Abstract
The quantification of Hepatitis C Virus (HCV) and Human Immunodeficiency Virus (HIV) in whole blood provides several advantages over the quantification in plasma samples. The application of small samples of capillary blood allows for application in point-of-care diagnostic testing methods.
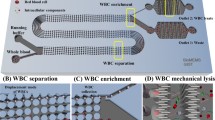
Here we describe two protocols of extracting viral RNA from small samples of whole blood by hybridization to biotinylated LNA-modified 2′-O-Methyl-RNA or to biotinylated DNA, indirect capturing to streptavidin-coated beads, and subsequent quantification by one-step non-nested qRT-PCR. Further, we provide some general guidelines on extraction and quantification of HIV and HCV in small volume whole blood samples.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Lavanchy D (2009) The global burden of hepatitis C. Liver Int 29(Suppl 1):74–81
Joint United Nations Programm on HIV/AIDS (UNAIDS) (2008) 2008 report on the global AIDS epidemic. World Health Organization
Mellors JW, Rinaldo CR, Gupta P, White RM, Todd JA, Kingsley LA (1996) Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 272(5265):1167–1170
Ghany MG, Strader DB, Thomas DL, Seeff LB (2009) Diagnosis, management, and treatment of hepatitis C: an update. Hepatology 49(4):1335–1374
O’Brien WA, Hartigan PM, Daar ES, Simberkoff MS, Hamilton JD (1997) Changes in plasma HIV RNA levels and CD4+ lymphocyte counts predict both response to antiretroviral therapy and therapeutic failure. Ann Intern Med 126(12):939–945
Gross R, Bilker WB, Friedman HM, Strom BL (2001) Effect of adherence to newly initiated antiretroviral therapy on plasma viral load. AIDS 15(16):2109–2117
Bouffard P, Hayashi PH, Acevedo R, Levy N, Zeldis JB (1992) Hepatitis C virus is detected in a monocyte/macrophage subpopulation of peripheral blood mononuclear cells of infected patients. J Infect Dis 166(6):1276–1280
Moldvay J, Deny P, Pol S, Brechot C, Lamas E (1994) Detection of hepatitis C virus RNA in peripheral blood mononuclear cells of infected patients by in situ hybridization. Blood 83(1):269–273
Manzin A, Solforosi L, Candela M, Cherubini G, Piccinini G, Brugia M, Gabrielli A, Clementi M (1996) Hepatitis C virus infection and mixed cryoglobulinaemia: assessment of HCV RNA copy numbers in supernatant, cryoprecipitate and non-liver cells. J Viral Hepat 3(6):285–292
Lotz G, Szalay F, Firneisz G, Abonyi M, Lengyel G, Telegdy L, Ibrányi E, Nemes B, Kury F, Schaff Z, Simon S (2002) Localization of hepatitis C virus RNA on human erythrocytes by RT in situ PCR technique. Scand J Gastroenterol 37(5):578–584
Hamaia S, Li C, Allain JP (2001) The dynamics of hepatitis C virus binding to platelets and 2 mononuclear cell lines. Blood 98(8):2293–2300
Sattentau QJ, Dalgleish AG, Weiss RA, Beverley PC (1986) Epitopes of the CD4 antigen and HIV infection. Science 234(4780):1120–1123
Kaiser P, Joos B, Niederöst B, Weber R, Günthard HF, Fischer M (2007) Productive human immunodeficiency virus type 1 infection in peripheral blood predominantly takes place in CD4/CD8 double-negative T lymphocytes. J Virol 81(18):9693–9706
Zhu T, Muthui D, Holte S, Nickle D, Feng F, Brodie S, Hwangbo Y, Mullins JI, Corey L (2002) Evidence for human immunodeficiency virus type 1 replication in vivo in CD14(+) monocytes and its potential role as a source of virus in patients on highly active antiretroviral therapy. J Virol 76(2):707–716
Hess C, Klimkait T, Schlapbach L, Del Zenero V, Sadallah S, Horakova E, Balestra G, Werder V, Schaefer C, Battegay M, Schifferli J (2002) Association of a pool of HIV-1 with erythrocytes in vivo: a cohort study. Lancet 359(9325):2230–2234
Bruisten S, van Gemen B, Koppelman M, Rasch M, van Strijp D, Schukkink R, Beyer R, Weigel H, Lens P, Huisman H (1993) Detection of HIV-1 distribution in different blood fractions by two nucleic acid amplification assays. AIDS Res Hum Retroviruses 9(3):259–265
Bruns T, Steinmetzer K, Ermantraut E, Stallmach A (2009) Hepatitis C virus RNA quantitation in venous and capillary small-volume whole-blood samples. J Clin Microbiol 47(10):3231–3240
Steinmetzer K, Seidel T, Stallmach A, Ermantraut E (2010) HIV load testing with small samples of whole blood. J Clin Microbiol 48(8):2786–2792
Cotten M, Oberhauser B, Brunar H, Holzner A, Issakides G, Noe CR, Schaffner G, Wagner E, Birnstiel ML (1991) 2′-O-methyl, 2′-O-ethyl oligoribonucleotides and phosphorothioate oligodeoxyribonucleotides as inhibitors of the in vitro U7 snRNP-dependent mRNA processing event. Nucleic Acids Res 19(10):2629–2635
Kumar R, Singh SK, Koshkin AA, Rajwanshi VK, Meldgaard M, Wengel J (1998) The first analogues of LNA (locked nucleic acids): phosphorothioate-LNA and 2′-thio-LNA. Bioorg Med Chem Lett 8(16):2219–2222
Saldanha J, Lelie N, Heath A (1999) Establishment of the first international standard for nucleic acid amplification technology (NAT) assays for HCV RNA. WHO Collaborative Study Group. Vox Sang 76(3):149–158
Holmes H, Davis C, Heath A, Hewlett I, Lelie N (2001) An international collaborative study to establish the 1st international standard for HIV-1 RNA for use in nucleic acid-based techniques. J Virol Methods 92(2):141–150
Al-Soud WA, Rådström P (2001) Purification and characterization of PCR-inhibitory components in blood cells. J Clin Microbiol 39(2):485–493
NCCLS (2003) Evaluation of the Linearity of quantitative measurement procedures: a statistical approach; approved guideline. NCCLS document EP6-A (ISBN 1-56238-498-8). NCCLS, Pennsylvania, USA
Acknowledgments
We wish to thank our colleagues from Alere Technologies Peter Slickers and Ralf Ehricht for the design of the oligonucleotides used to extract, amplify, and detect HCV and HIV RNA and Kornelia Kuhn and Monique Rüttger for performing most of the experiments to test oligonucleotide sequences and optimize procedures.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media New York
About this protocol
Cite this protocol
Bruns, T., Steinmetzer, K. (2012). Guidelines for the Quantification of HIV and HCV in Small Volume Whole Blood Samples. In: MacKenzie, C., Henrich, B. (eds) Diagnosis of Sexually Transmitted Diseases. Methods in Molecular Biology, vol 903. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-61779-937-2_3
Download citation
DOI: https://doi.org/10.1007/978-1-61779-937-2_3
Published:
Publisher Name: Humana Press, Totowa, NJ
Print ISBN: 978-1-61779-936-5
Online ISBN: 978-1-61779-937-2
eBook Packages: Springer Protocols