Abstract
Hydroponics is a method to grow crops without soil, and as such, these systems are added to aquaculture components to create aquaponics systems. Thus, together with the recirculating aquaculture system (RAS), hydroponic production forms a key part of the aqua-agricultural system of aquaponics. Many different existing hydroponic technologies can be applied when designing aquaponics systems. This depends on the environmental and financial circumstances, the type of crop that is cultivated and the available space. This chapter provides an overview of different hydroponic types, including substrates, nutrients and nutrient solutions, and disinfection methods of the recirculating nutrient solutions.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
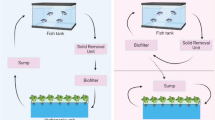
In horticultural crop production, the definition soilless cultivation encompasses all the systems that provide plant production in soilless conditions in which the supply of water and of minerals is carried out in nutrient solutions with or without a growing medium (e.g. stone wool, peat, perlite, pumice, coconut fibre, etc.). Soilless culture systems, commonly known as hydroponic systems, can further be divided into open systems, where the surplus nutrient solution is not recycled, and closed systems, where the excess flow of nutrients from the roots is collected and recycled back into the system (Fig. 4.1).
Soilless culture systems have evolved as one possible solution to avoid soil-borne diseases that have always been a problem in the greenhouse cultivation industry.
Nowadays, soilless growing systems are common in horticultural practice in most European countries, although not in every country does this occur on a large scale. The advantages of soilless systems compared to soil grown crops are:
-
Pathogen-free start with the use of substrates other than soil and/or easier control of soil-borne pathogens.
-
Growth and yield are independent of the soil type/quality of the cultivated area.
-
Better control of growth through a targeted supply of nutrient solution.
-
The potential for reusing the nutrient solution allowing for maximizing resources.
-
Increased quality of produce gained by the better control of other environmental parameters (temperature, relative humidity) and pests.
In most cases, open loop or run-to-waste systems rather than closed loop or recirculation systems are adopted, although in more and more European countries the latter are mandatory. In these open systems, the spent and/or superfluous nutrient solution is deposited into the ground and surface water bodies, or it is used in open field cultivation. However, regarding economics and environmental concerns, soilless systems should be as closed as possible, i.e. where recirculation of the nutrient solution occurs, where the substrate is reused and where more sustainable materials are used.
The advantages of closed systems are:
-
A reduction in the amount of waste material.
-
Less pollution of ground and surface water.
-
A more efficient use of water and fertilizers.
-
Increased production because of better management options.
-
Lower costs because of the savings in materials and higher production.
There are also a number of disadvantages such as:
-
The required high water quality.
-
High investments.
-
The risk of rapid dispersal of soil-borne pathogens by the recirculating nutrient solution.
-
Accumulation of potential phytotoxic metabolites and organic substances in the recirculating nutrient solution.
In commercial systems, the problems of pathogen dispersal are tackled by disinfecting the water through physical, chemical and/or biological filtration techniques. However, one of the main factors that hinder the use of recirculating nutrient solution culture for greenhouse crops is the accumulation of salts in the irrigation water. Typically, there is a steady increase in electrical conductivity (EC) due to the accumulation of ions, which are not fully absorbed by the crops. This may be especially true in aquaponic (AP) settings where sodium chloride (NaCl), incorporated in the fish feed, may accumulate in the system. To amend this problem, it has been suggested that an added desalination step could improve the nutrient balance in multi-loop AP systems (Goddek and Keesman 2018).
2 Soilless Systems
The intense research carried out in the field of hydroponic cultivation has led to the development of a large variety of cultivation systems (Hussain et al. 2014). In practical terms all of these can also be implemented in combination with aquaculture; however, for this purpose, some are more suitable than others (Maucieri et al. 2018). The great variety of systems that may be used necessitates a categorization of the different soilless systems (Table 4.1).
2.1 Solid Substrate Systems
At the start of soilless cultivation in the 1970s, many substrates were tested (Wallach 2008; Blok et al. 2008; Verwer 1978). Many failed for reasons such as being too wet, too dry, not sustainable, too expensive and releasing of toxic substances. Several solid substrates survived: stone wool, perlite, coir (coconut fibre), peat, polyurethane foam and bark. Solid substrate systems can be divided as follows:
Fibrous Substrates
These may be organic (e.g. peat, straw and coconut fibre) or inorganic (e.g. stone wool). They are characterized by the presence of fibres of different sizes, which give the substrate a high water-retention capacity (60–80%) and a modest air capacity (free porosity) (Wallach 2008). A high percentage of the retained water is easily available for the plant, which is directly reflected in the minimum volume of substrate per plant required to guarantee a sufficient water supply. In these substrates there are no obvious water and salinity gradients along the profile, and, consequently, the roots tend to grow faster, evenly and abundantly, using the entire available volume.
Granular Substrates
They are generally inorganic (e.g. sand, pumice, perlite, expanded clay) and are characterized by different particle sizes and thus textures; they have high porosity and are free draining. Water-holding capacity is rather poor (10–40%), and much of the water retained is not easily available to the plant (Maher et al. 2008). Therefore, the required volume of substrate per plant is higher compared to the fibrous ones. In granular substrates, a marked gradient of moisture is observed along the profile and this causes the roots to develop mainly on the bottom of containers. Smaller particle sizes, increase in the capacity for water retention, moisture homogeneity and greater EC and a lower volume of the substrate are required for the plant.
Substrates are usually enveloped in plastic coverings (so-called grow bags or slabs) or inserted in other types of containers of various sizes and of synthetic materials.
Before planting the substrate should be saturated in order to:
-
Provide adequate water and nutrients supply in the entire substrate slab.
-
Achieve uniform EC and pH levels.
-
Expel the presence of air and make a homogeneous wetting of the material.
It is equally important for a substrate dry phase after planting to stimulate the plants to evolve homogeneous substrate exploration by roots to obtain an abundant and well-distributed root system at the various levels and to expose the roots to air. Using a substrate for the second time by rewetting may be a problem because saturation is not possible due to drain holes in the plastic envelope. In an organic substrate (such as coir), adopting short and frequent irrigation turns, it is possible to recover the water-retention capability to use it for the second time, more easily than inert substrates (stone wool, perlite) (Perelli et al. 2009).
2.2 Substrates for Medium-Based Systems
A substrate is necessary for the anchorage of the roots, a support for the plant and also as a water-nutritional mechanism due to its microporosity and cation exchange capacity.
Plants grown in soilless systems are characterized by an unbalanced shoot/root ratio, demands for water, air and nutrients that are much greater than in open field conditions. In the latter case, the growth rates are slower, and the quantities of substrate are theoretically unlimited. To satisfy these requirements, it is necessary to resort to substrates which, alone or in mixture, ensure optimal and stable chemical–physical and nutritional conditions. An array of materials with different characteristics and costs can be used as substrates as illustrated in Fig. 4.2. However, as yet, there is no one substrate that can be used universally in all cultivation situations.
2.3 Characterization of Substrates
Bulk Density (BD)
BD is expressed by the dry weight of the substrate per unit of volume. It enables the anchorage of the roots and offers plant support. The optimum BD for crops in a container varies between 150 and 500 kg m−3 (Wallach 2008). Some substrates, because of their low BD and their looseness, as is the case of perlite (ca. 100 kg m−3), polystyrene in granules (ca. 35 kg m−3) and non-compressed sphagnum peat (ca. 60 kg m−3), are not suitable for use alone, especially with plants that grow vertically.
Porosity
The ideal substrate for potted crops should have a porosity of at least 75% with variable percentages of macropores (15–35%) and micropores (40–60%) depending on the cultivated species and the environmental and crop conditions (Wallach 2008; Blok et al. 2008; Maher et al. 2008). In small-sized containers, total porosity should reach 85% of the volume (Bunt 2012). The structure should be stable over time and should resist compaction and the reduction of volume during dehydration phases.
Water-Holding Capacity
Water-holding capacity ensures adequate levels of substrate moisture for crops, without having to resort to frequent irrigations. However, the water-holding capacity must not be too high in order to avoid root asphyxia and too much cooling. The water available for the plant is calculated by the difference between the quantity of water at the retention capacity and that retained at the wilting point. This should be around 30–40% of the apparent volume (Kipp et al. 2001). Finally, it must be considered that with the constant increase of the root system biomass during growth, the free porosity in the substrate is gradually reduced and the hydrological characteristics of the substrate are modified.
Cation Exchange Capacity (CEC)
CEC is a measure of how many cations can be retained on substrate particle surfaces. In general, organic materials have a higher CEC and a higher buffer capability than mineral ones (Wallach 2008; Blok et al. 2008) (Table 4.2).
pH
A suitable pH is required to suit the needs of the cultivated species. Substrates with a low pH are more suitable for crops in containers, as they are more easily modified towards the desired levels by adding calcium carbonate and also because they meet the needs of a wider number of species. Moreover, during cultivation the pH value tends to rise due to irrigation with water rich in carbonates. The pH may also vary in relation to the type of fertilizer used. It is more difficult to correct an alkaline substrate. This can however be achieved by adding sulphur or physiologically acid fertilizers (ammonium sulphate, potassium sulphate) or constitutionally acid fertilizers (mineral phosphate).
