Abstract
Aquaponics is a technology that is part of the broader integrated agri-aquaculture systems discipline which seeks to combine animal and plant culture technologies to confer advantages and conserve nutrients and other biological and economic resources. It emerged in the USA in the early 1970s and has recently seen a resurgence, especially in Europe. Whilst aquaponics broadly combines recirculating fish culture with hydroponic plant production, the application of the term aquaponic is broad and many technologies claim use of the name. Combining fish culture with aquatic-based, terrestrial plant culture via aquaponics may be better defined via its nutrient resource sharing credentials. Aquaponics applies several principles including, but not limited to, efficient water use, efficient nutrient use, lowered or negated environmental impact and the application of biological and ecological approaches to agricultural fish and plant production. Water sources are important so that the nutrients required for fish and plant production are available and balanced, and system water chemistry is paramount to optimised fish and plant production. Systems may be configured in several ways, including those that are fully recirculating and those that are decoupled. Aquaponics importantly seeks to apply methods that provide technical, biological, chemical, environmental and economic advantages.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Aquaponics is a technology that is a subset of a broader agricultural approach known as integrated agri-aquaculture systems (IAAS) (Gooley and Gavine 2003). This discipline consists of integrating aquaculture practices of various forms and styles (mostly fin fish farming) with plant-based agricultural production. The rationale of integrated agri-aquaculture systems is to take advantage of the resources shared between aquaculture and plant production, such as water and nutrients, to develop and achieve economically viable and environmentally more sustainable primary production practices (Gooley and Gavine 2003). In essence, both terrestrial plant and aquatic animal production systems share a common resource: water. Plants are generally consumptive of water via transpiration and release it to the surrounding gaseous environment, whereas fish are generally less consumptive of water, but their contained culture produces substantial waste water streams due to accumulated metabolic wastes. Therefore, aquaculture may be integrated within the water supply pathway of plant production in non-consumptive ways so that two crops (fish and plants) may be produced from a water source that is generally used to produce one crop (plants).
An interesting additional advantage of integrating aquaculture with the irrigation supply pathway for plant production is that aquaculture also produces waste nutrients via the dissolved and undissolved wastes produced from fish (and other aquatic animal) metabolism. Therefore, aquaculture may also produce waste nutrient streams that are suitable for, and assist, plant production by contributing to the plants nutrient requirements.
The advantages produced by integrating aquaculture with conventional terrestrial and aquatic plant production systems have been summarised by Gooley and Gavine (2003) as:
-
1.
An increase in farm productivity and profitability without any net increase in water consumption (Chap. 2).
-
2.
Farm diversification into higher-value crops, including high-value aquatic species.
-
3.
Reuse of otherwise wasted on-farm resources (e.g. capture and reuse of nutrients and water).
-
4.
Reduction of net environmental impacts of semi-intensive and intensive farming practices.
-
5.
Net economic benefits by offsetting existing farm capital and operating expenses (Chap. 18).
Aquaponics has been said to have evolved from relatively ancient agriculture practices associated with integrating fish culture with plant production, especially those developed within the South East Asian, flooded rice paddy farming context and South American Chinampa, floating island, agriculture practices (Komives and Junge 2015). In reality, historically, fish were rarely actively added to rice paddy fields until the nineteenth century (Halwart and Gupta 2004) and were present in very low densities which would not contribute to any substantial nutritive assistance to the plants. Chinampas were traditionally built on lakes in Mexico where nutrient advantages may have been supplied via the eutrophic or semi-eutrophic lake sediments rather than directly from any designed or actively integrated fish production system (Morehart 2016; Baquedano 1993).
Modern aquaponics started in the USA in the 1970s and was co-evolved by several institutions with an interest in more sustainable farming practices. Early important work was performed by several researchers, but ultimately, the progenitor of nearly all modern aquaponics is thought to be the work performed by, and the systems produced by, James Rakocy and his team at the University of the Virgin Islands (UVI) starting in the early 1980s (Lennard 2017).
Aquaponics is now considered a new and emerging industry with a relevant place in the broader, global agricultural production context and there are a number of variations of the technology of integrating fish culture with aquatic plant culture that are collectively defined under the aquaponics banner or name (Knaus and Palm 2017). Therefore, aquaponics seeks to integrate aquaculture animal production with hydroponic plant production using various methods to share water and nutrient resources between the major production components to produce commercial and saleable fish and plant products.
2 A Definition of Aquaponics
Aquaponics fits into the broader definition of integrated agri-aquaculture systems (IAAS). However, IAAS applies many different aquatic animal and plant production technologies in many contexts, whereas aquaponics is far more tightly associated with integrating tank-based fish culture technologies (e.g. recirculating aquaculture systems; RAS) with aquatic or hydroponic plant culture technologies (Lennard 2017). RAS technologies apply conserved and standard methods for the culture of fish in tanks with applied filtration to control and alter the water chemistry to make it suitable for fish (i.e. fast and efficient solid fish wastes removal, efficient, bacteria-mediated conversion of potentially toxic dissolved fish waste ammonia to less toxic nitrate and oxygen maintenance via assisted aeration or directly injected oxygen gas) (Timmons et al. 2002). Hydroponics and substrate culture technologies apply conserved and standard methods for the culture of edible terrestrial plants within aquatic environments (i.e. the plants gain access to the nutrients required for growth via a water-based delivery method) (Resh 2013).
The association of aquaponics with standard RAS aquaculture and hydroponics/substrate culture means that aquaponics is often defined simply as “… the combination of fish production (aquaculture) and soil-less plant cultivation hydroponics under coupled or decoupled water circulation” (Knaus and Palm 2017). This broad definition places an emphasis on the integration of hardware, equipment or technologies and places little, if any, emphasis on any other aspects of the method.
Because aquaponics is a relatively new industrial-scale technology that applies different methods and approaches, the applied definition appears very broad. Some define aquaponics within a recirculating context only (Cerozi and Fitzsimmons 2017), some concentrate on approaches that do not return the water from the plants to the fish (Delaide et al. 2016) and others include both recirculating and decoupled methods (Knaus and Palm 2017). Further still, some researchers are including the use of aquaculture effluents irrigated to soil-based crop production under the aquaponic title (Palm et al. 2018). Historically, aquaponics, as the breakdown of the word (aquaculture and hydroponics) suggests, was defined as only concerning aquaculture and hydroponic plant production (Rakocy and Hargreaves 1993), so current attempts at associations with soil-based culture seem incongruous.
Whilst aquaponic systems do integrate tank-based aquaculture technologies with hydroponic plant culture technologies, aquaponic systems work by supplying nutrients to, and partitioning nutrients between, the production inhabitants (fish and plants) and the inhabitants that perform biological and chemical services that assist the production inhabitant outcome (microflora) (Fig. 5.1) (Lennard 2017). Therefore, is aquaponics more a system associated with nutrient supply, dynamics and partitioning rather than one associated with the technology, equipment or hardware applied?
Schematic representation of the nutrient flows within an aquaponic system. Fish feed is the major nutrient entry point. The fish eat the feed, use what nutrients they need, release the rest as waste and this waste is then partitioned between the microbes, plants and system water. (adapted from Lennard 2017)
Over the past decades, the definition of aquaponics has included a similar theme, with subtle variations. The broadest definition has generally been provided in the scientific publications of Rakocy and his UVI team, for example:
This early definition was based on the assumption that one-loop, fully recirculating systems, consisting of a recirculating aquaculture component and a hydroponic component, represented all aquaponic systems, which at the time, they did. Graber and Junge (2009) expanded the definition, due to changes and developments in the approach, as follows:
Aquaponic is a special form of recirculating aquaculture systems (RAS), namely a polyculture consisting of fish tanks (aquaculture) and plants that are cultivated in the same water circle (hydroponic).
– Graber and Junge (2009)
Recent developments and methods ask for a reconsideration of this standpoint. In recent years a shifting of the focus of aquaponics towards a production system that tackles both ecological responsibility and economic sustainability has been present. Kloas et al. (2015) and Suhl et al. (2016) were one of the first to address this economic consideration:
[…] a unique and innovative double recirculating aquaponic system was developed as a prerequisite for a high productivity comparable to professional stand-alone fish/plant facilities.
– Suhl et al. (2016)
The definition issue, or clarifying “what can be defined as aquaponics”, has been a point of discussion over the past years. One of the main areas of development has been that of multi-loop (or decoupled) aquaponic systems that aim at providing additional fertilisers to the plants in order to expose them to an optimal nutrient concentration (Goddek 2017). There should be no opposition between the ideologies of fully recirculating and multi-loop aquaponic methodologies, both have their respective places and applications within the appropriate industrial context and a single common driving force of both should be that the technology, whilst being nutrient and water efficient, also needs to be economically competitive to establish itself in the market. In order to replace conventional practices, more than an ideology needs to be offered to potential clients/users – i.e. technical and economic feasibility.
The European COST sponsored Aquaponics Hub (COST FA1305 2017) applies the definition “…a production system of aquatic organisms and plants where the majority (> 50%) of nutrients sustaining the optimal plant growth derives from waste originating from feeding the aquatic organisms”, which clearly places an emphasis on the nutrient sharing aspect of the technology.
It must also be stated that the proportion of fish to plants should remain at a level that supports a core prospect of aquaponics; that plants are grown using fish wastes. A system containing one fish and several hectares of hydroponic plant cultivation, for example, should not be considered as aquaponics, simply because that one fish effectively contributes nothing to the nutrient requirements of the plants. Since the labelling of aquaponic products plays an increasingly important role in consumer choice, we want to encourage a discussion by redefining aquaponics based on these multiple developments of the technology. Even though we advocate closing the nutrient cycle to the highest possible degree in the context of best practicable means, a potential definition should also take all developments into consideration. Therefore, the definition should contain as a minimum, the requirement for a majority of aquaculture-derived nutrients for the plants. A new definition may therefore be represented as:
Aquaponics is defined as an integrated multi-trophic, aquatic food production approach comprising at least a recirculating aquaculture system (RAS) and a connected hydroponic unit, whereby the water for culture is shared in some configuration between the two units. Not less than 50% of the nutrients provided to the plants should be fish waste derived .
