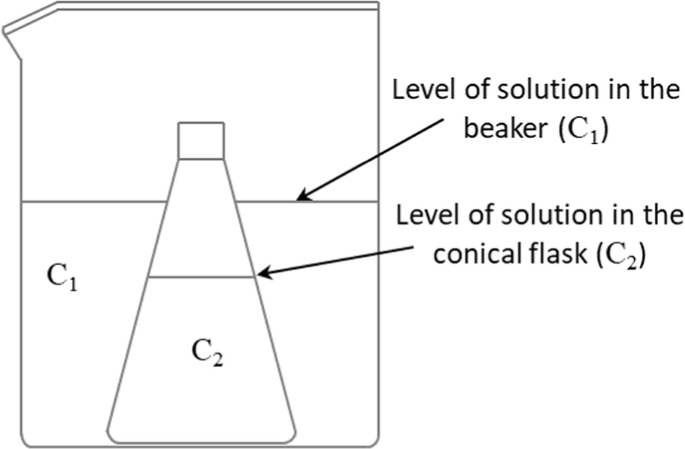
Assuming that water, but not solute, can go through the walls of the conical flask, the sketch above is drawn at equilibrium (initially at time zero, the level of the two solutions was identical). Choose the correct statements about the two solutions at time zero when the conical flask was first placed in the beaker unless if otherwise stated:
-
(a)
The two solutions were isosmotic.
-
(b)
The solution in the beaker was hyperosmotic as compared to the solution in the conical flask.
-
(c)
The solution in the beaker was hypoosmotic as compared to the solution in the conical flask.
-
(d)
The solution in the beaker was isotonic.
-
(e)
The solution in the beaker was hypotonic.
-
(f)
The solution in the conical flask was hypotonic.
-
(g)
The solute concentration was C2 > C1.
-
(h)
At equilibrium, water moves out of the conical flask into the beaker.
-
(i)
The water concentration in the beaker was higher than the water concentration in the conical flask.
-
(a)
-
7.6.
A solution in which the solute concentration is higher than the solute concentration inside the cell is called
-
(a)
Isotonic
-
(b)
Hypotonic
-
(c)
Hypertonic
-
(d)
Isosmotic
-
(e)
Hypoosmotic
-
(f)
Hyperosmotic
-
(g)
Cytotonic
-
(a)
-
7.7.
Calculate osmolarity for the following solutions:
-
(a)
0.25 M ZnCl2
-
(b)
0.4%w/v Al2(SO4)3
-
(c)
0.1 M tris hydroxyethyl amine (free base)
-
(d)
0.1 M tris hydroxyethyl amine hydrochloride
-
(e)
0.02%w/v Mg(OOCCH2)2N-CH2CH2-N(CH2COO)2Mg
-
(Answer: (a) 0.75; (b) 0.0585; (c) 0.1; (d) 0.2; (e) 0.00179)
-
-
(a)
-
7.8.
Calculate the sodium chloride equivalent (E-values ) for:
-
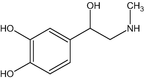
(a) Epinephrine (C9H13O3N).
-
(b) Epinephrine hydrochloride (C9H14O3NCl)

-
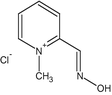
(c) Pralidoxime chloride (C7H9ON2Cl)
-
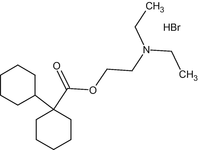
(d) Dicyclomine HCl (C19H36O2NBr)
-
(e) Acetazolamide (C4H6O3N4S2)
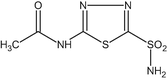
-
(f) Sodium bicarbonate (NaHCO3)
-
(g) Polyethyleneglycol (MW 5000), HO–[CH2CH2O]113–H
-
(h) Sodium carbonate (Na2CO3)
-
(i) Disodium cromoglycate (C23H14O11Na2)

-
(Answer: (a) 0.17; (b) 0.28; (c) 0.36; (d) 0.16; (e) 0.14; (f) 0.73; (g) 0.006; (h) 0.87; (i) 0.18)
-
-
7.9.