Electrical Conductivity (EC)
Substrates should have a known nutrient content and low EC values, (see also Table 4.4). It is often preferable to use a chemically inert substrate and to add the nutrients in relation to the specific crop requirements. Particular attention has to be paid to the EC levels. High EC levels indicate the presence of ions (e.g. Na+) that, although not being important as nutrients, can play a decisive role in the suitability of the substrate.
Health and Safety
Health in the systems and safety for operatives are provided by the absence of pathogens (nematodes, fungi, insects), potentially phytotoxic substances (pesticides) and weed seeds. Some industrially produced materials (expanded clay, perlite, stone wool, vermiculite and polystyrene) guarantee high levels of sterility due to the high temperatures applied during their processing.
Sustainability
Another important characteristic of a substrate is its sustainability profile. Many commonly used substrates face ecological challenges relating to their provenance, production process and/or subsequent processing and end-of-life footprint. In this regard, substrates originating from materials with a low ecological footprint (modified in an environmentally friendly way and ultimately biodegradable) are an extra characteristic to consider. Reusability of the substrate can also be an important aspect of the sustainability of a substrate.
Cost
Last but not least, the substrate must be inexpensive or at least cost-effective, readily available and standardized from the chemical–physical point of view.
2.4 Type of Substrates
The choice of substrates ranges from products of organic or mineral origin which are present in nature and which are subjected to special processing (e.g. peat, perlite, vermiculite), to those of organic origin derived from human activities (e.g. waste or by-products of agricultural, industrial and urban activities) and of industrial origin obtained by synthesis processes (e.g. polystyrene).
2.4.1 Organic Materials
This category includes natural organic substrates, including residues, waste and by-products of organic nature derived from agricultural (manure, straw, etc.) or, for example, industrial, by-products of the wood industry, etc. or from urban settlements, e.g. sewage sludge, etc. These materials can be subjected to additional processing, such as extraction and maturation.
All the materials that can be used in hydroponics can also be used in AP. However, as the bacterial load in an AP solution may be higher than in conventional hydroponic solutions, it can therefore be expected that organic substrates may be prone to an increased decomposition rate, causing substrate compaction and root aeration problems. Therefore, organic materials can be considered for crops with a shorter growth cycle, whilst mineral substrates may be preferred for crops with a long growth cycle.
Peat
Peat, used alone or with other substrates, is currently the most important material of organic origin for substrate preparation. The term peat refers to a product derived from residues of bryophytes (Sphagnum), Cyperaceae (Trichophorum, Eriophorum, Carex) and others (Calluna, Phragmites, etc.) transformed in anaerobic conditions.
Raised bogs are formed in cold and very rainy environments. Rainwater, without salts, is retained on the surface by mosses and vegetable residues, creating a saturated environment. In raised bogs we can distinguish a deeper, much decomposed layer of dark colour (brown peat) and a slightly decomposed, shallower layer of a light colour (blond peat). Both of the peats are characterized by good structural stability, very low availability of nutrients and acidic pH whilst they mainly differ in their structure (Table 4.2).
Brown peats, with very small pores, have a higher water-retention capacity and less free porosity for air and have higher CEC and buffer capability. Physical characteristics vary in relation to the particle size that allows water absorption from 4 up to 15 times its own weight. Raised bogs usually satisfy the requirements needed for a good substrate. Moreover, they have constant and homogenous properties, and so they can be industrially exploited. However, the use of these peats requires pH corrections with, e.g. calcium carbonate (CaCO3). Generally, for a sphagnum peat with a pH 3–4, 2 kg m−3 of CaCO3 should be added to increase the pH for one unit. Attention must be paid to avoid the complete drying of the substrate. It should also be taken into account that peat is subjected to microbiological decomposition processes which, over time, may increase water-retention capacity and reduce the free porosity.
Fen bogs are mainly present in temperate areas (e.g. Italy and western France), where Cyperaceae, Carex and Phragmites are dominant. These peats are formed in the presence of stagnant water. The oxygen, salts and calcium content in the water allow for a faster decomposition and humification, compared to that which occurs in the raised bogs. This results in a very dark, brown to black peat with a higher nutrient content, in particular nitrogen and calcium, a higher pH, higher bulk density and much lower free porosity (Table 4.2). They are rather fragile in the dry state, and have a remarkable plasticity in the humid state, which confers high susceptibility to compression and deformation. The carbon/nitrogen (C/N) ratio is generally between 15 and 48 (Kuhry and Vitt 1996; Abad et al. 2002). Because of its properties, black peat is of low value and is not suitable as a substrate, but can be mixed with other materials.
It should be noted that in some countries there is a drive to reduce peat use and extraction to reduce environmental effects and various peat substitutes have been identified with varied success.
Coconut Fibre
Coconut fibre (coir) is obtained from removing the fibrous husks of coconuts and is a by-product of the copra (coconut oil production) and fibre extraction industry, and is composed almost exclusively of lignin. Before use, it is composted for 2–3 years, and then it is dehydrated and compressed. Prior to its use, it must be rehydrated by adding up to 2–4 times of its compressed volume with water. Coconut fibre possesses chemical–physical characteristics that are similar to blond peat (Table 4.2), but with the advantages of having a higher pH. It also has a lower environmental impact than peat (excessive exploitation of peat bogs) and stone wool where there are problems with disposal. This is one of the reasons why it is increasingly preferred in soilless systems (Olle et al. 2012; Fornes et al. 2003).
Wood-Based Substrates
Organic substrates which are derived from wood or its by-products, such as bark, wood chips or saw dust, are also used in global commercial plant production (Maher et al. 2008). Substrates based on these materials generally possess good air content and high saturated hydraulic conductivities. The disadvantages can include low water-retention capacities, insufficient aeration caused by microbial activity, inappropriate particle-size distribution, nutrient immobilization or negative effects due to salt and toxic compound accumulations (Dorais et al. 2006).
2.4.2 Inorganic Materials
This category includes natural materials (e.g. sand, pumice) and mineral products derived from industrial processes (e.g. vermiculite, perlite) (Table 4.3).
Sand
Sands are natural inorganic material with particles between 0.05 and 2.0 mm diameter, originating from the weathering of different minerals. The chemical composition of sands may vary according to origin, but in general, it is constituted by 98.0–99.5% silica (SiO2) (Perelli et al. 2009). pH is mainly related to the carbonate content. Sands with lower calcium carbonate content and pH 6.4–7.0 are better suited as substrate material because they do not influence the solubility of phosphorus and some microelements (e.g. iron, manganese). Like all mineral-origin substrates, sands have a low CEC and low buffering capability (Table 4.3). Fine sands (0.05–0.5 mm) are the most suitable for use in hydroponic systems in mixtures 10–30% by volume with organic materials. Coarse sands (>0.5 mm) can be used in order to increase the drainage capacity of the substrate.
Pumice
Pumice comprises aluminium silicate of volcanic origin, being very light and porous, and may contain small amounts of sodium and potassium and traces of calcium, magnesium and iron depending on the place of origin. It is able to retain calcium, magnesium, potassium and phosphorus from the nutrient solutions and to gradually release these to the plant. It usually has a neutral pH, but some materials may have excessively high pH, good free porosity but low water-retention capacity (Table 4.3). The structure however tends to deteriorate fairly quickly, due to the easy breaking up of the particles. Pumice, added to peat, increases the drainage and aeration of the substrate. For horticulture use, pumice particles from 2 to 10 mm in diameter are preferred (Kipp et al. 2001).
Volcanic Tuffs
Tuffs derive from volcanic eruptions, with particles ranging between 2 and 10 mm diameter. They may have a bulk density ranging between 850 and 1100 kg m−3 and a water-retention capacity between 15% and 25% by volume (Kipp et al. 2001).
Vermiculite
Vermiculite comprises hydrous phyllosilicates of magnesium, aluminium and iron, which in the natural state have a thin lamellar structure that retains tiny drops of water. Exfoliated vermiculite is commonly used in the horticultural industry and is characterized by a high buffer capability and CEC values similar to those of the best peats (Table 4.3), but, compared to these, it has a higher nutrient availability (5–8% potassium and 9–12% magnesium) (Perelli et al. 2009). NH4 + is especially strongly retained by vermiculite; the activity of the nitrifying bacteria, however, allows the recovery of part of the fixed nitrogen. Similarly, vermiculite binds over 75% of phosphate in an irreversible form, whereas it has low absorbent capacity for Cl−, NO3 − and SO4 −. These characteristics should be carefully assessed when vermiculite is used as a substrate. The vermiculite structure is not very stable because of a low compression resistance and tends to deteriorate over time, reducing water drainage. It can be used alone; however, it is preferable to mix it with perlite or peat.
