Abstract
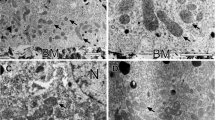
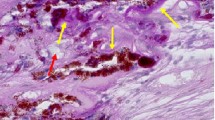
The aim of this chapter is to reveal the contribution of mitochondria and peroxisomes to the turnover of the photoreceptor outer segment and to describe the subsequent alteration of the retinal pigment epithelium and Bruch's membrane in normal aging and in age-related macular degeneration (AMD). Fifty-two surgically removed human eyes were involved in these histo-pathologic studies (25 female and 27 male, aged 56 to 87 years—mean age 68 years). Twenty-six of them were affected by early AMD, and 26 eyes were used as age-matched normal controls. For better visualization of lipids, osmium tetroxid postfixation was added to the standard electron microscopic technique. Polarization microscopy was also applied for the study of extracellular matrix components. Age-related changes of anisotropy were statistically analyzed using linear regression, and Fisher's transformation in both control and early AMD groups. Electron microscopy of retinal pigment epithelium both aged and early AMD showed a) accumulation of lipofuscin in the cytoplasm, b) focal or rarely diffuse alterations of mitochondrial cristae and matrix, and (c) accumulation of peroxisomes of variable size and electron density distributed throughout the cytoplasm. Electron and polarization microscopy of extracellular matrix showed a) an accumulation of amorphous, vacuolated and granular material in the collageneous layers of Bruch's membrane, b) the appearance of soft (rarely hard) Drusens, and c) a thickening of the basement membrane of retinal pigment epithelium (RPE) and choriocapillaries due to addition of axiparallel-oriented glycated fibrils and transversally oriented lipids. Although these processes were observed in both normal aging and early AMD, statistical analysis of anisotropy suggested that deposition of lipids and glycated fibrils was significantly different in AMD compared to normal aging.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Klaver ED, Wolfs RC, Vingerling JR, Hoffman A, de Jong PT (1998) Age-specific prevalence and causes of blindness and visual impairment in an older population: the Rotterdam Study. Arch Ophthalmol. 116:653–8
Feeney-Burns L, Ellersieck MR (1985) Age-related changes in the ultrastructure of Bruch's membrane. Amer J Ophthalmol. 100:686–97
Hageman GS, Mullins RF (1999) Molecular composition of drusens as related to substructural phenotype. Mol Vis.5:28
van der Shaft TL, Mooy CM, de Bruijn WC, Oron FG, Mulder PGH, de Jong PTVM (1992) Histologic features of the early stages of age-related macular degeneration. Ophthalmology. 99:278–286
Bird AC (1992) Pathophysiology of AMD. In CR Hampton and PT Nelson (eds) Age-Related Macular degeneration: Principles and Practice. Raven Press, New York, p 63–83
Starita C, Hussein AA, Patmore A, Marshall J (1997) Localization of the site of major resistence to fluid transport in Bruch's membrane. Invest Ophthamol Vis Sci.38:762–7
Holz FG, Sheraidah G, Pauleikhoff D et al. (1994) Analysis of lipid deposits extracted from human macular and peripheral Bruch's membrane. Arch Ophthalmol. 112:402–6
Spaide RF, Ho-Spaide WC, Browne RW, Armstrong D (1999) Characterization of lipids in Bruch's membrane. Retina 19:141–7
Curcio CA, Millican CL, Bailey T, Kruth HS (2001) Accumulation of cholesterol with age in human Bruch's membrane. Invest Ophthalmol Vis Sci. 42:263–74
Robison WG Jr, Kuwabara T (1975) Microperoxisomes in the retinal pigment epithelium. Invest Ophthalmol. 14; 866–72
Barreau E, Brossas, JY, Courtois Y, Treton JA (1996) Accumulation of mitochondrial DNA deletions in human retina during aging. Invest Ophthalmol Vis Sci. 37:384–91