Nutrient-based definitions are open and non-judgemental of the applied technology choice, or even the proportions of each component (fish and plants), as long as fish culture and some form of aquatic (hydroponic or substrate culture) plant production technology is utilised. However, it also focuses the definition on the nutrient dynamics and nutrient sharing aspects of the methods applied and therefore ensures, to at least some extent, that the advantages often associated with aquaponics (water saving, nutrient efficiency, lowered environmental impact, sustainability) are present in some proportion.
The nutrient association definition applied to aquaponics will always be a source of further contention among those who practise it. This is supported by the fact that the name aquaponics is applied to a vast array of different technologies with different nutrient supply motivations and usage outcomes: from system designs and methods that expect, if not demand, that the vast majority of the nutrients required to grow the plants arise from the fish wastes (in some cases, greater than 90%; Lennard 2017) to designs that share plant nutrient supplies between fish wastes and more substantial external additions (e.g. approximately 50:50 fish waste to external supplementation – as many modern, European decoupled aquaponic system designs do; COST FA1305 2017) to those designs that add so few fish that no discernible nutrient supply from the fish wastes to the plants is present (Lennard 2017).
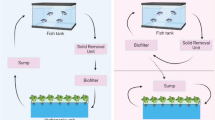
The name aquaponics, until relatively recently (i.e. the last 3–5 years), has been universally applied to coupled and fully recirculating system designs that seek to supply as much of the required plant nutrition from the fish wastes as possible (Rakocy and Hargreaves 1993; Lennard 2017) (Fig. 5.2).
However, decoupled approaches now represent a proportion of the systems being researched or commercially applied, especially in Europe, and in current practice do not supply plant nutrient requirements from the fish wastes to the same extent as fully recirculating systems do (Lennard 2017; Goddek and Keesman 2018). For example, Goddek and Keesman (2018) state that for 3 examples of current European decoupled aquaponic system designs, the relative addition requirements for external hydroponic-derived nutrients are 40–60% (NerBreen), 60% (Tilamur) and 38.1% (IGB Berlin). Because these decoupled designs are based on integrating existing RAS and hydroponic/substrate culture technologies, they are regarded as aquaponic in nature (Delaide et al. 2016) (Fig. 5.3) (see Chap. 8).
The definition of aquaponics is now being expanded beyond ecological, water and nutrient efficiency drivers and optimisation to also include economic drivers (Goddek and Körner 2019; Goddek and Keesman 2018; Goddek 2017; Kloas et al. 2015; Reyes Lastiri et al. 2016; Yogev et al. 2016) (Chap. 8). The benefits of such an approach are that a positive economic outcome from aquaponics technology is as important as its biological, chemical, engineering, ecological and sustainable credentials and therefore, the economic outcome should play a role within the overall definition (Chap. 8).
Many advantages are often associated with aquaponics, especially in terms of its water-use efficiency, its nutrient use efficiency, its sustainable nature, its ability to produce two crops from the one input source (fish feed) and its lowered environmental impact (Timmons, et al., 2002; Buzby and Lian-shin 2014; Wongkiew et al. 2017; Roosta and Hamidpour 2011; Suhl et al. 2016). These advantages are regularly quoted and applied by commercial aquaponic operators and are used as a marketing and price regulation pathway for the products (fish and plants), and therefore, the use of the name “aquaponics” directly and immediately associates that the products labelled as such have been produced with methods that contain or utilise the advantages listed. However, there is no formal regulation of the industry that dictates that the use of the word (aquaponics) only occurs when the advantages are apparent and present within the technology and methods applied. If the above advantages are assigned to aquaponics as a technology, then surely the technology should provide the prescribed advantages, and if the technology does not provide the advantages, then the word should not be applied (Lennard 2017).
Because aquaponics may be defined either in terms of its hardware equipment integration aspect (RAS with hydroponics), its nutrient sharing or partitioning properties or its ability to provide important advantages, there is still a wide spectrum of possible applications of the name to many different technical approaches that utilise different methods and demand different outcomes. Therefore, it appears that the actual definition of aquaponics is still unresolved.
It appears therefore that very important questions are yet to be answered: what is aquaponics and how is it defined?
This would suggest that one very important aspect for the aquaponic industry to consider is the development of a truthful and agreed-upon definition. The broader aquaponics industry will continue to be full of disagreement if a definition is not agreed upon, and more importantly, consumers of the products produced within aquaponic systems will become more and more confused about what aquaponics actually is – a state of affairs that will not assist the growth and evolution of the industry.
3 General Principles
Even though the definition of aquaponics has not been entirely resolved, there are some general principles that are associated with the broad range of aquaponic methods and technologies.
Using the nutrients added to the aquaponic system as optimally and efficiently as possible to produce the two main products of the enterprise (i.e. fish and plant biomass) is an important and shared first principle associated with the technology (Rakocy and Hargreaves 1993; Delaide et al. 2016; Knaus and Palm 2017). There is no use in adding nutrients (which possess an inherent cost in terms of money, time and value) to a system to watch a high percentage of those nutrients are partitioned into processes, requirements or outcomes that are not directly associated with the fish and plants produced, or any intermediary life forms that may assist nutrient access by the fish and plants (i.e. microorganisms – bacteria, fungi, etc.) (Lennard 2017). Therefore, probably the most important general principle associated with aquaponics is to use the applied nutrients as efficiently as possible to achieve the optimised production of both fish and plants.
This same argument may also be applied to the water requirement of the aquaponic system in question; again, the water added to the system should be utilised principally by the fish and plants and used as efficiently as possible and not allowed to leak to processes, life forms or outcomes that are not directly associated with fish and plant production or may impact on the surrounding environment (Lennard 2017).
In real terms, the efficient use of nutrients and water leads to several design principles that are broadly applied to the aquaponic method:
-
1.
The most important principle of aquaponics is to use the wastes produced by the fish as a principle nutrient source for the plants. In fact, this is the entire idea of aquaponics and so should be a first order driver for the method. Aquaponics was historically envisaged as a system to grow plants using fish aquaculture wastes so that those aquaculture wastes had less environmental impact and were seen as a positive and profitable commodity, rather than a troublesome waste product with an associated cost to meet environmental legislative requirements (Rakocy and Hargreaves 1993; Love et al. 2015a, b).
-
2.
The system design should encourage the use of fish keeping and plant culturing technologies that do not inherently uptake or destructively utilise the water or nutrient resources added. For example, fish keeping components based on using earthen ponds are discouraged, because the earthen pond has the ability to use and make unavailable water and nutrient resources to the associated fish and plants, thus lowering the water and nutrient use efficiency of the system. Similarly, hydroponic plant culturing methods should not use media that uptakes excessive amounts of nutrients or water and renders them unavailable to the plants (Lennard 2017).
-
3.
The system design should not waste nutrients or water via the production of external waste streams. Principally, if water and nutrients leave the system via a waste stream, then that water and those nutrients are not being used for fish or plant production, and therefore, that water and those nutrients are being wasted, and the system is not as efficient as possible. In addition, the production of a waste streams can have a potential environmental impact. If waste water and nutrients do leave the aquaponic system, they should be used in alternate, exterior-to-system plant production technologies so water and nutrients are not wasted, contribute to the overall production of edible or saleable biomass and do not present a broader environmental impact potential (Tyson et al. 2011).
-
4.
The system should be designed to lower or ideally, completely negate, direct environmental impact from water or nutrients. A first order goal of aquaponics is to use the wastes produced by the fish as a nutrient source for the plants so as to negate the release of those nutrients directly to the surrounding environment where they may cause impacts (Tyson et al. 2011).
-
5.
Aquaponic system designs should ideally lend themselves to being located within environmentally controlled structures and situations (e.g. greenhouses, fish rooms). This allows the potential to achieve the best productive rates of fish and plants from the system. Most aquaponic designs are relatively high in terms of capital costs and ongoing costs of production, and therefore, the ability to house the system in the perfect environment enhances profit potentials that financially justify the high capital and costs of production (Lennard 2017).
The above outlined principles of design directly associate with a set of general principles that are often, but not always, applied to the aquaponic production environment. These general principles relate to how the system operates and how nutrients are portioned among the system and its inhabitants.
The basic premise of aquaponics, in a nutrient dynamic context, is that fish are fed fish feed, fish metabolise and utilise the nutrients in the fish feed, fish release wastes based on the substances in the fish feed they do not utilise (including elements), microflora access those fish metabolic wastes and use small amounts of them, but transform the rest, and the plants then access and remove those microflora transformed, fish metabolic wastes as nutrient sources and, to some extent, clean the water medium of those wastes and counteract any associated accumulation (Rakocy and Hargreaves 1993; Love et al. 2015a, b).
Because earthen-based fish production systems remove nutrients themselves, aquaponics generally utilises what are known as recirculating aquaculture system (RAS) principles for the fish production component (Rakocy and Hargreaves 1993; Timmons et al. 2002). Fish are kept in tanks made of materials that do not remove nutrients from the water (plastic, fibreglass, concrete, etc.), the water is filtered to treat or remove the metabolic waste products of the fish (solids and dissolved ammonia gases) and the water (and associated nutrients) is then directed to a plant culturing component whereby the plants use the fish wastes as part of their nutrient resource (Timmons et al. 2002). As for the fish, earthen-based plant culturing components are not used because the soils involved remove nutrients and may not necessarily make them fully available to the plants. In addition, hydroponic plant culturing techniques do not use soil and are cleaner than soil-based systems and allow some passive control of the microorganism mixtures present.
Plants cultured in conventional hydroponics require the addition of what are known as mineral fertilisers: nutrients that are present in their basal, ionic forms (e.g. nitrate, phosphate, potassium, calcium, etc. as ions) (Resh 2013). Conversely, recirculating aquaculture systems must apply regular (daily) water exchanges to control the accumulation of fish waste metabolites (Timmons et al. 2002). Aquaponics seeks to combine the two separate enterprises to produce an outcome that achieves the best of the two technologies while negating the worst (Goddek et al. 2015).