Perlite
Perlite comprises aluminium silicate of volcanic origin containing 75% SiO2 and 13% Al2O3. The raw material is crushed, sieved, compressed and heated to 700–1000 °C. At these temperatures, the little water contained in the raw material turns into vapour by expanding the particles into small whitish-grey aggregates which, unlike vermiculite, have a closed cell structure. It is very light and possesses high free porosity even after the soaking. It contains no nutrients, has negligible CEC and is virtually neutral (Table 4.3) (Verdonk et al. 1983). pH, however, can vary easily, because the buffer capacity is insignificant. pH ought to be controlled via the quality of the irrigation water and should not fall below 5.0 in order to avoid the phytotoxic effects of the aluminium. The closed cell structure allows water to be held only on the surface and in the spaces between the agglomerations, so the water-retention capacity is variable in relation to the dimensions of the agglomerations. It is marketed in different sizes, but the most suitable for horticulture are 2–5 mm diameter. It can be used as a substrate in rooting beds, because it ensures good aeration. In mixtures with organic materials, it enhances the softness, permeability and aeration of the substrate. Perlite can be reused for several years as long as it is sterilized between uses.
Expanded Clay
Expanded clay is obtained by treating clay powder at about 700 °C. Stable aggregates are formed, and, depending on the used clay material, they have variable values with regard to CEC, pH and bulk density (Table 4.3). Expanded clay can be used in mixtures with organic materials in the amounts of about 10–35% by volume, to which it provides more aeration and drainage (Lamanna et al. 1990). Expanded clays with pH values above 7.0 are not suitable for use in soilless systems.
Stone Wool
Stone wool is the most used substrate in soilless cultivation. It originates from the fusion of aluminium, calcium and magnesium silicates and carbon coke at 1500–2000 °C. The liquefied mixture is extruded in 0.05 mm diameter strands and, after compression and addition of special resins, the material assumes a very light fibrous structure with a high porosity (Table 4.3).
Stone wool is chemically inert and, when added to a substrate, it improves its aeration and drainage and also offers an excellent anchorage for plant roots. It is used alone, as a sowing substrate and for soilless cultivation. The slabs used for the cultivation can be employed for several production cycles depending on quality, as long as the structure is able to guarantee enough porosity and oxygen availability for root systems. Usually, after several crop cycles, the greater part of substrate porosity is filled with old, dead roots, and this is due to the compaction of the substrate over time. The result is a then a reduced depth of substrate where irrigation strategies may need adaptation.
Zeolites
Zeolites comprise hydrated aluminium silicates characterized by the capacity to absorb gaseous elements; they are high in macro- and microelements, they have high absorbent power and they have high internal surface (structures with 0.5 mm pores). This substrate is of great interest as it absorbs and slowly releases K+ and NH4 + ions, whilst it is not able to absorb Cl− and Na+, which are hazardous to plants. Zeolites are marketed in formulations which differ in the N and P content and which can be used in seed sowing, for the rooting of cuttings or during the cultivation phase (Pickering et al. 2002).
2.4.3 Synthetic materials
Synthetic materials include both low-density plastic materials and ion-exchange synthetic resins. These materials, called “expanded”, because they are obtained by a process of dilation at high temperatures, are not yet widely used, but they possess physical properties suitable to balance the characteristics of other substrates.
Expanded Polystyrene
Expanded polystyrene is produced in granules of 4–10 mm diameter with a closed cell structure. It does not decompose, is very light and has a very high porosity but with an extremely low water-retention capacity (Table 4.3). It has no CEC and virtually zero buffer capability, so it is added to the substrate (e.g. peat) exclusively to improve its porosity and drainage. The preferred particle size is 4–5 mm (Bunt 2012).
Polyurethane Foam
Polyurethane foam is a low-density material (12–18 kg m−3) with a porous structure that allows absorption of water equal to 70% of its volume. It is chemically inert, has an almost neutral pH (6.5–7.0), does not contain useful nutrients available to plants and does not undergo decomposition (Kipp et al. 2001). In the market it is possible to find it in the form of granules, rooting cubes or blocks. Like a stone wool, it can also be used for soilless cultivation.
2.5 Preparation of Mixed Cultivation Substrates
Mixed substrates can be useful to reduce overall substrate costs and/or to improve some characteristics of the original materials. For example, peat, vermiculite and coir can be added to increase water-retention capacity; perlite, polystyrene, coarse sand and expanded clay to increase free porosity and drainage; blonde peat to raise the acidity; higher quantities of organic material or suitable amounts of clay soil to increase CEC and buffer capability; and low decomposable substrates for increased durability and stability. The characteristics of the mixtures rarely represent the average of the components because with the mixing the structures are modified between the individual particles and consequently the relationship of physical and chemical characteristics. In general, mixtures with a low nutrient content are preferable, in order to be able to better manage cultivation. The right relationship among the different constituents of a mixture also varies with the environmental conditions in which it operates. At high temperatures it is rational to use components that possess a higher water-retention capacity and do not allow fast evaporation (e.g. peat) and, at the same time, are resilient to decomposition. In contrast, in humid environments, with low solar radiation, the components characterized by high porosity are preferred to ensure good drainage. In this case, it will be necessary to add coarse substrates such as sand, pumice, expanded clay and expanded polystyrene (Bunt 2012).
3 Types of Hydroponic Systems According to Water/Nutrient Distribution
3.1 Deep Flow Technique (DFT)
Deep flow technique (DFT), also known as deep water technique, is the cultivation of plants on floating or hanging support (rafts, panels, boards) in containers filled with 10–20 cm nutrient solution (Van Os et al. 2008) (Fig. 4.3). In AP this can be up to 30 cm. There are different forms of application that can be distinguished mainly by the depth and volume of the solution, and the methods of recirculation and oxygenation.
One of the simplest systems comprises 20–30 cm deep tanks, which can be constructed of different materials and waterproofed with polyethylene films. The tanks are equipped with floating rafts (several types are available from suppliers) that serve to support the plants above the water whilst the plants’ roots penetrate the water. The system is particularly interesting as it minimizes costs and management. For example, there is a limited need for the automation of the control and correction of the nutrient solution, particularly in short duration crops such as lettuce, where the relatively high volume of solution facilitates the replenishment of the nutrient solution only at the end of each cycle, and only the oxygen content needs to be monitored periodically. Oxygen levels should be above 4–5 mg L−1; otherwise, nutrient deficiencies may appear due to root systems uptake low performance. Circulation of the solution will normally add oxygen, or Venturi systems can be added which dramatically increase air into the system. This is especially important when water temperatures are greater than 23 °C, as such high temperatures may stimulate lettuce bolting.
3.2 Nutrient Film Technique (NFT)
The NFT technique is used ubiquitously and can be considered the classic hydroponic cultivation system, where a nutrient solution flows along and circulates in troughs with a 1–2 cm layer of water (Cooper 1979; Jensen and Collins 1985; Van Os et al. 2008) (Fig. 4.4). The recirculation of the nutrient solution and the absence of substrate represent one of the main advantages of the NFT system. An additional advantage is its great potential for automation to save on labour costs (planting & harvesting) and the opportunity to manage the optimal plant density during crop cycle. On the other hand, the lack of substrate and low water levels makes the NFT vulnerable to the failure of pumps, due to e.g. clogging or a failure in the power supply. Temperature fluctuations in the nutrient solution can cause plant stress followed by diseases.
The development of the root system, part of which remains suspended in air above the nutrient flow and which is exposed to an early ageing and loss of functionality, represents a major constraint as it prevents the production of long-cycle crops (over 4–5 months). Because of its high susceptibility to temperature variations, this system is not suitable for cultivation environments characterized by high levels of irradiation and temperature (e.g. southern areas of the Mediterranean basin). However, in response to these challenges, a multilayer NFT trough has been designed which allows for longer production cycles without clogging problems (NGS). It is made of a series of interconnected layers placed in a cascade, so that even in strong rooting plant species, such as tomatoes, the nutrient solution will still find its way to the roots by by-passing the root-clogged layer via a lower positioned layer.
3.3 Aeroponic Systems
The aeroponic technique is mainly aimed at smaller horticultural species, and has not yet been widely used due to the high investment and management costs. Plants are supported by plastic panels or by polystyrene, arranged horizontally or on inclined tops of growing boxes. These panels are supported by a structure made with inert materials (plastic, steel coated with plastic film, polystyrene boards), in order to form closed boxes where the suspended root system can develop (Fig. 4.5).
The nutrient solution is directly sprayed on the roots, which are suspended in the box in air, with static sprinklers (sprayers), inserted on pipes housed inside the box module. The spray duration is from 30 to 60 s, whilst the frequency varies depending on the cultivation period, the growth stage of the plants, the species and the time of day. Some systems use vibrating plates to create micro droplets of water which form a steam which condenses on the roots. The leachate is collected on the bottom of the box modules and conveyed to the storage tank, for reuse.
4 Plant Physiology
4.1 Mechanisms of Absorption
Amongst the main mechanisms involved in plant nutrition, the most important is the absorption which, for the majority of the nutrients, takes place in ionic form following the hydrolysis of salts dissolved in the nutrient solution.
Active roots are the main organ of the plant involved in nutrient absorption. Anions and cations are absorbed from the nutrient solution, and, once inside the plant, they cause the protons (H+) or hydroxyls (OH−) to exit which maintains the balance between the electric charges (Haynes 1990). This process, whilst maintaining the ionic equilibrium, can cause changes in the pH of the solution in relation to the quantity and quality of the nutrients absorbed (Fig. 4.6).