Vingerling JR, Dielemams I, Bots ML, Hof nan A, Grobbee DE, de Jong PTVM (1996) Age-related macular degeneration is associated with atherosclerosis: The Rotterdam Study. Am J Epidemiology, 142:404–9
Klein R, Clegg L, Cooper LS, et al. (1999) Prevalence of age-related maculopathy in the Atherosclerosis Risk Communities Study. Arch Ophthalmol. 117:1203–10
van der Schaft TL, Mooy CM,de Bruijn WC, Bosman FT, de Jong PTVM (1994) Immunohistochemical light and electron microscopy of basal laminar deposits. Graefe's Arch Clin Exp Opthalmol.232:40–6
Romhanyi G, Deak G, Fisher J (1975) Aldehyde Bisulfite-Toluidine Blue (ABT) staining as a topooptical reaction for demonstration of linear order of vicinal OH groups in biological structures. Histochemistry 43:333–48
Missmahl H P (1957) Doppelbrechung der retikularen Faser and sich hieraus ergebender Nachweis von genchtet eingelagerten Lipoiden in die Faser. Z Zellforsh.45:612–7
Feher J, Valu L (1969) Structure of the Descemets membrane (German). Graefe's Arch Ophthalmol 179:65–73
Kroemer G. Redd JC (2000) Mitochondrial control of cell death. Natare Medicine. 6:513–9
Modis L (1974) Topo-optical invesstipbons of mucopolysaccharidm In: Gm mlann W & Neumann (eds): Handbuch der Histochemie, vol II, Part 4. G. Fisher, Stuttgart, p 3–35
Zingsheim HP, Plattner H (1976) Electron microscopic methods in membrane biology. In: Korn ED; (eds) Methods in Membrane Biology. Plenum Press, New York, p 1–146
Young RW (1967) The renewal of rod and cone outer segments. J Cell Biol 33:61–72
Ryeom SW, Sparrow JR, Silverstein RL (1996) CD36 partecipates in the phagocytosis of rod outer segments by retinal pigment epithelium. J Cell Sci 109:387–395
Rodriguez de Turco EB, Parkins N, Ershov AV, Bazan NG (1999) Selective retinal pigment epithelial cell lipid metabolism and remodelling conserves photoreceptor decosahexaenoic acid following phagocytosis. J Neurosci Res 57:479–86
Noske UM, Schmidt-Erfurth U, Meyer C, Diddens H (1998) Lipid metabolism in retinal pigment epithelium. Possible significance of lipoprotein receptors. Ophthalmologie 95:814–9
Wang N, Anderson R (1993) Transport of 22:6n-3 in the plasma and uptake into retinal pigment epithelium and retina. Exp Eye Res 57:225–3
Kennedy CJ, Rakoczy PE, Constable IJ (1995) Lipofuscin of the retinal pigment epithelium: A review. Eye 9:763–71
Feeney-Burns L, Hilderbrand ES (1984) Eldridge S Aging human RPE: morphometric analysis of macular, equatorial and peripheral cells. Invest Ophthalmol Vis Sci 25;195–200
Katz ML, Rice LM, Gao CL (1999) Reversible accumulation of lipofuscin-like inclusions in the retinal pigment epithelium. Invest Ophthalmol Vis Sci 40:175–81
Sundelin S, Wihlmark U, Nilsson SE, Brunk UT (1998) Lipofuscin accumulation in cultured retinal pigment epithelial cells reduces their phogocitic capacity. Curr Eye Res 17:851–7
Schraermeyer U, Heiman K (1999) Current understanding on the role of retinal pigment epithelium and its pigmentation. Pigment Cell Res 12:219–36
Thumann G, Bartz-Schmidt KU, Kociok N, Heilmamm K, Schraermeyer U (1999) Ultimate fate of rod outer segments in the retinal pigment epithelium. Pigment Cell Res 12:311–7
Hiltunen J K, Qin YM (2000) Beta-oxidation-strategies for the metabolism of a wide variety of acyl-CoA esters. Reviwe. Biochem Biophys Acta 1484:117–28
Andrews RM, Griffiths PG, Johnson MA, Turnbull DM (1999) Histochemical localization of mitochondrial enzyme activity in human optic nerve and retina.Br J Ophthalmol 83:231–5
Hruszkewycz AM (1988) Evidence for mitochondrial DNA damage by lipid peroxidation. Biochem Biophys Res Com 153:191–7