Use the sodium chloride equivalent method to prepare the IV infusion solution shown below:
Epinephrine sulfate
0.01%w/v
Na2HPO4
q.s.
Water for injection
q.s. ad.
1.0 L
How many grams of sodium phosphate dibasic are required to make 1 L of the solution above isotonic?
MW (epinephrine sulfate) = 281 g/mol, E-value (epinephrine sulfate) = 0.22, MW (Na2HPO4) = 142 g/mol, MW (NaCl) = 58.5 g/mol, E-value (sodium phosphate dibasic) = 0.65.
-
(Answer: 13.81 g)
-
7.10.
Classify the following commercial products according to their tonicity.
-
(a)
Dextrose 5%w/v (E-value = 0.17)
-
(b)
Sodium bicarbonate 5%w/v (E-value = 0.73)
-
(c)
Neomycin sulfate 8.2%w/v (C23H46N6O13 ∙ 3H2SO4; MW = 908, i = 4)
-
(d)
Diazepam 5 μg/mL (C16H13ClN2O, i = 1, E-value (diazepam) = 0.11)
-
(e)
Valproic acid 125 mg/tsp ((CH3CH2CH2)2CHCOOH; i = 1)
-
(f)
Human serum albumin 25%w/v (MW 66 kDa; hint: use ΔTf = − i ⋅ kf ⋅ m, where kf is the cryoscopic constant (1.86) and m is the molality of the protein solution)
-
(Answer: (a) isotonic; (b) hypertonic; (c) hypertonic; (d) hypotonic; (e) hypotonic; (f) hypotonic)
-
-
(a)
-
7.11.
You wish to prepare 250 mL of HBS (HEPES buffer saline) for a protein stability experiment. The HBS is an isotonic solution of HEPES and NaCl. Based on the concentration of the therapeutic protein, you decided to use 25 mM HEPES (2-hydroxyethylpiperazino-N′-2-ethanosulfonic acid, monosodium salt; MW = 261; i= 2). How much sodium chloride you should use in order to make the solution isotonic in the absence of protein?
-
(Answer: 1.865 g)
-
-
7.12.
Use the sodium chloride equivalent method to compound the following ophthalmic isotonic solution.
Rx
Drug Z (hydrochloride salt)
0.55%w/v
NaCl
q.s.
Sterile deionized water
q.s. ad.
25 mL
M.et. ft. sterile, isotonic solution
Sig. 2 gtt o.u. q.4 h for 5 days
Empirical formula of drug Z: C17H18FN3O3∙HCl , E-value = 0.17
-
(a)
How much sodium chloride is needed to fill the prescription?
-
(b)
Calculate the dose (in μg) if the dropper delivered 20 drops/mL?
(Answer: (a) 0.2 g; (b) 550 μg)
-
(a)
-
7.13.
Local anesthetics like proparacaine produce local anesthesia by blocking the sodium channels of the excitable membrane of nerve axons, thus stabilizing the neuronal membrane and preventing the transmission of nerve pulses. Proparacaine hydrochloride isotonic solution 0.5%w/v (Sig. 2 gtt q.15 min during eye examination) is indicated for topical anesthesia during eye examination. Local anesthesia begins within 30 s after instillation and lasts about 15 min. The empirical formula of the drug is C16H27N2O3Cl and MW = 331 g/mol.

-
(a)
How much water should you use to prepare 45 mL of isotonic proparacaine HCl solution if 1.8%w/v NaCl, pH 6 is available in your lab.
-
(b)
If the eye examination lasted 50 min, how much proparacaine hydrochloride had the patient actually received during the examination (assume 19 drops/mL)?
-
(Answer: (a) 24.826 mL; (b) 2.11 mg)
-
-
(a)
-
7.14.
Among other uses, the benzodiazepine midazolam is useful for patient premedication before an operation. You received a medication order for 10 mL of midazolam 6 mg/mL for IM administration. You have midazolam and midazolam hydrochloride available in powder form. Describe how you should prepare the solution; what form of midazolam you should use?
Midazolam empirical formula: C18H13ClFN3∙HCl; MW = 362; E-value = 0.17.