The practical implications of this process for the horticulturist are two-fold: to provide adequate buffer capability to the nutrient solution (adding bicarbonates if needed) and to induce slight pH changes with the choice of fertilizer. The effect of fertilizers on the pH relates to the different chemical forms of the used compounds. In the case of N, for example, the most commonly used form is nitric nitrogen (NO3 −), but when the pH should be lowered, nitrogen can be supplied as ammonium nitrogen (NH4 +). This form, when absorbed, induces the release of H+ and consequently an acidification of the medium.
Climatic conditions, especially air and substrate temperature and relative humidity, exert a major influence on the absorption of nutrients (Pregitzer and King 2005; Masclaux-Daubresse et al. 2010; Marschner 2012; Cortella et al. 2014). In general, the best growth occurs where there are few differences between substrate and air temperature. However, persistently high temperature levels in the root system have a negative effect. Sub-optimal temperatures reduce the absorption of N (Dong et al. 2001). Whilst NH4 + is effectively used at optimum temperatures, at low temperatures, the bacterial oxidation is reduced, causing accumulation within the plant that can produce symptoms of toxicity and damage to the root system and the aerial biomass. Low temperatures at the root level also inhibit the assimilation of K and P, as well as the P translocation. Although the available information regarding the effect of low temperatures on the absorption of micronutrients is less clear, it appears that Mn, Zn, Cu, and Mo uptake are most affected (Tindall et al. 1990; Fageria et al. 2002).
4.2 Essential Nutrients, Their Role and Possible Antagonisms
The appropriate management of plant nutrition must be based on basic aspects that are influenced by uptake and use of macro, and micro-nutrients (Sonneveld and Voogt 2009). Macro-nutrients are needed in relatively large amounts, whilst micro-nutrients or trace elements are needed in small amounts. Furthermore, nutrient availability to the plant in the case of the soilless systems presents more or less consistent phenomena of synergy and antagonism (Fig. 4.7).
Nitrogen (N)
Nitrogen is absorbed by plants to produce amino acids, proteins, enzymes and chlorophyll. The most used nitrogen forms for plant fertilization are nitrate and ammonium. Nitrates are quickly absorbed by the roots, are highly movable inside the plants and can be stored without toxic effects. Ammonium can be absorbed by plants only in low quantities and cannot be stored at high quantities because it exerts toxic effects. Quantities higher than 10 mg L−1 inhibit plant calcium and copper uptake, increase the shoot growth compared to root growth and result in a strong green colour of the leaves. Further excesses in ammonia concentration result in phytotoxic effects such as chlorosis along the leaves’ margins. Excess in nitrogen supply causes high vegetative growth, increase of crop cycle length, strong green leaf colour, low fruit set, high content of water in the tissues, low tissue lignification and high tissue nitrate accumulation. Commonly, nitrogen deficiency is characterized by a pale green colour of the older leaves (chlorosis), reduced growth and senescence advance.
Potassium (K)
Potassium is fundamental for cell division and extension, protein synthesis, enzyme activation and photosynthesis and also acts as a transporter of other elements and carbohydrates through the cell membrane. It has an important role in keeping the osmotic potential of the cell in equilibrium and regulating the stomatal opening. The first signs of deficiency are manifested in the form of yellowish spots that very quickly necrotize on the margins of the older leaves. Potassium deficient plants are more susceptible to sudden temperature drops, water stress and fungal attacks (Wang et al. 2013).
Phosphorus (P)
Phosphorus stimulates roots development, the rapid growth of buds and flower quantity. P is absorbed very easily and can be accumulated without damage to the plant. Its fundamental role is linked to the formation of high-energy compounds (ATP) necessary for plant metabolism. The average quantities requested by plants are rather modest (10–15% of the needs of N and K) (Le Bot et al. 1998). However, unlike what occurs in soil, P is easily leachable in soilless crops. The absorption of P appears to be reduced by low substrate temperatures (< 13 °C) or at increasing pH values (> 6.5) which can lead to deficiency symptoms (Vance et al. 2003). Under these conditions a temperature increase and/or pH reduction is more effective than additional amendments of phosphorus fertilizers. P excess can reduce or block the absorption of some other nutrients (e.g. K, Cu, Fe) (Fig. 4.7). Phosphorus deficiency manifests in a green-violet colour of the older leaves, which may follow chlorosis and necrosis in addition to the stunted growth of the vegetative apex. However, these symptoms are non-specific and make P deficiencies difficult to be identified (Uchida 2000).
Calcium (Ca)
Calcium is involved in cell wall formation, membrane permeability, cell division and extension. Good availability gives the plant greater resistance to fungal attacks and bacterial infections (Liu et al. 2014). The absorption is very closely linked to the water flow between roots and aerial parts. Its movement occurs through the xylem and is therefore particularly influenced by low temperatures at the root level, by reduced water supply (drought or salinity of the solution) or by excessive relative humidity of the air. As Ca is not mobile within the plant, deficiencies start from the most recently formed parts (Adams 1991; Adams and Ho 1992; Ho et al. 1993). The main symptoms are plant growth being stunted, deformation of the margins of the younger leaves, light green or sometimes chlorotic colouring of new tissues and a stunted root system without fine roots. The deficiencies are displayed in different ways, e.g. apical rot in tomato and/or marginal browning of leaves in lettuce.
Magnesium (Mg)
Magnesium is involved in the constitution of chlorophyll molecules. It is immobilized at pH values below 5.5 and enters into competition with the absorption of K and Ca (Fig. 4.7). Symptoms of deficiency are yellowing between leaf veins and internal chlorosis of the basal leaves. As Mg can be easily mobilized, magnesium-deficient plants will first break down chlorophyll in the older leaves and transport the Mg to younger leaves. Therefore, the first sign of magnesium deficiency is the interveinal chlorosis in older leaves, contrary to iron deficiency where interveinal chlorosis first appears in the youngest leaves (Sonneveld and Voogt 2009).
Sulphur (S)
Sulphur is required by the plant in quantities comparable to those of phosphorus, and in order to optimize its absorption, it must be present in a 1:10 ratio with nitrogen (McCutchan et al. 2003). It is absorbed as sulphate. The deficiencies are not easily detected, as the symptoms can be confused with those of nitrogen deficiency, except that the deficiency of nitrogen begins to manifest itself from the older leaves, whilst that of sulphur from the youngest ones (Schnug and Haneklaus 2005). S nutrition has a significant role in ameliorating the damages in photosynthetic apparatus caused by Fe-deficiency (Muneer et al. 2014).
Iron (Fe)
Iron is one of the most important micro-nutrients because it is key in many biological processes such as photosynthesis (Briat et al. 2015; Heuvelink and Kierkels 2016). To improve its absorption, the nutrient solution pH should be around 5.5–6.0, and the Mn content should not be allowed to become too high because the two elements subsequently enter into competition (Fig. 4.7). The optimal ratio of Fe–Mn is around 2:1 for most crops (Sonneveld and Voogt 2009). At low temperatures, the assimilation efficiency is reduced. The deficiency symptoms are characterized by interveinal chlorosis from the young leaves towards the older basal ones, and by reduced root system growth. Symptoms of deficiency are not always due to the low presence of Fe in the nutrient solution, but often they are due to the Fe unavailability for the plant. The use of chelating agents guarantees constant availability of Fe for the plant.
Chlorine (Cl)
Chlorine has been recently considered a micro-nutrient, even if its content in plants (0.2–2.0% dw) is quite high. It is easily absorbed by the plant and is very mobile within it. It is involved in the photosynthetic process and the regulation of the stomata opening. Deficiencies, which are rather infrequent, occur with typical symptoms of leaves drying out, especially at the margins. Much more widespread is the damage due to an excess of Cl that leads to conspicuous plant shrinkage which is relative to the different sensitivities of different species. To avoid crop damage, it is always advisable to check the Cl content in the water used to prepare nutrient solutions and choose suitable fertilizers (e.g. K2SO4 rather than KCl).
Sodium (Na)
Sodium, if in excess, is harmful to plants, as it is toxic and interferes with the absorption of other ions. The antagonism with K (Fig. 4.7), for example, is not always harmful because in some species (e.g. tomatoes), it improves the fruit taste, whereas in others (e.g. beans), it can reduce plant growth. Similar to Cl, it is important to know the concentration in the water used to prepare the nutrient solution (Sonneveld and Voogt 2009).
Manganese (Mn)
Manganese forms part of many coenzymes and is involved in the extension of root cells and their resistance to pathogens. Its availability is controlled by the pH of the nutrient solution and by competition with other nutrients (Fig. 4.7). Symptoms of deficiency are similar to those of the Fe except for the appearance of slightly sunken areas in the interveinal areas (Uchida 2000). Corrections can be made by adding MnSO4 or by lowering the pH of the nutrient solution.
Boron (B)
Boron is essential for fruit setting and seed development. The absorption methods are similar to those already described for Ca with which it can compete. The pH of the nutrient solution must be below 6.0 and the optimal level seems to be between 4.5 and 5.5. Symptoms of deficiency can be detected in the new structures that appear dark green, the young leaves greatly increase their thickness and have a leathery consistency. Subsequently they can appear chlorotic and then necrotic, with rusty colouring.