Plants require a suite of macro and micro elements for optimal and efficient growth. In aquaponics, the majority of these nutrients arise from the fish wastes (Rakocy and Hargreaves 1993; Lennard 2017; COST FA1305 2017). However, fish feeds (the major source of aquaponic system nutrients) do not contain all the nutrients required for optimised plant growth, and therefore, external nutrition, to varying extents, is required.
Standard hydroponics and substrate culture add nutrients to the water in forms that are directly plant-available (i.e. ionic, inorganic forms produced via designed salt variety additions) (Resh 2013). A proportion of the wastes released by fish are in forms that are directly plant-available (e.g. ammonia) but potentially toxic to the fish (Timmons et al. 2002). These dissolved, ionic fish waste metabolites, like ammonia, are transformed by ubiquitous bacterial species that replace hydrogen ions with oxygen ions, the product from ammonia being nitrate, which is far less toxic to the fish and the preferred nitrogen source for the plants (Lennard 2017). Other nutrients appropriate to plant uptake are bound in the solid fraction of the fish waste as organic compounds and require further treatment via microbial interaction to render the nutrients available to plant uptake (Goddek et al. 2015). Therefore, aquaponic systems require a suite of microflora to be present to perform these transformations.
The key to optimised aquaponic integration is determining the ratio between fish waste output (as directly influenced by fish feed addition) and plant nutrient utilisation (Rakocy and Hargreaves 1993; Lennard and Leonard 2006; Goddek et al. 2015). Various rules of thumb and models have been developed in an attempt to define this balance. Rakocy et al. (2006) developed an approach that matches the plant growing area requirement with the daily fish feed input and called it “The Aquaponic Feeding Rate Ratio”. The feeding rate ratio is set between 60 and 100 grams of fish feed added per day, per square meter of plant growing area (60–100 g/m2/day). This feeding rate ratio was developed using Tilapia spp. fish eating a standard, 32% protein commercial diet (Rakocy and Hargreaves 1993). In addition, the aquaponic system this ratio is particular to (known as the University of the Virgin Islands Aquaponic System – UVI System) does not utilise the solid fish waste fraction, is over-supplied with nitrogen and requires in-system, passive de-nitrification to control the nitrogen accumulation rate (Lennard 2017). Others have determined alternate ratios based on different fish and plant combinations, tested in different specific conditions (e.g. Endut et al. 2010 – 15–42 g/m2/day for African catfish, Clarias gariepinus and water spinach plants, Ipomoea aquatica).
The UVI feeding rate ratio was developed by Rakocy and his team as an approximate approach; hence why it is stated as a range (Rakocy and Hargreaves 1993). The UVI ratio tries to account for the fact that different plants require different nutrient amounts and mixtures and therefore a “generic” aquaponic design approach is a difficult prospect. Lennard (2017) has developed an alternate approach that seeks to directly match individual fish waste nutrient production rates (based on the fish feed utilised and the fish conversion and utilisation of that feed) with specific plant nutrient uptake rates so that exacting fish to plant ratio matching for any fish or plant species chosen may be realised and accounted for in the aquaponic system design. He matches this design approach with a specific management approach that also utilises all the nutrients available within the fish solid waste fraction (via aerobic remineralisation of the fish solid wastes) and only adds the nutrients required by the chosen plant species for culture that are missing from the fish waste production fractions. Therefore, this substantially lowers the associated feeding rate ratio (e.g. less than 11 g/m2/day for some leafy green varieties as a UVI equivalent) and allows any fish species to be specifically and exactly matched to any plant species chosen (Lennard 2017). Similarly, Goddek et al. (2016) have proposed models that allow more exacting fish to plant component ratio determination for decoupled aquaponic systems.
The general principles of efficient nutrient use, low and efficient water use, low or negated environmental impact, ability to be located away from traditional soil resources and sustainability of resource use are the general principles applied to aquaponic system design and configuration and their ongoing application should be encouraged within the field and industry.
4 Water Sources
Water is the key medium used in aquaponic systems because it is shared between the two major components of the system (fish and plant components), it is the major carrier of the nutrient resources within the system and it sets the overall chemical environment the fish and plants are cultured within. Therefore, it is a vital ingredient that may have a substantial influence over the system.
In an aquaponic system, water-based environment context, the source of water and what that source water contains chemically, physically and biologically are a major influence over the system because it sets a baseline for what is required to be added to the system by the various inputs of the system. These inputs, in turn, effect and set the environment that the fish and plants are cultured within. For example, some of the major inputs in terms of nutrients to any aquaponic system include, but are not limited to, the fish feed (a primary nutrient resource for the system), the buffers applied (which assist to control and set the pH values associated with both the fish and plant components) and any external nutrient additions or supplementations required to meet the nutrient needs of the fish and plants (Lennard 2017).
Fish feeds are designed to provide the nutrition required for fish growth and health and therefore contain nutrient mixtures and quantities primarily to aid the fish being cultured (Timmons et al. 2002; Rakocy et al. 2006). Plants, on the other hand, have different nutrient requirements to fish, and fish feeds rarely, if ever, meet the total nutrient requirements of the plants (Rakocy et al. 2006). Because of this, aquaponic systems that culture fish and plants solely using fish feed-derived nutrient resources may efficiently and optimally produce fish, but they rarely do so for the plants. The best aquaponic system designs recognise that the ultimate outcome is to produce both fish and plants at optimal and efficient growth rates and therefore, also recognise that some form of additional nutrition is required to meet the total plant nutrient requirement (Rakocy et al. 2006; Suhl et al. 2016).
Classical, fully recirculating aquaponic systems generally rely on fish feeds (after the fish have consumed that feed, metabolised it and utilised the nutrients within it) as the major nutrient source for the plants and supplement any missing nutrients required by the plants via some form of buffering regime (Rakocy et al. 2006) or via additional nutrient supplementation (e.g. adding chelated nutrient forms directly to the culture water or by adding nutrients via foliar sprays) (Roosta and Hamidpour 2011).
The best example of this classical recirculating aquaponic approach is the UVI (University of the Virgin Islands) aquaponic system developed by Dr. James Rakocy and his UVI team (Rakocy and Hargreaves 1993; Rakocy et al. 2006). The UVI design principally adds nutrients for both fish and plant culture via fish feed additions. However, fish feeds do not contain enough calcium (Ca+) and potassium (K+) for optimal plant culture. The bacteria-mediated conversion of fish waste-dissolved ammonia to nitrate causes system-wide production of hydrogen ions within the water column, and the proliferation of these hydrogen ions results in a constant fall in the system water pH towards acid. The buffering regime employed adds the missing calcium and potassium by adding basic salts (often salts based on carbonate, bicarbonate or hydroxyl ions paired with calcium or potassium) to the system that assist to control the system water pH at a level that meets both the shared pH environmental requirements of the fish and the plants, whilst providing the additional calcium and potassium the plants require (Rakocy et al. 2006). In addition, the UVI system adds another major nutrient for plant growth that is not available in standard fish feeds, iron (Fe), via regular and controlled iron chelate additions. Therefore, the potassium, calcium and iron the plants require that are not found in the fish feed are available via these two additional nutrient supply mechanisms (Rakocy et al. 2006).
Decoupled aquaponic designs adopt an approach to culture the fish and plants in a way whereby the water is used by the fish and the fish waste nutrients are supplied to the plants, without recirculation of the water back to the fish (Karimanzira et al. 2016). Decoupled designs therefore allow more flexibility in customising the water chemistry, after fish use, for optimised plant production because supplementation of the nutrients not present in the fish feed (and fish waste) may be achieved with no concerns of the water returning to the fish (Goddek et al. 2016). This means decoupled designs potentially may apply more exacting nutrient mixtures and strengths to the culture water, post fish use, for plant culture, and this may be achieved with more exacting and intense nutrient supplementation.
In both cases (recirculating and decoupled aquaponic system designs), an understanding of the chemical quality of the source water is vital so that as close to optimal nutrient concentrations for the plants may be achieved. If, for example, the source water contains calcium (a case often seen when ground water resources are utilised), this will affect and change the buffering regime applied to recirculating aquaponic designs and the extent of the nutrient supplementation applied to a decoupled design because the calcium present in the source water will offset any required supplementation required for plant calcium needs (Lennard 2017). Or, if the source water contains elevated sodium (Na+) concentrations (again, often seen with ground water resources and a nutrient plants do not use and which can accumulate in system waters), it is important to know how much is present so management methods may be applied to avoid potential plant nutrient toxicity (Rakocy et al. 2006). The chemical nature of the source water, therefore, is vital to overall aquaponic system health and management.
Ultimately, because source water chemistry can affect aquaponic system nutrient management and because aquaponic operators like to have the ability to manipulate aquaponic water and nutrient chemistry to a high degree, a water source with little, if any, associated water chemistry is highly desirable (Lennard 2017). In this sense, rainwater or water treated for chemical removal (e.g. reverse osmosis) is the best source water for aquaponics in a water chemistry context (Rakocy et al. 2004a, b; Lennard 2017). Ground waters are also suitable, but it must be ensured that they do not contain chemicals or salts in concentrations that are too high to be practical (e.g. high magnesium or iron concentrations) or contain chemical species that are not used by the fish or plants (e.g. high sodium concentrations) (Lennard 2017). River waters may also be suitable as aquaponic source water, but as for other water sources, they should be tested for chemical presence and concentrations. Town water sources (i.e. water reticulated and supplied for domestic and consumptive purposes) are broadly applied in aquaponics (Love et al. 2015a, b) and are also acceptable if they contain acceptable nutrient, salt or chemical concentrations. In the case of town- or municipal-supplied water resources, it should be noted that many supplies have some form of sterilisation applied to make the water drinkable for humans. If this source of water is to be used for aquaponics, then it is important to ensure that any chemicals that may be applied to achieve sterilisation (e.g. chlorine, chloramine, etc.) are not present in concentrations that could harm the fish, plants or microorganisms within the aquaponic system (Lennard 2017).