-
(Answer: use midazolam hydrochloride and 78.66 mg of NaCl)
-
7.15.
Given the following prescription:
Rx
Fluconazole
10 mg/5 mL
Lactose
q.s.
Sterile water
q.s. ad.
1 L
M.et.ft. infusion solution, D.T.D # 4
Sig. Infuse 1400 mg on first day, then 600 mg daily for 3 days.
Fluconazole: C13H12F2N6O, MW = 306; i = 1; E-value = 0.10
Lactose : C12H22O11
-
(a)
Calculate the amount of drug needed to fill the prescription.
-
(b)
How many grams of lactose are there in 1 L of isotonic solution?
-
(c)
How many milliliters must be infused at the end of the second day?
-
(Answer: (a) 8 g; (b) 97.78 g; (c) 1000 mL)
-
-
(a)
-
7.16.
A 33-pound pediatric patient was prescribed the medication order below for respiratory tract infection:
Rx
Cefuroxime
125 mg/mL
ZnCl2
q.s.
Sterile water
q.s. ad.
7 mL
M.et.ft. isotonic solution
Sig. 60 mg/kg/day IM in three divided doses
Cefuroxime : C20H22N4O10S, MW = 510; i = 1; E-value = 0.06.
-
(a)
Calculate the osmolarity of cefuroxime in the solution.
-
(b)
What is the contribution of zinc chloride to the total osmotic strength of the solution?
-
(c)
How many milliliters should the patient receive per dose?
-
(Answer: (a) 0.245 osmol/L; (b) 48.7 mOsm/L; (c) 2.4 mL)
-
-
(a)
-
7.17.
Given the following formula:
Scopolamine HCl
0.25%w/v
NaCl
0.45%w/v
Boric acid (5%w/v)
q.s.
Sterile water
q.s. ad.
15 mL
M.et. ft. sterile, isotonic solution
Calculate the volume of 5%w/v boric acid needed to make the above solution isotonic, using the freezing point depression data given below:
-
ΔTf 1%w/v boric acid = 0.29 °C
-
ΔTf 0.9%w/v sodium chloride = 0.52 °C
-
ΔTf 0.25%w/v scopolamine HCl = 0.08 °C
-
(Answer: 1.86 mL)
-
-
7.18.
Given the following:
Dextrose (60% w/v)
Sterile water for injection
q.s. ad.
1 L
E-value (dextrose) = 0.17
-
(a)
How many milliliters should be used from the 60%w/v dextrose stock solution to make the final solution isotonic?
-
(b)
What is the concentration of dextrose in the final isotonic solution?
-
(Answer: (a) 88.2 mL; (b) 5.3%w/v)
-
-
(a)
Additional Exercises
-
7.19.
You were asked to prepare 1.5 L of 0.12%w/v epinephrine sulfate isotonic solution for injection. The epinephrine sulfate chemical formula is C9H13O3NH+, HSO4− (MW = 281 g/mol). How much of NaCl should you use to adjust the tonicity of the solution?
-
(Answer: 13.1 g)
-
-
7.20.
Ciprofloxacin is a broad spectrum fluoroquinolone-based antimicrobial agent. Explain how you should compound the following prescription for eye infections.
Rx
Ciprofloxacin HCl monohydrate
0.45%w/v
NaCl
q.s.
Sterile water
15 mL
M. et. Ft. sterile isotonic solution
Sig. 2 gtt o.u. q.1 h for 8 h. Then 2 gtt o.u. q.8 h for 6 days.
Empirical formula of the drug is C17H18FN3O3∙HCl H2O, MW = 386 g/mol, i = 2.
The structural formula of ciprofloxacin HCl is shown below:
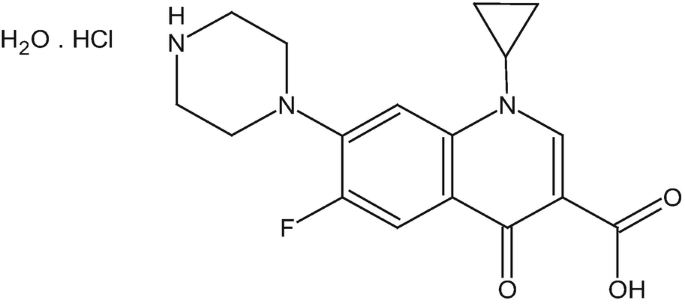
-
(a)
How much sodium chloride should you use to make the solution isotonic?