Zinc (Zn)
Zinc plays an important role in certain enzymatic reactions. Its absorption is strongly influenced by the pH and the P supply of the nutrient solution. pH values between 5.5 and 6.5 promote the absorption of Zn. Low temperature and high P levels reduce the amount of zinc absorbed by the plant. Zinc deficiencies occur rarely, and are represented by chlorotic spots in the interveinal areas of the leaves, very short internodes, leaf epinasty and poor growth (Gibson 2007).
Copper (Cu)
Copper is involved in respiratory and photosynthetic processes. Its absorption is reduced at pH values higher than 6.5, whilst pH values lower than 5.5 may result in toxic effects (Rooney et al. 2006). High levels of ammonium and phosphorus interact with Cu reducing the availability of the latter. The excessive presence of Cu interferes with the absorption of Fe, Mn and Mo. The deficiencies are manifested by interveinal chlorosis which leads to the collapse of the leaf tissues that look like desiccated (Gibson 2007).
Molybdenum (Mo)
Molybdenum is essential in protein synthesis and in nitrogen metabolism. Contrary to other micro-nutrients, it is better available at neutral pH values. Symptoms of deficiency start with chlorosis and necrosis along the main rib of old leaves, whilst the young leaves appear deformed (Gibson 2007).
4.3 Nutrient Management in Relation to the Requirements of Plants
Since the development of soilless horticulture systems in the 1970s (Verwer 1978; Cooper 1979), different nutrient solutions have been developed and adjusted according to the growers’ preferences (Table 4.4; De Kreij et al. 1999). All mixes follow the principles of excess availability of all elements to prevent deficiencies and balance between (bivalent) cations to avoid competition between cations in plant nutrient uptake (Hoagland and Arnon 1950; Steiner 1961; Steiner 1984; Sonneveld and Voogt 2009). Commonly, the EC is allowed to rise in the root zone to a limited degree. In tomatoes, for example, the nutrient solution typically has an EC of ca. 3 dS m−1, whilst in the root zone in the stone wool slabs, the EC may rise to 4–5 dS m−1. However, in northern European countries, for the first irrigation of new stone wool slabs at the beginning of the production cycle, the nutrient solution may have an EC as high as 5 dS m−1, saturating the stone wool substrate with ions up to an EC of 10 dS m−1, which will subsequently be flushed after 2 weeks. To provide sufficient flushing of the root zone, in a typical drip-irrigation stone wool slab system, about 20–50% of the dosed water is collected as drainage water. The drainage water is then recycled, filtered, mixed with fresh water and topped up with nutrients for use in the next cycle (Van Os 1994).
In tomato production, increasing the EC can be applied to enhance lycopene synthesis (promoting the bright red coloration of the fruits), total soluble solids (TSS) and fructose and glucose content (Fanasca et al. 2006; Wu and Kubota 2008). Furthermore, tomato plants have higher absorption rates for N, P, Ca and Mg and low absorption of K during the early (vegetative) stages. Once the plants start developing fruits, leaf production is slowed down leading to a reduction in N and Ca requirements, whilst K requirement increases (e.g. Zekki et al. 1996; Silber, Bar-Tal 2008). In lettuce, on the other hand, an increased EC may promote tip-burn disease during hot growing conditions. Huett (1994) showed a significant decrease in the number of leaves with tip-burn disease per plant when the EC was dropped from 3.6 to 0.4 dS m−1, as well as when the nutrient formulation K/Ca was reduced from 3.5:1 to 1.25:1. In AP the management of nutrients is more difficult than in hydroponics since they mainly depend on fish stock density, feed type and feeding rates.
4.4 Nutrient Solution Properties
Phosphorus is an element which occurs in forms that are strongly dependent on environmental pH. In the root zone, this element can be found as PO4 3−, HPO4 2− and H2PO4 − ions, where the last two ions are the main forms of P taken up by the plants. Thus, when the pH is slightly acidic (pH 5–6), the largest amount of P is presented in a nutrient solution (De Rijck and Schrevens 1997).
Potassium, calcium and magnesium are available to plants in a wide range of pH. However, the presence of other ions may interfere in their plant availability due to the formation of compounds with different grades of solubility. At a pH above 8.3, Ca2+ and Mg2+ ions easily precipitate as carbonates by reacting with CO3 2−. Also sulphate forms relatively strong complexes with Ca2+ and Mg2+ (De Rijck and Schrevens 1998). As pH increases from 2 to 9, the amount of SO4 2− forming soluble complexes with Mg2+ as MgSO4 and with K+ as KSO4 − increases (De Rijck and Schrevens 1999). In general, nutrient availability for plant uptake at pH above 7 may be restricted due to a precipitation of Boron, Fe2+, Mn2+, PO4 3−, Ca2+ and Mg2+ due to insoluble and unavailable salts. The most appropriate pH values of the nutrient solution for the development of crops lie between 5.5 and 6.5 (Sonneveld and Voogt 2009).
4.5 Water Quality and Nutrients
The quality of the supplied water is extremely important in hydroponic and AP systems. For long-term recirculation, the chemical composition should be well known and monitored frequently to avoid an imbalance in nutrient supply but also to avoid the accumulation of certain elements leading to toxicity. De Kreij et al. (1999) made an overview of the chemical demands on water quality for hydroponic systems.
Before starting, an analysis of the water supply has to be made on the macro- and microelements. Based on the analysis, a scheme for the nutrient solution can be made. For example, if rainwater is used, special attention has to be made for Zn when collection takes place via untreated gutters. In tap water, problems may appear with Na, Ca, Mg, SO4 and HCO3. Furthermore, surface and bore hole water may be used which may also contain amounts of Na, Cl, K, Ca, Mg, SO4 and Fe but also microelements as Mn, Zn, B and Cu. It should be noted that all valves and pipes should be made of synthetic materials such as PVC and PE, and not containing Ni or Cu parts.
It often happens that water supplies contain a certain amount of Ca and Mg; therefore, the contents have to be subtracted from the amount in the nutrient solution to avoid accumulation of these ions. HCO3 has to be compensated preferably by nitric acid, about 0.5 mmol L−1 that can be maintained as a pH buffer in the nutrient solution. Phosphoric and sulphuric acid can also be possibly used to compensate pH, but both will rapidly give a surplus of H2PO4 − or SO4 2− in the nutrient solution. In AP systems nitric acid (HNO3) and potassium hydroxide (KOH) can be also used to regulate pH and at same time supply macronutrients in the system (Nozzi et al. 2018).
4.5.1 Water Quality Management
For the formulation of nutrient solutions, simple fertilizers (granular, powder or liquid) and substances (e.g. acid compounds) that affect the pH are preferably used. The integration of the nutrient elements into the solution takes into account the optimum values of the quantities of each element. This has to be made in relation to the requirements of the species and its cultivars considering phenological phases and substrate. The calculation of nutrient supplements must be carried out considering the conditions of the water used, according to a strict set of priorities. On the priority scale, magnesium and sulphates are positioned at the bottom, at the same level, because they have less nutritional importance and the plants do not present damage even if their presence is abundant in the nutrient solution. This characteristic has an advantageous practical feedback as it allows an exploitation of the two elements in order to balance the nutritional composition with respect to other macronutrients whose deficiency or excess may be negative for production. As an example, we can consider a nutrient solution where an integration of only potassium or only nitrate is required. The salts to be used, in this case, are respectively potassium sulphate or magnesium nitrate. In fact, if the most common potassium nitrate or calcium nitrate were used, the levels of nitrate, in the first case, and calcium, in the second case, would automatically increase. Moreover, when the analysis of the water used shows an imbalance between cations and anions, and in order to be able to calculate a nutrient solution with the EC in equilibrium, the correction of the water values is carried out reducing the levels of magnesium and/or sulphates.
The following points provide guidelines for the formulation of nutrient solutions:
-
1.
Definition of the species and cultivar requirements. Consideration of the cultivation environment and the characteristics of water need to be taken into account. In order to satisfy the needs of the plants in warm periods and with intense radiation, the solution must possess a lower EC and K content, which contrasts to a higher quantity of Ca. Instead, when the temperature and brightness reach sub-optimal levels, it is advisable to raise the values of the EC and K by reducing those of the Ca. It is important to note regarding cultivars that there are substantial variations, especially for the values of the NO3 −, due to the different vegetative vigorousness of the cultivars. For tomatoes, in fact, 15 mmol L−1 of NO3 − is used on average (Table 4.4), and in the case of cultivars characterized by low vegetative vigour and in certain phenological phases (e.g. fruit setting of the fourth trusses), up to 20 mM L−1 of NO3 − is adopted. In case that some elements such as Na are present in the water, in order to reduce its effect, which is particularly negative for some crops, it will be necessary to increase the amount of NO3 − and Ca and possibly decrease the K, keeping the EC at the same level.
-
2.
Nutrient requirement calculations should be obtained by subtracting the values of the chemical elements of the water from the chemical elements defined above. For example, the established need for Mg of peppers (Capsicum sp.) is 1.5 mM L−1, having the water at 0.5 mM L−1, and 1.0 mM L−1 of Mg should be added to the water (1.5 requirement – 0.5 water supply = 1.0).