The chemistry associated with source water is not the only factor that needs consideration when supplying source water for aquaponic use. Many natural waters may also contain microbial and other microorganisms that may affect the overall ecological health of the aquaponic system or present a discernible human health risk. Rainwaters rarely contain microbes themselves; however, the vessels or tanks the rainwater may be stored within may contain or allow microbial proliferation. Ground waters are usually good in terms of microbial presence but may also contain high microbial loads, especially if sourced from areas associated with animal farming or human waste treatment. River waters may also contain high microbial loads due to farming or human waste treatment outflows and again should be checked via detailed microbial analysis (Lennard 2017).
Because the chemical and microbial nature of the source water used in aquaponic systems can have potential effects on system water chemistry and microbiology, it is recommended that any applied water source be sterilised and treated for chemical removal (e.g. reverse osmosis, distillation, etc.) before being used in an aquaponic system (Lennard 2017). If sterilisation is universally applied, the chance of introducing any foreign and unwanted microbes to the system is substantially lowered. If water treatment and filtration is applied, any chemicals, salts, unwanted nutrients, pesticides, herbicides, etc. will be removed and therefore cannot contribute negatively to the system.
A clean water source, free of microbes, salts, nutrients and other chemicals allows the aquaponic operator to manipulate the system water to contain the nutrient mixture and strength they require without the fear that any external influences may affect the operation of the system or the health and strength of the fish and plants and is a vital requirement for any commercial aquaponic operation.
5 Water Quality Requirements
Aquaponics represents an effort to control water quality so that all the present life forms (fish, plants and microbes) are being cultured in as close to ideal water chemistry conditions as possible (Goddek et al. 2015). If water chemistry can be matched to the requirements of these three sets of important life forms, efficiency and optimisation of growth and health of all may be aspired to (Lennard 2017). Optimisation is important to commercial aquaponic production because it is only through optimisation that commercial success (i.e. financial profitability) may be realised. Therefore, water chemistry and water quality requirements within the aquaponic system are pivotal to the ultimate commercial and economic success of the enterprise (Goddek et al. 2015).
There is currently disagreement within the broader aquaponic industry and community in terms of what represents good or acceptable water quality within aquaponic systems. It appears that it is universally accepted that the natural water chemistry requirements of the individual life form subsets (fish, plants and microbes) are broadly agreed upon (Rakocy and Hargreaves 1993; Rakocy et al. 2006; Goddek et al. 2015; Delaide et al. 2016; Lennard 2017). However, the presence of a broad range of approaches, methods and technology choices that are called aquaponics and the background or history of the associated, stand-alone technologies of recirculating aquaculture systems (RAS) and hydroponic plant culture (including substrate culture) appears to lead to disagreements among operators, scientists and designers. For example, taking only one single water chemistry parameter into consideration, pH, some argue that the pH requirements of hydroponically cultured plants are very different to the pH requirements of RAS-cultured freshwater fish species (Suhl et al. 2016). The hydroponic industry generally applies pH settings between 4.5 and 6.0 for water-based plant culture (Resh 2013), whereas the RAS industry typically applies pH settings between 7.0 and 8.0 (Timmons et al. 2002) to meet the requirements of the fish and the microbes present (which perform important transformations of potentially toxic fish waste metabolites to less toxic forms). The argument, therefore, is that any pH set point is a compromise between the requirements of the plants, the fish and the microbes and that therefore an optimal pH for all life forms is not achievable which leads to suboptimal plant production (Suhl et al. 2016). Others argue, however, that a closer scrutiny of the complexities of the nutrient dynamics of plant nutrient uptake may elucidate a different opinion (Lennard 2017).
Hydroponic (and substrate culture) systems feed nutrients to the plants in their basal, ionic forms by adding nutrient salts to the water that dissociate to release the available nutrient ions (Resh 2013). Research has demonstrated that these ionic nutrient forms exist in a window of availability to the plant, based on the available system water pH. Therefore, in a standard hydroponic context, with no present microbial flora (i.e. sterilised – as most hydroponic systems are), it is important to set the pH of the system water to a level that makes the mixture of ionic nutrients the plant requires as available as possible (Resh 2013). Within any hydroponic system, this is a compromise itself, because as any ionic nutrient availability chart demonstrates (see Fig. 5.4), different ionic nutrient forms are the most available at differing pHs (Resh 2013). It is this standard ionic nutrient availability association that the hydroponic industry uses as its primer for pH set points and explains why the desired hydroponic operational pH is somewhere between 4.5 and 6.0 (an acid environment) in sterilised hydroponic and substrate culture systems.
Alternatively, RAS applies a water pH set point based on what is natural for the fish being cultured and the microbes treating and converting the fish waste products (Timmons et al. 2002; Goddek et al. 2015; Suhl et al. 2016). In natural freshwater environments, most fish species require an environmental pH (i.e. water pH) that closely matches the internal pH of the fish, which is often close to a pH of 7.4 (Lennard 2017). In addition, the major microbes associated with dissolved metabolite transformation in RAS culture (the nitrification bacteria of several species) also require a pH around 7.5 for optimal ammonia transformation to nitrate (Goddek et al. 2015; Suhl et al. 2016). Therefore, RAS operators apply a pH set point of approximately 7.5 to RAS freshwater fish culture.
There is an obvious difference between a pH of 5.5 (an average for standard, sterilised, hydroponic plant culture) and a pH of 7.5 (an average standard for RAS fish culture). Therefore, it is argued broadly that pH represents one of the largest water quality compromises present in aquaponic science (Goddek et al. 2015; Suhl et al. 2016). Advocates of decoupled aquaponic designs often cite this difference in optimal pH requirement as an argument for the decoupled design approach, stating that fully recirculating designs must find a pH compromise when decoupled designs have the luxury of applying different water pH set points to the fish and plant components (Suhl et al. 2016; Goddek et al. 2016). However, what this argument ignores is that aquaponic systems, as opposed to hydroponic systems, are not sterile and employ ecological aquatic techniques that encourage a diverse population of microflora to be present within the aquaponic system (Eck 2017; Lennard 2017). This results in a broad variety of present microbes, many of which form intricate and complex associations with the plants, especially the plant roots, within the aquaponic system (Lennard 2017). It is well known and established in plant physiology that many microbes, associated with the soil medium and matrix, closely associate with plant roots and that many of these microbes assist plants to access and uptake vital nutrients (Vimal et al. 2017). It is also known that some of these microbes produce organic molecules that directly further assist plant growth, assist plant immunity development and assist to outcompete plant (especially root) pathogens (Vimal et al. 2017; Srivastava et al. 2017). In essence, these microbes assist plants in many ways that are simply not present in the sterilised environment applied in standard hydroponic culture.
With these diverse microbes present, the plants gain access to nutrients in many ways that are not possible in systems that rely on aquatic pH settings alone to enable plant nutrient access (e.g. standard hydroponics and substrate culture). Many of these microbes operate at broad pH levels, just like other soil-based microbes, such as the nitrification bacteria (pH of 6.5–8.0, Timmons et al. 2002). Therefore, with these microbes present in aquaponic systems, the pH set point may be raised above what is normally applied in hydroponic or substrate culture techniques (i.e. pH of 4.5–6.0) while advanced and efficient plant growth is still present (Lennard 2017). This is evidenced in the work of several aquaponic researchers who have demonstrated better plant growth rates in aquaponics than in standard hydroponics (Nichols and Lennard 2010).
Other water quality requirements in aquaponic systems relate to physical/chemical parameters and more specifically, plant nutrient requirement parameters. In terms of physical/chemical requirements, plants, fish and microbes share many commonalities. Dissolved oxygen (DO) is vital to fish, plant roots and microflora and must be maintained in aquaponic systems (Rakocy and Hargreaves 1993; Rakocy et al. 2006). Plant roots and microflora generally require relatively lower DO concentrations than most fish; plant roots and microbes can survive with DO below 3 mg/L (Goto et al. 1996), whereas most fish require above 5 mg/L (Timmons et al. 2002). Therefore, if the DO concentration within the aquaponic system is set and maintained for the fish requirement, the plant and microbe requirement is also met (Lennard 2017). Different fish species require different DO concentrations: warm water fish (e.g. Tilapia spp., barramundi) can generally tolerate lower DO concentrations than cool water fish species (e.g. salmonids like rainbow trout and arctic char); because the fish DO requirement is almost always greater than the plant roots and microfloral requirement, DO should be set for the specific fish species being cultured (Lennard 2017).
Water-carbon dioxide (CO2) concentrations, like that for DO, are generally set by the fish because the plant roots and microbes can tolerate higher concentrations than the fish. Carbon dioxide concentrations are important to optimal fish health and growth and are often ignored in aquaponic designs. Parameters and set points for CO2 concentrations should be the same as for the same fish species cultured in fish-only, RAS systems and in general, should be kept below 20 mg/L (Masser et al. 1992).
Water temperature is important to all the present life forms within an aquaponic system. Fish and plant species should be matched as closely as possible for water temperature requirements (e.g. Tilapia spp. of fish like 25° C plus, and plants like basil thrive in this relatively high water temperature; lettuce varieties like cooler water, and therefore, a better matched fish candidate is rainbow trout) (Lennard 2017). However, as for other water physical and chemistry parameters, meeting the fish’s requirement for water temperature is paramount because the microbes have the ability to undergo specific species selection based on the ambient conditions (e.g. nitrification bacterial species differentiation occurs at different water temperatures and the species that matches best to the particular water temperature will dominate the nitrification bacterial biomass of the system) and many plants can grow very well at a broader range of water temperatures (Lennard 2017). Matching the water temperature, and maintaining it within plus or minus 2° C (i.e. a high-level temperature control) to the fish, is an important requirement in aquaponics because when water temperature is correct and does not deviate from the ideal average, the fish achieve efficient and optimised metabolism and eat and convert feed efficiently, leading to better fish growth rates and stable and predictable waste load releases, which assists plant culture (Timmons et al. 2002).