-
(b)
How much ciprofloxacin has the patient received after 8 h? (assume 20 drops/mL)
-
(c)
How much ciprofloxacin has the patient received after 6 days if he started taking the medication on day 1 at 8 am? (assume 20 drops/mL)
-
(Answer: (a) 0.124 g; (b) 7.2 mg; (c) 22.5 mg)
-
-
(a)
-
7.21.
The following formula can be used to prepare isotonic ciprofloxacin solution (pH 6) for intravenous infusion.
Ciprofloxacin lactate
0.25%w/v
HCl
0.006 M
Dextrose
q.s.
Sterile water
q.s. ad.
1 L
In stock: Hydrochloric acid is 37% (ρ = 1.2)
Chemical formula of lactic acid: CH3CH(OH)COOH, MW = 90, pKa (lactic acid) = 3.85
Ciprofloxacin lactate (MW = 422, i = 2, E-value = 0.146)
-
(a)
Calculate the osmolarity of the solution with respect to ciprofloxacin HCl.
-
(b)
How many microliters of HCl 37% are there in the formula?
-
(c)
How many grams of dextrose are needed to make the solution isotonic?
-
(Answer: (a) 0.012 Osm/L; (b) 493 μL; (c) 48.52 g)
-
-
(a)
-
7.22.
Pneumococcal pneumonia can be effectively treated with intravenous administration of the first-generation cephalosporin, cefazolin.
Rx
Cefazolin sodium
2.25 g
NaCl
0.2%w/v
Dextrose
q.s.
Bacteriostatic water
q.s. ad.
45 mL
M. et. Ft. isotonic solution
Sig. Inject 10 mL IV slowly, within no less than 3 min, q.12 h for 2 days.
The chemical formula of cefazolin sodium salt is shown below (MW = 476 g/mol):
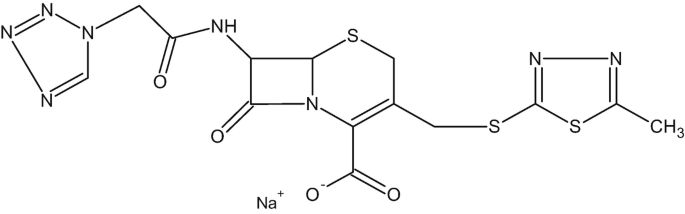
-
(a)
Use the E-value for cefazolin sodium to calculate the NaCl osmotically equivalent to 2.25 g of drug.
-
(b)
How many milligrams of dextrose are needed to fill the prescription?
-
(c)
How much of cefazolin sodium will the patient receive in a day?
-
(Answer: (a) 0.291 g; (b) 141 mg; (c) 1 g)
-
-
(a)
-
7.23.
Given the following formula:
Epinephrine
Sodium metabisulfite
0.25 g
Sodium chloride
q.s.
Sterile water
q.s. ad.
0.5 L
M. et. Ft. sterile isotonic solution so that when 12.5 mL of the solution is diluted to 0.5 L yields epinephrine concentration of 1:10,000 g/mL.
Epinephrine : MW = 183 g/mol, i = 1, E-value = 0.168
Sodium metabisulfite: Na2S2O5, i = 3.
-
(a)
Calculate the osmolarity of epinephrine.
-
(b)
How many grams of sodium chloride are needed to compound the formula?
-
(Answer: (a) 0.022 Osm/L; (b) 4.0425 g)
-
-
(a)
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Savva, M. (2019). Isotonic Solutions. In: Pharmaceutical Calculations. Springer, Cham. https://doi.org/10.1007/978-3-030-20335-1_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-20335-1_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-20334-4
Online ISBN: 978-3-030-20335-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)