-
3.
Choice and calculation of fertilizers and acids to be used. For example, having to provide Mg, as in the example of point 2 above, MgSO4 or Mg(NO3)2 can be used. A decision will be made taking into account the collateral contribution of sulphate or nitrate as well.
4.6 Comparison Between Hydroponic and Aquaponic Production
During their life cycle, plants need several essential macro- and microelements for regular development (boron, calcium, carbon, chlorine, copper, hydrogen, iron, magnesium, manganese, molybdenum, nitrogen, oxygen, phosphorous, potassium, sulphur, zinc), usually absorbed from the nutrient solution (Bittsanszky et al. 2016). The nutrient concentration and ratio amongst them are the most important variables capable to influence plant uptake. In AP systems fish metabolic wastes contain nutrients for the plants, but it must be taken into account, especially at commercial scales, that the nutrient concentrations supplied by the fish in AP systems are significantly lower and unbalanced for most nutrients compared to hydroponic systems (Nicoletto et al. 2018). Usually, in AP, with appropriate fish stocking rates, the levels of nitrate are sufficient for good plant growth, whereas the levels of K and P are generally insufficient for maximum plant growth. Furthermore, calcium and iron could also be limited. This can reduce the crop yield and quality and so nutrient integration should be carried out to support an efficient nutrient reuse. Microbial communities play a crucial role in the nutrient dynamic of AP systems (Schmautz et al. 2017), converting ammonium to nitrate, but also contributing to the processing of particulate matter and dissolved waste in the system (Bittsanszky et al. 2016). Plant uptake of N and P represents only a fraction of the amount removed from the water (Trang and Brix 2014), indicating that microbial processes in the root zone of the plants, and in the substrate (if present) and throughout the whole system, play a major role.
The composition of fish feeds depends on the type of fish and this influences nutrient release from fish’s metabolic output. Typically, fish feed contains an energy source (carbohydrates and/or lipids), essential amino acids, vitamins, as well as other organic molecules that are necessary for normal metabolism but some that the fish’s cells cannot synthesize. Furthermore, it must be taken into account that a plant’s nutritional requirements vary with species (Nozzi et al. 2018), variety, life cycle stage, day length and weather conditions and that recently (Parent et al. 2013; Baxter 2015), the Liebig’s law (plant growth is controlled by the scarcest resource) has been superseded by complex algorithms that consider the interactions between the individual nutrients. Both these aspects do not allow a simple evaluation of the effects of changes in nutrient concentrations in hydroponic or AP systems.
The question thus arises whether it is necessary and effective to add nutrients to AP systems. As reported by Bittsanszky et al. (2016), AP systems can only be operated efficiently and thus successfully, if special care is taken through the continuous monitoring of the chemical composition of the recirculating water for adequate concentrations and ratios of nutrients and of the potentially toxic component, ammonium. The necessity to add nutrients depends on plant species and growth stage. Frequently, although fish density is optimal for nitrogen supply, the addition of P and K with mineral fertilizers, at least, should be carried out (Nicoletto et al. 2018). In contrast to, for example, lettuce, tomatoes which need to bear fruit, mature and ripen, need supplemental nutrients. In order to calculate these needs, a software can be used, such as HydroBuddy which is a free software (Fernandez 2016) that is used to calculate the amount of required mineral nutrient supplements.
5 Disinfection of the Recirculating Nutrient Solution
To minimize the risk of spreading soil-borne pathogens, disinfection of the circulating nutrient solution is required (Postma et al. 2008). Heat treatment (Runia et al. 1988) was the first method used. Van Os (2009) made an overview for the most important methods and a summary is given below. Recirculating of the nutrient solution opens possibilities to save on water and fertilizers (Van Os 1999). The big disadvantage of the recirculation of the nutrient solution is the increasing risk of spreading root-borne pathogens all over the production system. To minimize such risks, the solution should be treated before reuse. The use of pesticides for such a treatment is limited as effective pesticides are not available for all such pathogens, and if available, resistance may appear, and environmental legislation restricts discharge of water with pesticides (and nutrients) into the environment (European Parliament and European Council 2000). In addition, in AP systems, the use of pesticides exerts negative effects on fish health and cannot be carried out, even if hydroponic and AP parts of the system are in different rooms, because spraying of chemicals may enter the nutrient solution via condensation water or via direct spraying on the substrate slabs. In view of this, a biological control approach can be adopted to manage pest diseases, and this can be accessed via the EU Aquaponics Hub Fact Sheet (EU Aquaponics Hub). At the same time, similar problems can be observed for fish treatment using veterinary drugs that are not compatible with the plant’s cycle.
5.1 Description of Disinfection Methods
Disinfection of the circulating nutrient solution should take place continuously. All drain returned (10–12 h during daytime) has to be treated within 24 h. For a greenhouse of 1000 m2 in a substrate cultivation (stone wool, coir, perlite), a disinfection capability of about 1–3 m3 per day is needed to disinfect an estimated needed surplus of 30% of the water supplied with drip irrigation to tomato plants during a 24-h period in summer conditions. Because of the variable return rate of drain water, a sufficiently large catchment tank for drain water is needed in which the water is stored before it is pumped to the disinfection unit. After disinfection another tank is required to store the clean water before adjusting EC and pH and blending with new water to supply to the plants. Both tanks have an average size of 5 m3 per 1000 m2. In a nutrient film system (NFT), about 10 m3 per day should be disinfected daily. It is generally considered that such a capacity is uneconomical to disinfect (Ruijs 1994). DFT requires similar treatment. This is the main reason why NFT and DFT production units do not normally disinfect the nutrient solution. Disinfection is carried out either by non-chemical or chemical methods as follows:
5.1.1 Non-chemical Methods
In general these methods do not alter the chemical composition of the solution, and there is no build-up of residuals:
-
1.
Heat treatment. Heating the drain water to temperatures high enough to eradicate bacteria and pathogens is the most reliable method for disinfection. Each type of organism has its own lethal temperature. Non-spore-forming bacteria have lethal temperatures between 40 and 60 °C, fungi between 40 and 85 °C, nematodes between 45 and 55 °C and viruses between 80 and 95°C (Runia et al. 1988) at an exposure time of 10 s. Generally, the temperature set point of 95 °C is high enough to kill most of the organisms that are likely to cause diseases with a minimum time of 10 s. Whilst this may seem very energy intensive, it should be noted that the energy is recovered and reused with heat exchangers. Availability of a cheap energy source is of greater importance for practical application.
-
2.
UV radiation. UV radiation is electromagnetic radiation with a wavelength between 200 and 400 nm. Wavelengths between 200 and 280 nm (UV-C), with an optimum at 254 nm, has a strong killing effect on micro-organisms, because it minimizes the multiplication of DNA chains. Different levels of radiation are needed for different organisms so as to achieve the same level of efficacy. Runia (1995) recommends a dose which varies from 100 mJ cm−2 for eliminating bacteria and fungi to 250 mJ cm−2 for eliminating viruses. These relatively high doses are needed to compensate for variations in water turbidity and variations in penetration of the energy into the solution due to low turbulence around the UV lamp or variations in output from the UV lamp. Zoschke et al. (2014) reviewed that UV irradiation at 185 and 254 nm offers water organic contaminant control and disinfection. Moreover, Moriarty et al. (2018) reported that UV radiation efficiently inactivated coliforms in AP systems.
-
3.
Filtration. Filtration can be used to remove any undissolved material out of the nutrient solution. Various types of filters are available relative to the range of particle sizes. Rapid sand filters are often used to remove large particles from the drain water before adding, measuring and control of EC, pH and application of new fertilizers. After passing the fertilizer unit, often a fine synthetic filter (50–80 um) is built in the water flow to remove undissolved fertilizer salts or precipitates to avoid clogging of the irrigation drippers. These synthetic filters are also used as a pretreatment for disinfection methods with heat treatment, ozone treatment or UV radiation. With reductions in filtration pore size, the flow is inhibited, so that removal of very small particles requires a combination of adequate filters and high pressure followed by frequent cleaning of the filter(s). Removal of pathogens requires relatively small pore sizes (<10 μm; so-called micro-, ultra- or nanofiltration).
5.1.2 Chemical Methods
-
1.
Ozone (O 3). Ozone is produced from dry air and electricity using an ozone-generator (converting 3O2 ➔ 2O3). The ozone-enriched air is injected into the water that is being sanitized and stored for a period of 1 h. Runia (1995) concluded that an ozone supply of 10 g per hour per m3 drain water with an exposure time of 1 h is sufficient to eliminate all pathogens, including viruses. The reduction of microbial populations in vegetable production in soilless systems managed with ozone has also been observed by Nicoletto et al. (2017). Human exposure to the ozone that vents from the system or the storage tanks should be avoided since even a short exposure time of a concentration of 0.1 mg L−1 of ozone may cause irritation of mucous membranes. A drawback of the use of ozone is that it reacts with iron chelate, as UV does. Consequently, higher dosages of iron are required and measures need to be taken to deal with iron deposits in the system. Recent research (Van Os 2017) with contemporary ozone installations looks promising, where complete elimination of pathogens and breakdown of remaining pesticides is achieved, with no safety problems.