Maintaining water clarity (low turbidity) is another important parameter in aquaponic culture (Rakocy et al. 2006). Most water turbidity is due to suspended solids loads that have not been adequately filtered, and these solids may affect fish by adhering to their gills, which may lower potential oxygen transfer rates and ammonia release rates (Timmons et al. 2002). Suspended solids loads less than 30 mg/L are recommended for aquaponically cultured fish (Masser et al. 1992; Timmons et al. 2002). High suspended solids loads also affect plant roots because they have the ability to adhere to the roots which may cause nutrient uptake inefficiency, but more commonly provides increased potential for pathogenic organism colonisation, which leads to poor root health and ultimate plant death (Rakocy et al. 2006). These suspended solids also encourage the prevalence of heterotrophic bacteria (species that break down and metabolise organic carbon) which, if allowed to dominate systems, may outcompete other required species, such as nitrification bacteria.
Electrical conductivity (EC) is a measure often applied in hydroponics to gain an understanding of the amount of total nutrient present in the water. It, however, cannot provide information on the nutrient mix, the presence or absence of individual nutrient species or the amount of individual nutrient species present (Resh 2013). It is not often applied in aquaponics because it only measures the presence of ionic (charged) nutrient forms, and it has been argued that aquaponics is an organic nutrient supply method, and therefore, EC is not a relevant measure (Hallam 2017). However, plants generally only source ionic forms of nutrients, and therefore, EC can be used as a general tool or guide to the total amount of plant-available nutrient in an aquaponic system (Lennard 2017).
For fully recirculating aquaponic systems, in terms of physical and chemical parameters, it is the fish that are more exacting in their requirements, and therefore, if systems are managed to maintain the requirements of the fish, the plants and microbes are having their requirements more than satisfied (Lennard 2017). The difference when it comes to the plants, however, is their requirement for the correct mixture and strength of nutrients to be present to allow optimised nutrient access and uptake (whether stand-alone or microbial-assisted) which leads to efficient and fast growth. Decoupled aquaponic systems may therefore be more attractive because of the perception that they allow more exacting nutrient delivery to the plants (Goddek et al. 2016). Fish feed and, therefore, fish waste do not contain the correct mixture of nutrients to meet the plant requirements (Rakocy et al. 2006). Therefore, the aquaponic system design must account for those missing nutrients and supplement them. Fully recirculating aquaponic systems generally supplement nutrients by adding them in the salt species used to manage the daily pH buffering regime; the basic portion of the salt adjusts the pH and the positive portion of the salt allows the supplementation of missing plant nutrients (e.g. potassium, calcium, magnesium) (Rakocy et al. 2006). Decoupled aquaponic designs take the waste water and associated solid wastes from the fish component and adjust the water to contain the nutrients required for plant production by adding nutrients in different forms (Goddek et al. 2016). These nutrient additions are generally based on using standard hydroponic salt species that do not necessarily provide any pH adjustment outcome (e.g. calcium phosphate, calcium sulphate, potassium phosphate, etc.).
The pathway to efficient plant growth in aquaponic systems is to provide an aquatic nutrient profile that provides all the nutrients the plant requires (mixture) at the strengths required (concentration) (Lennard 2017). In fully recirculating aquaponic designs, or decoupled aquaponic designs that do not apply sterilisation methods, there appears to be less of a requirement to meet the nutrient concentrations or strengths applied in standard hydroponics, because the ecological nature of the system associates many diverse microflora with the plant roots and these microflora assist plant nutrient access (Lennard 2017). For decoupled, or other, aquaponic designs that apply sterilisation to the plant component and follow a standard hydroponic analogue approach, there appears to be a requirement to try and approach standard hydroponic nutrient concentrations (Suhl et al. 2016; Karimanzira et al. 2016). The compromise, however, with the decoupled approach is that it leads to external supplementation ratios far beyond those of fully recirculating aquaponic designs; European decoupled designs currently average 50% or greater external nutrient additions (COST FA1305 2017; Goddek and Keesman 2018), while the UVI method supplies less than 20%, and other systems may supply less than 10% external nutrient supplementation (Lennard 2017).
No matter the method, all aquaponic systems should strive to supply the plants with the nutrition required for optimised growth so as to provide the enterprise with the greatest chance of financial viability. In this context, the nutrient content and strength of the water being delivered to the plants is very important and regular nutrient testing of the water should be employed so that nutrient mixture and strength may be maintained and managed as a very important water quality requirement.
6 Applicable Fish Culture Technologies
In aquaponics, the aquaculture portion of the integration equation is broadly applied in a tank-based context, where the fish are kept in tanks, the water is filtered via mechanical (solids removal) and biological (ammonia transformation to nitrate) mechanisms and dissolved oxygen is maintained, either via aeration or direct oxygen injection (Rakocy et al. 2006; Lennard 2017).
As has been argued in Sect. 5.0 (Introduction) of this chapter, historical examples of chinampas (Somerville et al. 2014) and Asian rice paddy farming (Halwart and Gupta 2004) as early iterations of aquaponics are unfounded and inappropriate examples of aquaponic principles, because modern aquaponics relies on designed additions of fish and fish feeds to supply a designed level of nutrition to the plants, and therefore, these historical examples cannot be considered in any way similar (Lennard 2017).
The above historical examples, which rely on soil-based plant culturing systems, lead to the question of what aquaculture technologies are suitable for aquaponic integration. Soil-based, extensive, freshwater pond aquaculture of fin fish is the largest culturing method applied to produce freshwater fish for human consumption (Boyd and Tucker 2012). A pond approach relies on the earthen base of the pond, and the associated microflora present in that soil, to treat and remediate the wastes produced from fish culture so the fish are not living in water that has a potential to be toxic to them (Boyd and Tucker 2012). Because this system relies on the inherent treatment capacity of the earthen pond itself, fish densities are relatively low compared to other aquaculture methods. Because the fish densities are low (and therefore the associated feeding rates are low) and the pond itself treats and uptakes the waste nutrients produced by the fish, pond waters exhibit extremely low water nutrient concentrations. These pond system aquatic nutrient concentrations are so low that they are often inappropriate as nutrient sources for substantial, commercial aquatic plant production methods (Lennard 2017). Therefore, ponds are not an appropriate aquaculture method to be integrated with hydroponics in terms of acceptable plant production rates.
Similarly, raceway fin fish culture methods (as regularly applied for freshwater Salmonid production), which supply very large volumes of water at high turnover rates, or low residence times, through controlled raceway fish culture tanks, are not appropriate for aquaponic integration because the high water turnover rates do not allow adequate nutrient accumulations to meet plant nutrient requirements (Rakocy and Hargreaves 1993).
The most appropriate fish culture technologies to apply within an aquaponic integration context are those that culture fish in tanks and allow a level of fish waste accumulation (plant nutrient accumulation) that has the potential to lead to water nutrient concentrations that are applicable for efficient hydroponic plant production (Rakocy et al. 2006). Recirculating Aquaculture System (RAS) principles are broadly applied to aquaponics because they provide the methodologies to successfully keep and grow the fish, in controlled volumes of water, with low daily water replacement rates, that allow fish waste (plant nutrient) accumulations that approach those required to efficiently hydroponically culture the plants (Rakocy and Hargreaves 1993; Lennard 2017). The complexities and design requirements of RAS are discussed in Chap. 3 of this book. Suffice to say that RAS fish culture is the only real appropriate method to apply for fish culturing components in an aquaponic context and as discussed above, soil-based aquaculture systems, such as extensive pond systems and raceway culture systems, cannot provide the nutrient requirements of the plants and therefore should not be considered.
7 Nutrient Sources
The major input to any aquaponic system are the nutrients added because aquaponic systems are designed to efficiently partition the nutrients added to them to the three important forms of life present: the fish and plants (which are the main products of the system) and the microflora (which assist to make the added nutrients available to the fish and plants) (Lennard 2017).
In classical, fully recirculating aquaponic designs, one of the key design drivers is to use the main nutrient input source, the fish feed, as efficiently as possible and therefore fully recirculating designs strive to supply as many of the nutrients required for the plants from the fish feed (Lennard 2017). Decoupled designs, on the other hand, place an emphasis on optimised plant growth by directly comparing the nutrient mixtures and strengths applied in standard hydroponics and substrate culture and trying to replicate those within the aquaponic context and therefore do not strive to supply as many of the nutrients required for the plants from the fish feed and utilise substantial external nutrient supplementations to achieve the required plant growth rates (Delaide et al. 2016). This means that a different emphasis is placed on the origin of the nutrients added, based on the technical design approach, and this, therefore, affects the main nutrient supply source of the aquaponic system; for fully recirculating designs, the major plant nutrient source is fish feed (via fish waste production), and for decoupled designs the major nutrient supply source for the plants is external supplements (e.g. nutrient salts) (Lennard 2017).
Fully recirculating aquaponic designs, such as the UVI aquaponic system model, rely on the fish feed as the major nutrient source for the system (Rakocy et al. 2006). The fish feed is added to the fish, which eat it, metabolise it and use the nutrients from it as required and then produce a waste stream (both solids and dissolved). This waste stream from the fish becomes the major nutrient source for the plants, and hence, the fish feed is the major nutrient source for the plants. The UVI system provides approximately 80% or more of the nutrients required to grow the plants from the fish feed alone (Lennard 2017). The remaining nutrients required for plant growth, because the fish feed does not contain them in the amounts required, are added via a nutrient supplementation method that provides the dual role of supplementing the additional nutrients and controlling the system aquatic pH (Rakocy et al. 2006). This dual role approach is referred to as “buffering” and the supplement is referred to as “buffer”. For the UVI model, the two important plant nutrients identified as lacking in fish feed and which require supplementation are potassium (K) and calcium (Ca) and are supplemented daily via the buffering regime. In addition, plant-required iron (Fe) is also lacking in the fish feed and is supplemented in a chelated form via direct addition to the system water at a frequency measured in weeks (i.e. every 2–4 weeks based on weekly aquatic iron analysis) (Rakocy et al. 2006).