-
2.
Hydrogen peroxide (H 2O 2). Hydrogen peroxide is a strong, unstable oxidizing agent that reacts to form H2O and an O·- radical. Commercially so-called activators are added to the solution to stabilize the original solution and to increase efficacy. Activators are mostly formic acid or acetic acid, which decrease pH in the nutrient solution. Different dosages are recommended (Runia 1995) against Pythium spp. (0.005%), other fungi (0.01%), such as Fusarium, and viruses (0.05%). The 0.05% concentration is also harmful to plant roots. Hydrogen peroxide is especially helpful for cleaning the watering system, whilst the use for disinfection has been taken over by other methods. The method is considered inexpensive, but not efficient.
-
3.
Sodium hypochlorite (NaOCl). Sodium hypochlorite is a compound having different commercial names (e.g. household bleach) with different concentrations but with the same chemical structure (NaOCl). It is widely used for water treatment, especially in swimming pools. The product is relatively inexpensive. When added to water, sodium hypochlorite decomposes to HOCl and NaOH− and depending on the pH to OCl−; the latter decomposes to Cl− and O. for strong oxidation. It reacts directly with any organic substance, and if there is enough hypochlorite, it also reacts with pathogens. Le Quillec et al. (2003) showed that the tenability of hypochlorite depends on the climatic conditions and the related decomposing reactions. High temperatures and contact with air cause rapid decomposition, at which NaClO3 is formed with phytotoxic properties. Runia (1995) showed that hypochlorite is not effective for eliminating viruses. Chlorination with a concentration of 1–5 mg Cl L−1 and an exposure time of 2 h achieved a reduction of 90–99.9% of Fusarium oxysporum, but some spores survived at all concentrations. Safety measures have to be taken for safe storage and handling. Hypochlorite might work against a number of pathogens, but not all, but at the same time, Na+ and Cl− concentration is increased in a closed growing system which will also lead to levels which decrease productivity of the crop and at which time the nutrient solution has to be leached. Despite the above-mentioned drawbacks, the product is used and recommended by commercial operatives as a cheap and useful method.
5.2 Chemical Versus Non-chemical Methods
Growers prefer disinfection methods with excellent performance in combination with low costs. A good performance can be described by eliminating pathogens with a reduction of 99.9% (or a log 3 reduction) combined with a clear, understandable and controllable process. Low costs are preferably combined with low investments, low maintenance costs and no need for the grower to perform as a laboratory specialist. Heat treatment, UV radiation and ozone treatment show a good performance. However, investments in ozone treatment are very high, resulting in high annual costs. Heat treatment and UV radiation also have high annual costs, but investments are lower, whilst the eliminating process is easy to control. The latter two methods are most popular among growers, especially at nurseries larger than 1 or 2 ha. Slow sand filtration is less perfect in performance but has considerably lower annual costs. This method could be recommended for producers smaller than 1 ha and for growers with lower investment capital, as sand filters can be constructed by the grower themselves. Sodium hypochlorite and hydrogen peroxide are also cheap methods, but performance is insufficient to eliminate all pathogens. Besides it is a biocide and not a pesticide, which means by law, in the EU at least, it is legally forbidden to use it for the elimination of pathogens.
5.3 Biofouling and Pretreatment
Disinfection methods are not very selective between pathogens and other organic material in the solution. Therefore, pretreatment (rapid sand filters, or 50–80 um mechanical filters) of the solution before disinfection is recommended at heat treatment, UV radiation and ozone treatment. If after disinfection residuals of the chemical methods remain in the water, they may react with the biofilms which have been formed in the pipe lines of the watering systems. If the biofilm is released from the walls of the pipes, they will be transported to the drippers and cause clogging. Several oxidizing methods (sodium hypochlorite, hydrogen peroxide with activators, chlorine dioxide) are mainly used to clean pipe lines and equipment, and these create a special risk for clogging drippers over time.
References
Abad M, Noguera P, Puchades R, Maquieira A, Noguera V (2002) Physico-chemical and chemical properties of some coconut coir dusts for use as a peat substitute for containerised ornamental plants. Bioresour Technol 82:241–245
Adams P (1991) Effect of increasing the salinity of the nutrient solution with major nutrients or sodium chloride on the yield, quality and composition of tomatoes grown in rockwool. J Hortic Sci 66:201–207
Adams P, Ho LC (1992) The susceptibility of modern tomato cultivars to blossom-end rot in relation to salinity. J Hortic Sci 67:827–839
Baxter I (2015) Should we treat the ionome as a combination of individual elements, or should we be deriving novel combined traits? J Exp Bot 66:2127–2131
Bittsanszky A, Uzinger N, Gyulai G, Mathis A, Junge R, Villarroel M, Kotzen B, Komives T (2016) Nutrient supply of plants in aquaponic systems. Ecocycles 2:17–20
Blok C, de Kreij C, Baas R, Wever G (2008) Analytical methods used in soilless cultivation. In: Raviv, Lieth (eds) Soilless culture, theory and practice. Elsevier, Amsterdam, pp 245–290
Briat JF, Dubos C, Gaymard F (2015) Iron nutrition, biomass production, and plant product quality. Trends Plant Sci 20:33–40
Bunt BR (2012) Media and mixes for container-grown plants: a manual on the preparation and use of growing media for pot plants. Springer, Dordrecht
Cooper A (1979) The ABC of NFT. Grower Books, London
Cortella G, Saro O, De Angelis A, Ceccotti L, Tomasi N, Dalla Costa L, Manzocco L, Pinton R, Mimmo T, Cesco S (2014) Temperature control of nutrient solution in floating system cultivation. Appl Therm Eng 73:1055–1065
De Kreij C, Voogt W, Baas R (1999) Nutrient solutions and water quality for soilless cultures, report 196. Research station for Floriculture and Glasshouse Vegetables, Naaldwijk, p 36
De Rijck G, Schrevens E (1997) pH influenced by the elemental composition of nutrient solutions. J Plant Nutr 20:911–923
De Rijck G, Schrevens E (1998) Elemental bioavailability in nutrient solutions in relation to complexation reactions. J Plant Nutr 21:2103–2113
De Rijck G, Schrevens E (1999) Anion speciation in nutrient solutions as a function of pH. J Plant Nutr 22:269–279
Dong S, Scagel CF, Cheng L, Fuchigami LH, Rygiewicz PT (2001) Soil temperature and plant growth stage influence nitrogen uptake and amino acid concentration of apple during early spring growth. Tree Physiol 21:541–547
Dorais M, Menard C, Begin G (2006) Risk of phytotoxicity of sawdust substrates for greenhouse vegetables. In: XXVII International Horticultural Congress-IHC2006: International symposium on advances in environmental control, automation 761, pp 589–595
Enzo M, Gianquinto G, Lazzarin R, Pimpini F, Sambo P (2001) Principi tecnico-agronomici della fertirrigazione e del fuori suolo. In: Tipografia-Garbin. Padova, Italy
EU Aquaponics Hub. Plant Protection Fact Sheet. http://euaquaponicshub.com/hub/wp-content/uploads/2016/05/Plant-protection-factsheet.pdf
European Parliament and European Council (2000) Directive 2000/60/EC of the European Parliament and the Council of 23 October 2000 establishing a framework for Community action in the field of water policy (short: Water Framework Directive) Off J Eur Communities, p 327/1
Fageria NK, Baligar VC, Clark RB (2002) Micronutrients in crop production. Adv Agron 77:185–268
Fanasca S, Colla G, Maiani G, Venneria E, Rouphael Y, Azzini E, Saccardo F (2006) Changes in antioxidant content of tomato fruits in response to cultivar and nutrient solution composition. J Agric Food Chem 54:4319–4325
Fernandez D (2016) HydroBuddy v1.50: The first free Open source hydroponic nutrient calculator program Available Online [WWW document]. Sci Hydroponics. http://scienceinhydroponics.com/
Fornes F, Belda RM, Abad M, Noguera P, Puchades R, Maquieira A, Noguera V (2003) The microstructure of coconut coir dusts for use as alternatives to peat in soilless growing media. Aust J Exp Agr 43:1171–1179
Gibson JL (2007) Nutrient deficiencies in bedding plants. Ball Publishing, Batavia
Goddek S, Keesman KJ (2018) The necessity of desalination technology for designing and sizing multi-loop aquaponics systems. Desalination 428:76–85
Haynes RJ (1990) Active ion uptake and maintenance of cation-anion balance: a critical examination of their role in regulating rhizosphere pH. Plant Soil 126:247–264
Heuvelink E, Kierkels T (2016) Iron is essential for photosynthesis and respiration: iron deficiency. In Greenhouses: the international magazine for greenhouse growers 5, pp 48–49
Ho LC, Belda R, Brown M (1993) Uptake and transport of calcium and the possible causes of blossom-end rot in tomato. J Exp Bot 44:509–518
Hoagland DR, Arnon DI (1950) The water culture method for growing plants without soil, Circular 347, Agricultural Experimental station, University of California, Berkeley, CA, USA
Huett DO (1994) Growth, nutrient uptake and tipburn severity of hydroponic lettuce in response to electrical conductivity and K:Ca ratio in solution. Aust J Agric Res 45:251–267
Hussain A, Iqbal K, Aziem S, Mahato P, Negi AK (2014) A review on the science of growing crops without soil (soilless culture)-a novel alternative for growing crops. Int J Agric Crop Sci 7:833–842
Jensen MH, Collins WL (1985) Hydroponic vegetable production. Hortic Rev 7:483–558
Kipp JA, Wever G, de Kreij C (2001) International substrate manual. Elsevier, Amsterdam
Kuhry P, Vitt DH (1996) Fossil carbon/nitrogen ratios as a measure of peat decomposition. Ecology 77:271–275
Lamanna D, Castelnuovo M, D’Angelo G (1990) Compost-based media as alternative to peat on ten pot ornamentals. Acta Hortic 294:125–130
Le Bot J, Adamowicz S, Robin P (1998) Modelling plant nutrition of horticultural crops: a review. Sci Hortic 74:47–82
Le Quillec S, Fabre R, Lesourd D (2003) Phytotoxicité sur tomate et chlorate de sodium. Infos-ctifl, pp 40–43
Liu S, Hou Y, Chen X, Gao Y, Li H, Sun S (2014) Combination of fluconazole with non-antifungal agents: a promising approach to cope with resistant Candida albicans infections and insight into new antifungal agent discovery. Int J Antimicrob Agents 43:395–402
Maher MJ, Prasad M, Raviv M (2008) Organic soilless media components. In: Raviv, Lieth (eds) Soilless culture, theory and practice. Elsevier, Amsterdam, pp 459–504
Marschner P (2012) Rhizosphere biology. In: Marschner’s mineral nutrition of higher plants, 3rd edn. Academic, Amsterdam, pp 369–388
Masclaux-Daubresse C, Daniel-Vedele F, Dechorgnat J, Chardon F, Gaufichon L, Suzuki A (2010) Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Ann Bot 105:1141–1157
Maucieri C, Nicoletto C, Junge R, Schmautz Z, Sambo P, Borin M (2018) Hydroponic systems and water management in aquaponics: a review. Ital J Agron 13:1–11
McCutchan JH, Lewis WM, Kendall C, McGrath CC (2003) Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102:378–390
Moriarty MJ, Semmens K, Bissonnette GK, Jaczynski J (2018) Inactivation with UV-radiation and internalization assessment of coliforms and Escherichia coli in aquaponically grown lettuce. LWT 89:624–630
Muneer S, Lee BR, Kim KY, Park SH, Zhang Q, Kim TH (2014) Involvement of Sulphur nutrition in modulating iron deficiency responses in photosynthetic organelles of oilseed rape (Brassica napus L.). Photosynth Res 119:319–329
NGS, Multilayer. http://ngsystem.com/en/ngs/multibanda
Nicoletto C, Maucieri C, Sambo P (2017) Effects on water management and quality characteristics of ozone application in chicory forcing process: a pilot system. Agronomy 7:29
Nicoletto C, Maucieri C, Mathis A, Schmautz Z, Komives T, Sambo P, Junge R (2018) Extension of aquaponic water use for NFT baby-leaf production: mizuna and rocket salad. Agronomy 8:75
Nozzi V, Graber A, Schmautz Z, Mathis A, Junge R (2018) Nutrient management in aquaponics: comparison of three approaches for cultivating lettuce, mint and mushroom herb. Agronomy 8:27
Olle M, Ngouajio M, Siomos A (2012) Vegetable quality and productivity as influenced by growing medium: a review. Agriculture 99:399–408
Parent S-É, Parent LE, Egozcue JJ, Rozane D-E, Hernandes A, Lapointe L, Hébert-Gentile V, Naess K, Marchand S, Lafond J, Mattos D, Barlow P, Natale W (2013) The plant ionome revisited by the nutrient balance concept. Front Plant Sci 4:39
Perelli M, Graziano PL, Calzavara R (2009) Nutrire le piante. Arvan Ed., Venice.
Pickering HW, Menzies NW, Hunter MN (2002) Zeolite/rock phosphate – a novel slow release phosphorus fertiliser for potted plant production. Sci Hortic 94:333–343
Postma J, van Os EA, Bonants PJM (2008) Pathogen detection and management strategies in soilless plant growing systems. In: Raviv, Lieth (eds) Soilless culture, theory and practice. Elsevier, Amsterdam, pp 425–458
Pregitzer KS, King JS (2005) Effects of soil temperature on nutrient uptake. In: Nutrient acquisition by plants. Springer, Berlin/Heidelberg, pp 277–310
Rooney CP, Zhao FJ, McGrath SP (2006) Soil factors controlling the expression of copper toxicity to plants in a wide range of European soils. Environ Toxicol Chem 25:726–732
Ruijs MNA (1994) Economic evaluation of closed production systems in glasshouse horticulture. Acta Hortic 340:87–94
Runia WT (1995) A review of possibilities for disinfection of recirculation water from soilless culture. Acta Hortic 382:221–229
Runia WT, van Os EA, Bollen GJ (1988) Disinfection of drain water from soilless cultures by heat treatment. Neth J Agric Sci 36:231–238
Schmautz Z, Graber A, Jaenicke S, Goesmann A, Junge R, Smits TH (2017) Microbial diversity in different compartments of an aquaponics system. Arch Microbiol 199:613–620
Schnug E, Haneklaus S (2005) Sulphur deficiency symptoms in oilseed rape (Brassica napus L.)-the aesthetics of starvation. Phyton 45:79–95
Silber A, Bar-Tal A (2008) Nutrition of substrate-grown plants. In: Raviv, Lieth (eds) Soilless culture, theory and practice. Elsevier, Amsterdam, pp 292–342
Sonneveld C, Voogt W (2009) Plant nutrition of greenhouse crops. Springer, Dordrecht, p 403
Steiner AA (1961) A universal method for preparing nutrient solutions of a certain desired composition. Plant Soil 15:134–154
Steiner AA (1984) The universal nutrient solution. In: Proceeding 6th International congress on soilless culture. Lunteren, Netherlands, ISOSC, pp 633–649
Tindall JA, Mills HA, Radcliffe DE (1990) The effect of root zone temperature on nutrient uptake of tomato. J Plant Nutr 13:939–956
Trang NTD, Brix H (2014) Use of planted biofilters in integrated recirculating aquaculture-hydroponics systems in the Mekong Delta, Vietnam. Aquac Res 45:460–469
Uchida R (2000) Essential nutrients for plant growth: nutrient functions and deficiency symptoms. In: Silva JA, Uchida R (eds) Plant nutrient management in Hawaii’s soils, approaches for tropical and subtropical agriculture. College of Tropical Agriculture and Human Resources, University of Hawaii at Manoa, Honolulu, pp 31–55
Van Os EA (1994) Closed growing systems for more efficient and environmental friendly production. Acta Hortic 361:194–200
Van Os EA (1999) Closed soilless growing systems: a sustainable solution for Dutch greenhouse horticulture. Water Sci Technol 39:105–112
Van Os EA (2009) Comparison of some chemical and non-chemical treatments to disinfect a recirculating nutrient solution. Acta Hortic 843:229–234
Van Os EA (2017) Recent advances in soilless culture in Europe. Acta Hortic 1176:1–8
Van Os EA, Gieling TH, Lieth JH (2008) Technical equipment in soilless production systems. In: Raviv, Lieth (eds) Soilless culture, theory and practice. Elsevier, Amsterdam, pp 157–207
Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157:423–447
Verdonck O, Penninck RD, De Boodt M (1983) The physical properties of different horticultural substrates. Acta Hortic 150:155–160
Verwer FLJA (1978) Research and results with horticultural crops grown in rockwool and nutrient film. Acta Hortic 82:141–148
Wallach J (2008) Physical characteristics of soilless media. In: Raviv, Lieth (eds) Soilless culture, theory and practice. Elsevier, Amsterdam, pp 41–116
Wang M, Zheng Q, Shen Q, Guo S (2013) The critical role of potassium in plant stress response. Int J Mol Sci 14:7370–7390
Wu M, Kubota C (2008) Effects of high electrical conductivity of nutrient solution and its application timing on lycopene, chlorophyll and sugar concentrations of hydroponic tomatoes during ripening. Sci Hortic 116:122–129
Zekki H, Gauthier L, Gosselin A (1996) Growth, productivity, and mineral composition of hydroponically cultivated greenhouse tomatoes, with or without nutrient solution recycling. J Am Soc Hortic Sci 121:1082–1088
Zoschke K, Börnick H, Worch E (2014) Vacuum-UV radiation at 185 nm in water treatment–a review. Water Res 52:131–145
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2019 The Author(s)
About this chapter
Cite this chapter
Maucieri, C., Nicoletto, C., Os, E.v., Anseeuw, D., Havermaet, R.V., Junge, R. (2019). Hydroponic Technologies. In: Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M. (eds) Aquaponics Food Production Systems. Springer, Cham. https://doi.org/10.1007/978-3-030-15943-6_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-15943-6_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-15942-9
Online ISBN: 978-3-030-15943-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)