Other fully recirculating aquaponic design approaches or methods place an even higher emphasis on providing nutrients via the fish feed. Lennard (2017) has developed a method for fully recirculating systems that supplies greater than 90% of the nutrients required for plant growth from the fish feed added. The increase in the efficiency of nutrients supplied via the fish feed of this method when compared to the UVI method is that this approach remineralises the solid fish wastes (via external, bacteria-mediated biodigestion) and adds these nutrients back into the aquaponic system for plant utilisation, whereas the UVI method sends the majority of the solid fish wastes to an external waste stream (Rakocy et al. 2006; Lennard 2017). This approach also adds nutrients deficient in the fish feed for plant growth via a buffering regime; however, this regime is far more exacting and allows greater manipulation of nutrient strengths and mixtures than the UVI approach (Lennard 2017).
Therefore, the major nutrient addition pathways for most fully recirculating aquaponic system designs are the fish feed (major route), buffer external supplementation for added potassium and calcium (minor route) and direct supplementation of iron chelate (minor route).
Decoupled aquaponic system designs, such as those being widely adopted currently in Europe, rely on a mixture of fish feed nutrients and active, external supplementation to provide the nutrients required for plant growth (Suhl et al. 2016). Because decoupled designs do not return water from the plant component to the fish component, it is possible to customise the nutrient profile within the water specifically to the plant requirement (Goddek et al. 2016). Therefore, decoupled aquaponic designs almost always rely on substantial external nutrient supplementation to meet the plant requirement and place far less emphasis on providing as much nutrition as possible for the plants from the fish wastes. In addition, the amount of external supplementation is substantial when compared to fully recirculating approaches (Lennard 2017) with external fractions regularly 50% or more of the total plant nutrient requirement or greater (Goddek 2017). The external nutrients supplemented to decoupled aquaponic systems are most often hydroponic nutrient salt analogues or derivatives (Delaide et al. 2016; Karimanzira et al. 2016). This reliance on a source of substantial additional nutrients other than those that arise from fish wastes (fish feeds) that are hydroponic salt in nature, for plant supply of the European decoupled approach, has even directly affected the definition of aquaponics that the European aquaponics community currently applies, with the EU COST, EU Aquaponics Hub, defining aquaponics as “…a production system of aquatic organisms and plants where the majority (> 50%) of nutrients sustaining the plants derives from wastes originating from feeding the aquatic organisms” (Goddek 2017; COST FA 1305, 2017) compared to Lennard (2017) who defines aquaponics as requiring at least 80% nutrient supply from fish wastes. Some also argue whether a method that relies on 50% of the nutrients required for plant growth originating from external sources other than fish feeds is actually aquaponic in nature or rather, a hydroponic method with some fish integrated or added (Lennard 2017)?
Another proposed supply source for nutrients to aquaponic systems is that of external nutrient supplementation via the application of foliar plant sprays (Tyson et al. 2008; Roosta and Hamidpour 2011; Roosta and Hamidpour 2013; Roosta 2014). These foliar sprays are again, an aquatic delivery of standard hydroponic nutrient salts or derivatives. The difference is that in the decoupled examples above, the nutrient salts are added directly to the culture water and are therefore accessed by the plants via root uptake (Resh 2013), whereas foliar sprays, as the name implies, add dissolved nutrient salts to the plant leaves and uptake is achieved via plant leaf stomatal or cuticle access (Fernandez et al. 2013).
There are therefore several major nutrient sources applied in aquaponics: fish feeds, buffering systems (via basic, pH-adjusting salt species added to the water column), nutrient salt additions (hydroponic nutrient salts added to the water column) and foliar sprays (hydroponic nutrient salts added to the leaf surface). All of which supply nutrients to the aquaponic system for the health and growth of the fish and plants that are cultured.
8 Aquaponics as an Ecological Approach
Aquaponics, until recently, has been dominated by fully recirculating (or coupled) design approaches that share and recirculate the water resource constantly between the two major components (fish and plant culture) (Rakocy et al. 2006; Lennard 2017). In addition, the low to medium technology approaches historically applied to aquaponics have driven a desire to remove costly components so as to increase the potential of a positive economic outcome. One of the filtration components almost always applied to standard RAS and hydroponics/substrate culture technologies, that of aquatic sterilisation, has regularly not been included by aquaponic designers.
Sterilisation in a RAS and hydroponic/substrate culture context is universally applied because the high densities of either the fish or plants cultured usually attract pressure from aquatic, pathogenic organisms that substantially lower overall production rates (Van Os 1999; Timmons et al. 2002). The major reason for this increased aquatic pest pressure in both technologies is that each concentrates on providing minimal biotic, ecological resources and therefore allows considerable “ecological space” within the system water for biotic colonisation. In these “open” biological conditions, pest and pathogenic species proliferate and tend to colonise quickly to take advantage of the species present (i.e. fish and plants) (Lennard 2017). In this context, sterilisation or disinfection of the culture water has historically be seen as an engineered approach to counteract the issue (Van Os 1999; Timmons et al. 2002). This means that both RAS and hydroponic/substrate culture industries adopt a sterilisation approach to control pathogenic organisms within the associated culture water.
Aquaponics has always placed an emphasis on the importance of the associated microbiology to perform important biological services. In all the coupled aquaponic designs of Rakocy and his UVI team, a biological filter was not included because they demonstrated that the raft culture, hydroponic component provided more than enough surface area to support the nitrifying bacteria colony size to treat all the ammonia produced by the fish as a dissolved waste product and convert it to nitrate (Rakocy et al. 2006, 2011). Rakocy and his team therefore did not advocate applied sterilisation of the system water because it may have affected the nitrifying bacterial colonies. This historical UVI/Rakocy perspective dictated aquaponic system design into the future. Other advantages of not including aquatic sterilisation to aquaponic systems were identified and discussed, especially in the context of assistive plant microbiota (Savidov 2005; Goddek et al. 2016).
The current thinking in aquaponic research and industry is that not applying any form of aquatic sterilisation or disinfection allows the system water to develop a complex aquatic ecology that consists of many different microbiological life forms (Goddek et al. 2016; Lennard 2017). This produces a situation similar to a natural ecosystem whereby a high diversity of microflora interacts with each other and the other associated life forms within the system (i.e. fish and plants). The proposed outcome is that this diversity leads to a situation in which no single pathogenic organism can dominate due to the presence of all of the other microflora and can therefore not cause devastating effects to fish or plant production. It has been demonstrated that aquaponic systems contain a high diversity of microflora (Eck 2017) and via the proposed ecological diversity mechanism outlined above, assistance to both fish and plant health and growth is provided via this microbial diversity (Lennard 2017).
The non-sterilised, ecologically diverse approach to aquaponics has been historically applied to coupled or fully recirculating aquaponic designs (Rakocy et al. 2006), whereas a sterilised, hydroponic analogy has been proposed for some decoupled aquaponic design approaches (Monsees et al. 2016; Priva 2009; Goddek 2017). However, it appears more decoupled designers are now applying principles that take an ecological, non-sterilised approach into consideration (Goddek et al. 2016; Suhl et al. 2016; Karimanzira et al. 2016) and therefore acknowledge there is a positive effect associated with a diverse aquaponic microflora (Goddek et al. 2016; Lennard 2017).
9 Advantages of Aquaponics
Because there are two separate, existing, analogous technologies that produce fish and plants at high rates (RAS fish culture and hydroponic/substrate culture plant production), a reason for their integration seems pertinent. RAS produces fish at productive rates in terms of individual biomass gain, for the feed weight added, that rivals, if not betters, other aquaculture methods (Lennard 2017). In addition, the high fish densities that RAS allows lead to higher collective biomass gains (Rakocy et al. 2006; Lennard 2017). Hydroponics and substrate culture possess, within a controlled environment context, advanced production rates of plants that better most other agriculture and horticulture methods (Resh 2013). Therefore, initially, there is a requirement for aquaponics to produce fish and plants at rates that equal these two separate productive technologies; if not, then any loss of productive effort counts against any integration argument. If the productive rate of the fish and plants in an aquaponic system can equal, or better, the RAS and hydroponic industries, then a further case may be made for other advantages that may occur due to the integration process.
Standard hydroponics or substrate culture has been directly compared with aquaponics in terms of the plant growth rates of the two technologies. Lennard (2005) compared aquaponic system lettuce production to a hydroponic control in several replicated laboratory experiments. He demonstrated that aquaponic lettuce production was statistically lower in aquaponics (4.10 kg/m2) when compared to hydroponics (6.52 kg/m2) when a standard approach to media bed aquaponic system design and management was applied. However, he then performed a series of experiments that isolated specific parameters of the design (e.g. reciprocal vs constant hydroponic subunit water delivery, applied water flow rate to the hydroponic subunit and comparing different hydroponic subunits) or comparing specific management drivers (e.g. buffering methodologies and species and the overall starting nutrient concentrations) to achieve optimisation and then demonstrated that aquaponics (5.77 kg/m2) was statistically identical to hydroponic lettuce production (5.46 kg/m2) after optimisation of the aquaponic system based on the improvements suggested by his earlier experiments, the result suggesting that improvements to coupled or fully recirculating aquaponic designs can equal standard hydroponic plant production rates. Lennard (2005) also demonstrated fish survival, SGR, FCR and growth rates equal to those exhibited in standard RAS and extensive pond aquaculture for the fish species tested (Australian Murray Cod).
Pantanella et al. (2010) also demonstrated statistically similar lettuce production results within high fish density (5.7 kg/m2 lettuce production) and low fish density (5.6 kg/m2 lettuce production) aquaponic systems compared to a standard hydroponic control (6.0 kg/m2).
Lennard (Nichols and Lennard 2010) demonstrated statistically equal or better results for all lettuce varieties and almost all herb variety production tested in a nutrient film technique (NFT) aquaponic system when compared to a hydroponic NFT system within the same greenhouse.
Delaide et al. (2016) compared nutrient-complemented RAS production water (nutrients were added to match one water nutrient mixture and strength example by Rakocy – denoted as an aquaponic analogue), fully nutrient-complemented RAS production water (RAS production water with added hydroponic nutrient salts to meet a water nutrient mixture and strength as used for standard hydroponics – denoted as a decoupled analogue) and a hydroponic control (standard hydroponic nutrient solution) in terms of plant growth rate and showed the aquaponic water analogue equalled the hydroponic control and the decoupled analogue water bettered the hydroponic control. However, it must be noted that these were not fully operating aquaponic systems containing fish (and the associated, full and active microbial content) that were compared, but simply water removed from an operating RAS and complemented, then compared to a hydroponic control water.
Rakocy and his UVI team have demonstrated with several studies that Tilapia spp. fish growth rates equal industry standards set by standard aquaculture production practices (Rakocy and Hargreaves 1993; Rakocy et al. 2004a, b, 2006, 2011).
These and other studies have demonstrated that aquaponics, no matter the configuration (coupled and decoupled), has the potential to produce plant production rates equal to, or better than, standard hydroponics and fish production rates of a similar standard to RAS. Therefore, the above discussed requirement for aquaponics to equal its industry analogues (RAS and hydroponics) seems to have been adequately proven, and therefore, the other advantages of aquaponics should be considered.
Efficient water use is regularly attributed to aquaponics. Lennard (2005) stated that the water savings associated with an optimised aquaponic test system (laboratory) were 90% or greater when compared to a standard RAS aquaculture control system where water was exchanged to control nitrate accumulations, whereas the plants in the aquaponics performed the same requirement. Therefore, he demonstrated that aquaponics provides a substantial water-saving benefit compared to standard RAS aquaculture. Interestingly, this 90% water-saving figure has subsequently been stated broadly within the global aquaponics community in a plant use context (e.g. aquaponics uses 90% less water than soil-based plant production (Graber and Junge 2009)) – an example of how scientific argument can be incorrectly adopted by nonscientific industry participants.
McMurtry (1990) demonstrated a water consumption rate in his aquaponic system of approximately 1% of that required in a similar pond culture system. Rakocy (1989) has demonstrated similar 1% water consumption rates compared to pond-based aquaculture. Rakocy and Hargreaves (1993) stated that the daily water replacement rate for the UVI aquaponic system was approximately 1.5% of the total system volume and Love et al. (2015a, b) stated an approximate 1% of system volume water loss rate per day for their aquaponic research system.
The comparison of aquaponics to RAS can realise substantial water savings and aquaponics uses small amounts of replacement water on a daily basis. A well-designed aquaponic system will seek to use water as efficiently as possible and therefore only replace that water lost via plant evapotranspiration (Lennard 2017). In fact, it has been proposed that water may even be recovered from that lost due to plant evapotranspiration via employing some form of air water content harvesting scheme or technology (Kalantari et al. 2017). Coupled aquaponic systems appear to provide a greater potential to conserve and lower water use (Lennard 2017). If the nutrient dynamics between fish production and plant use can be balanced, the only water loss is via plant evapotranspiration, and because the water is integrally shared between the fish and plant components, daily makeup water volumes simply represent all the water lost from the system plants (Lennard 2017). Decoupled aquaponic designs present a more difficult proposition because the two components are not integrally linked and the daily water use of the fish component does not match the daily water use of the plant component (Goddek et al. 2016; Goddek and Keesman 2018). Therefore, water use and replacement rates for aquaponic systems are not completely resolved and probably never will be due to the broad differences in system design approaches.
Efficient nutrient utilisation is assigned to the aquaponic method and cited as an advantage of the aquaponic approach (Rakocy et al. 2006; Blidariu and Grozea 2011; Suhl et al. 2016; Goddek et al. 2015). This is generally because standard RAS aquaculture utilises the nutrients within the fish feed to grow the fish, with the remainder being sent to waste. Fish metabolise much of the feed they are fed, but only utilise approximately 25–35% of the nutrients added (Timmons et al. 2002; Lennard 2017). This means up to 75% of the nutrients added to fish-only RAS are wasted and not utilised. Aquaponics seeks to use the nutrients wasted in RAS for plant production, and therefore, aquaponics is said to use the nutrients added more efficiently because two crops are produced from the one input source (Rakocy and Hargreaves 1993; Timmons et al. 2002; Rakocy et al. 2006; Lennard 2017). The extent of fish waste nutrient use does differ between the various aquaponic methods. The fully recirculating UVI model does not utilise the majority of the solid fish wastes generated in the fish component and sends them to waste (Rakocy et al. 2006), the fully recirculating Lennard model takes this a step further by using all the wastes generated by the fish component (dissolved wastes directly and solids via external microbial remineralisation with main system replacement) (Lennard 2017). Many decoupled approaches also attempt to utilise all the wastes generated by the fish component, via direct use of dissolved wastes and again, via external microbial remineralisation with main system replacement (Goddek et al. 2016; Goddek and Keesman 2018). All of these methods and approaches demonstrate that a primary driver for the aquaponic method is to utilise as many of the added nutrients as possible and therefore attempt to use the added nutrients as efficiently as possible.
Independence from soil has been cited as an advantage of the aquaponic method (Blidariu and Grozea 2011; Love et al. 2015a, b). The advantage perceived is that because soil is not required, the aquaponic system or facility may be located where the operator chooses, rather than where suitable soil is present (Love et al. 2015a, b). Therefore, the aquaponic method is independent of location based on soil availability, which is an advantage over soil-based agriculture.
It has been argued that aquaponics provides an advantage by mimicking natural systems (Blidariu and Grozea 2011; Love et al. 2014). This is supported by the ecological nature of the aquaponic approach/method, as outlined in Sect. 5.7 above, with the associated advantages related to diverse and dense microfloral communities (Lennard 2017).
Aquaculture has a potential direct environmental impact due to the release of nutrient-rich waste waters to the surrounding environment – generally, aquatic environments (Boyd and Tucker 2012). Some hydroponic methods may also possess this potential. However, aquaponics can exhibit lowered or negated direct environmental impact from nutrient-rich waste streams because the main waste-generating component (i.e. the fish) is integrated with a nutrient use component (i.e. the plants) (Rakocy et al. 2006; Blidariu and Grozea 2011; Goddek et al. 2015; Lennard 2017). However, some aquaponic methods do produce wastes (e.g. the UVI model); but these are generally treated and reused for other agricultural practices at the aquaponic facility site (Timmons et al. 2002; Rakocy et al. 2006). Many aquaponic methods rely on the use of standard aquaculture feeds, which contain varying concentrations of sodium, usually via the use of fish meal or fish oil as an ingredient (Timmons et al. 2002). Sodium is not utilised by plants and therefore may accumulate over time in aquaponic systems, which may lead to a requirement for some form of water replacement so sodium does not accumulate to concentrations that affect the plants (Lennard 2017). However, it has been reported that some species of lettuce had the ability to take up sodium, when being exposed to aquaculture water (Goddek and Vermeulen 2018).
Coupled or fully recirculating aquaponic systems integrally share the water resource between the two main components (fish and plant). Because of this fully connected and recirculating aquatic nature, coupled aquaponic systems exhibit a self-controlling mechanism in terms of an inability to safely apply herbicides and pesticides to the plants; if they are applied, their presence may negatively affect the fish (Blidariu and Grozea 2011). Fully recirculating advocates see this inability to applying pesticides and herbicides as an advantage, the argument being that it guarantees a spray-free product (Blidariu and Grozea 2011). Decoupled aquaponics advocates also seek to not apply herbicides or pesticides; however, due to the fact that the water is not recirculated back to the fish from the plants, the ability to apply pesticides and herbicides to the plants is present (Goddek 2017). Therefore, the application or lack of application of pesticides and herbicides to the plant component of aquaponic designs is seen differently by groups who advocate for different design approaches.
There is a perception that the presence of both fish and plants in the same aquatic system provides positive synergistic effects to fish and plant health (Blidariu and Grozea 2011). This has been indirectly demonstrated by the ability of aquaponics in some studies to produce plant growth rates greater than those seen in standard hydroponics (Nichols and Lennard 2010; Delaide et al. 2016). However, no direct causal link has been established between the presence of both fish and plants and any positive outcome to fish or plant health.
References
Baquedano E (1993) Aztec Inca & Maya. A Dorling Kindersley Book, Singapore
Blidariu F, Grozea A (2011) Increasing the economic efficiency and sustainability of indoor fish farming by means of aquaponics – review. Anim Sci Biotechnol 44(2):1–8
Boyd CE, Tucker CS (2012) Pond aquaculture water quality management. Springer Science and Business Media
Buzby KM, Lian-shin L (2014) Scaling aquaponic systems: balancing plant uptake with fish output. Aquac Eng 63:39–44
Cerozi BS, Fitzsimmons K (2017) Phosphorous dynamics modelling and mass balance in an aquaponics system. Agric Syst 153:94–100
COST FA1305 (2017) The EU aquaponics hub – realising sustainable integrated fish and vegetable production for the EU. https://euaquaponicshub.com
Delaide B, Goddek S, Gott J, Soyeurt H, Haissam Jijakli M (2016) Lettuce (Lactuca sativa L. var. Sucrine) (2016). Growth performance in complemented aquaponic solution outperforms hydroponics. Water 8(10):467
Eck M (2017) Taxonomic characterisation of bacteria communities from water of diversified aquaponic systems. Thesis for the partial fulfillment of a Masters Degree. Université de Liège, Liège
Endut A, Jusoh A, Ali N, Wan Nik WB, Hassan A (2010) A study on the optimal hydraulic loading rate and plant ratios in recirculation aquaponic system. Technol 101:1511–1517
Fernandez V, Sotiropoulos T, Brown P (2013) Foliar fertilization: scientific principles and field practices, 1st edn. International Fertilizer Industry Association (IFA), Paris
Goddek S (2017) Opportunities and challenges of multi-loop aquaponic systems. Wageningen University, Wageningen
Goddek S, Keesman KJ (2018) The necessity of desalination technology for designing and sizing multi-loop aquaponics systems. Desalination 428:76–85
Goddek S, Körner O (2019) A fully integrated simulation model of multi-loop aquaponics: a case study for system sizing in different environments. Agric Syst 171:143–154
Goddek S, Vermeulen T (2018) Comparison of Lactuca sativa growth performance in conventional and RAS-based hydroponic systems. Aquac Int 26:1–10. https://doi.org/10.1007/s10499-018-0293-8
Goddek S, Delaide B, Mankasingh U, Vala Ragnarsdottir K, Jijakli H, Thorarinsdottir R (2015) Challenges of sustainable and commercial aquaponics. Sustainability 7(4):4199–4224
Goddek S, Espinal CA, Delaide B, Jikali MH, Schmautz Z, Wuertz S, Keesman J (2016) Navigating towards decoupled aquaponic systems: a system dynamics design approach. Water 8(7):303. 1–29
Gooley GJ, Gavine FM (2003) Integrated agri-aquaculture systems: a resource handbook for Australian industry development. RIRDC Publication No. 03/012
Goto E, Both AJ, Albright LD, Langhans RW, Leed AR (1996) Effect of dissolved oxygen concentration on lettuce growth in floating hydroponics. Proceedings of the International Symposium in Plant Production in Closed Systems. Acta Hortic 440:205–210
Graber A, Junge R (2009) Aquaponic systems: nutrient recycling from fish wastewater by vegetable production. Desalination 246:147–156
Hallam M (2017) EC. Murray Hallam’s practical aquaponics. https://aquaponics.net.au/ec/
Halwart M, Gupta MV (eds) (2004) Culture of fish in rice fields. FAO and The WorldFish Center, Penang
Kalantari F, Tahir OM, Lahijani AM, Kalantari S (2017) A review of vertical farming technology: a guide for implementation. Adv Eng Forum 24:76–91
Karimanzira D, Keesman KJ, Kloas W, Baganz D, Raushenbach T (2016) Dynamic modelling of the INAPRO aquaponic system. Aquac Eng 75:29–45
Kloas W, Groß R, Baganz D, Graupner J, Monsees H, Schmidt U, Staaks G, Suhl J, Tschirner M, Wittstock B, Wuertz S, Zikova A, Rennert B (2015) A new concept for aquaponic systems to improve sustainability, increase productivity, and reduce environmental impacts. Aquac Environ Interact 7:179–192. https://doi.org/10.3354/aei00146
Knaus U, Palm HW (2017) Effects of the fish species choice on vegetables in aquaponics under spring-summer conditions in northern Germany (Mecklenburg Western Pomerania). Aquaculture 473:62–73
Komives T, Junge R (2015) Editorial: on the “aquaponic corner” section of the journal. Ecocycles 1(2):1–2
Lennard WA (2005) Aquaponic integration of Murray Cod (Maccullochella peelii peelii) aquaculture and lettuce (Lactuca sativa) hydroponics. Thesis (Ph.D.). RMIT University, 2005
Lennard W (2017) Commercial aquaponic systems: integrating recirculating fish culture with hydroponic plant production. In press
Lennard WA, Leonard BV (2006) A comparison of three different hydroponic sub-systems (gravel bed, floating and nutrient film technique) in an aquaponic test system. Aquac Int 14:539–550
Love DC, Fry JP, Genello G, Hill ES, Frederick JA, Li X, Semmens K (2014) An international survey of aquaponics practitioners. PLoS One 9(7):E102662
Love DC, Fry JP, Li X, Hill ES, Genello L, Semmens K, Thompson RE (2015a) Commercial aquaponics production and profitability: findings from an international survey. Aquaculture 435:67–74
Love DC, Uhl MS, Genello L (2015b) Energy and water use of a small-scale raft aquaponics system in Baltimore, Maryland, United States. Aquac Eng 68:19–27
Masser MP, Rakocy J, Losordo TM (1992) Recirculating aquaculture tank production systems. SRAC Publication No. 452. Southern Regional Aquaculture Center. USA
McMurtry M (1990) Performance of an integrated aquaculture-olericulture system as influenced by component ratio. PhD. dissertation, North Carolina State University, Raleigh, North Carolina, USA
Monsees H, Kloas W, Wuertz S (2016) Comparison of coupled and decoupled aquaponics - Implications for future system design. Abstract from aquaculture Europe, 2016. Edinburgh, Scotland
Morehart CT (2016) Chinampa agriculture, surplus production and political change at Xaltocan, Mexico. Anc Mesoam 27(1):183–196
Nichols MA, Lennard W (2010) Aquaponics in New Zealand. Practical Hydroponics and Greenhouses, 115: 46–51
Palm HW et al (2018) Towards commercial aquaponics: a review of systems, designs, scales and nomenclature. Aquac Int 26(3):813–842
Pantanella E, Cardarelli M, Colla G, Rea A, Marcucci A (2010) Aquaponic vs hydroponic: production and quality of lettuce crop. Acta Hortic 927:887–893
Priva (2009) Eindrapport project EcoFutura, visteelt in de glastuinbouw. Priva B.V., Aqua-Terra Nova B.V., Green Q Group B.V., Groen Agro Control. www.ecofutura.nl
Rakocy JE (1989) Vegetable hydroponics and fish culture, a productive interface. World Aquacult 20:42–47
Rakocy JE, Hargreaves JA (1993) Integration of vegetable hydroponics with fish culture: a review. In: Wang J (ed) Techniques for modern aquaculture. American Society of Agricultural Engineers, St Joseph
Rakocy JE, Bailey DS, Shultz RC, Thoman ES (2004a) Update on tilapia and vegetable production in the UVI aquaponic system. In: New Dimensions on Farmed Tilapia: Proceedings of the Sixth International Symposium on Tilapia in Aquaculture, Manila, pp 676–690
Rakocy JE, Shultz RC, Bailey DS, Thoman ES (2004b) Aquaponic integration of Tilapia and Basil: Comparing a batch and staggered cropping system. Acta Hortic 648:63–69
Rakocy JE, Masser MP, Losordo TM (2006) Recirculating aquaculture tank production systems: aquaponics – integrating fish and plant culture. SRAC Publication No. 454. Southern Regional Aquaculture Center. USA
Rakocy JE, Bailey DS, Shultz RC, Danaher JJ (2011) A commercial scale aquaponic system developed at the University of the Virgin Islands. Proceedings of the 9th International Symposium on Tilapia in Aquaculture
Resh HM (2013) Hydroponic food production, 7th edn. CRC Press, Boca Raton
Reyes Lastiri D, Slinkert T, Cappon HJ, Baganz D, Staaks G, Keesman KJ (2016) Model of an aquaponic system for minimised water, energy and nitrogen requirements. Water Sci Technol 74:1. https://doi.org/10.2166/wst.2016.127
Roosta HR (2014) Effects of foliar spray of K on mint, radish, parsley and coriander plants in aquaponic system. J Plant Nutr 37(14):2236–2254
Roosta HR, Hamidpour M (2011) Effects of foliar application of some macro- and micro-nutrients on tomato plants in aquaponic and hydroponic systems. Sci Hortic 129:396–402
Roosta HR, Hamidpour M (2013) Mineral nutrient content of tomato plants in aquaponic and hydroponic systems: Effect of foliar application of some macro-and micro-nutrients. J Plant Nutr 36(13):2070–2083
Savidov N (2005) Comparative study of aquaponically and hydroponically grown plants in model system. In: Evaluation and development of aquaponics production and product market capabilities in Alberta. Chapter 3.2., Phase II, pp 21–31
Somerville C, Cohen M, Pantanella E, Stankus A, Lovatelli A (2014) Small-scale aquaponic food production: integrated fish and plant farming. FAO Fisheries and Aquaculture Technical Paper No. 589
Srivastava JK, Chandra H, Kalra SJ, Mishra P, Khan H, Yadav P (2017) Plant–microbe interaction in aquatic system and their role in the management of water quality: a review. Appl Water Sci 7:1079–1090
Suhl J, Dannehl D, Kloas W, Baganz D, Jobs S, Schiebe G, Schmidt U (2016) Advanced Aquaponics: evaluation of intensive tomato production in aquaponics vs conventional hydroponics. Agric Water Manag 178:335–344
Timmons MB, Ebeling JM, Wheaton FW, Summerfelt ST, Vinci BJ (2002) Recirculating aquaculture systems, 2nd edn. Cayuga Aqua Ventures, Ithaca
Tyson RV, Simonne EH, Treadwell DD, Davis M, White JM (2008) Effect of water pH on yield and nutritional status of greenhouse cucumber grown in recirculating hydroponics. J Plant Nutr 31(11):2018–2030
Tyson RV, Treadwell DD, Simonne EH (2011) Opportunities and challenges to sustainability in aquaponic systems. Hort Technol 21(1):6–13
Van Os E (1999) Design of sustainable hydroponic systems in relation to environment-friendly disinfection methods. Acta Hortic 548:197–205
Vimal SR, Singh JS, Arora NK, Singh S (2017) Soil-plant-microbe interactions in stressed agriculture management: a review. Pedosphere 27(2):177–192
Wongkiew S, Zhen H, Chandran K, Woo Lee J, Khanal SK (2017) Nitrogen transformations in aquaponic systems: a review. Aquac Eng 76:9–19
Yogev U, Barnes A, Gross A (2016) Nutrients and energy balance analysis for a conceptual model of a three loops off grid, aquaponics. Water 8:589. https://doi.org/10.3390/W8120589
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2019 The Author(s)
About this chapter
Cite this chapter
Lennard, W., Goddek, S. (2019). Aquaponics: The Basics. In: Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M. (eds) Aquaponics Food Production Systems. Springer, Cham. https://doi.org/10.1007/978-3-030-15943-6_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-15943-6_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-15942-9
Online ISBN: 978-3-030-15943-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)