Winkler BS, Boulton ME, Gottsch JD, Stemberg P (1999) Oxidative damage and age-related macular degeneration. Mol Vis 5:32
Kersten S, Desvergne B, Wahli W (2000) Roles of PPARs in heath and disease. Nature 405:4214
Nagy L, Tontonoz P, Alvarez JGA, Chen H, Evans RM (1998) Oxidized LDL regulates macrophage gene expression through ligand activation of PPAR-gamma. Cell 93:22940
Ershov AV, Bazan NG (2000) Photoreceptor phagocytosis selectively activates PPAR-gamma expression in retinal pigment epithelial cells. J Neurosci Res 60:328–37
Young RW (1987) Pathophysiology of age-related macular degeneration. Sury Ophthalmol 31:291–306
François J, Feher J (1973) Arcus senilis. Doc. Ophthalmol 34:165–82
Tokura T, Ito S, Nishikawa M, Yamane A, Miki H. (1999) Changes in Bruch's membrane in experimental hypercholesteremia in rats. Nippon Ganka Gakkai Zasshi 203:85–91
Miceli M V, Newsome DA, Tate DJ, Sarohie TG (2000) Pathologic changes in the retinal pigment epithelium and Bruch's membrane of fat-fed atherogenic mice. Current Eye Res. 20:8–16
Olsson U, Bondjers G, Camejo G (1999) Fatty acids modulate the composition of extracellular matrix in cultured human arterial smooth muscle cells by altering the expression of genes for proteoglycan core proteins. Diabetes 48:616–22
Nerlich AG, Schleicher ED (1999) N(epsilon)-(carboxymethyl)lysine in atherosclerotic vascular lesion as a marker for local oxidative stress. Atherosclerosis 144:41–7
Oak J, Nakagawa K, Miyazawa T (2000) Synthetically prepared Amadori-glycated phosphatidylethanol-amine can trigger lipid peroxidation via free radical resction. FEBS Lett 481:26–30
Fu MX, Requena JR, Jenkins AJ, Lyons TJ, Baynes JW, Thorpe SR (1996) The advanced glycation end product, Nepsilon-(carboxymethyl)lysisne, is a product of both lipid peroxidation and glycoxidation reaction. J Biol Chem 27:9982–6
Ishibashi T, Murata T, Hangai M, Nagai R, Horiuchi S, Lopez PF, Hinton DR, Ryan SJ (1998) Advanced glycation end products in age-related macular degeneration. Arch Ophthalmol 116:1629–32
Handa JT, Verzijl N, Matsunaga H, Aotaki-Keen A, Lutty GA, Koeppele JM, Miyata T, Hjelmeland LM (1999) Increase in advanced glycation end product pentosidine in Bruch's membrane with age. Invest Ophthalmol Vis Sci 40:755–9
Hammes HP, Hoerauf H, Alt A, Schleicher E, Clausen JT, Bretzel RG, Laqua H (1999) N(ep silon)(carboxymethyl)lysin and AGE receptor RAGE colonize in age-related macular degeneration. Invest Ophthalmol Vis S6 40:1855–9
Hagemann GS, Mullins RF, Russell SR, Johnson LV, Anderson DH (1999) Vitronectin is a constituent of ocular drusen and the vitronectin gene is expressed in human retinal pigmented epithelial cells. FESEB J. 13:477–84
Hammes HP, Weiss A, Hess S, Araki N, Horiuchi S, Brownlee M, Preissner KT (1998) Modification of vitronectin by advanced glycation alters functional properties in vitro and in the diabetic retina. Lab Invest. 75:325–38
Ozawa T (1997) Genetic and functional changes in mitochondria associated with aging. Physiol Rev. 77: 425–64
Acknowledgments
Acknowledgements The authors thank Ida Bozso for her excellent technical assistance, and Livia Feher and Alessandro Mariani for their contribution to this chapter.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2008 Humana Press, a part of Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Kovacs, I., Feher, J., Cavallotti, C.A.P. (2008). Macular Degeneration: Ultrastructural Age-Related Changes . In: Cavallotti, C.A.P., Cerulli, L. (eds) Age-Related Changes of the Human Eye. Aging Medicine. Humana Press. https://doi.org/10.1007/978-1-59745-507-7_15
Download citation
DOI: https://doi.org/10.1007/978-1-59745-507-7_15
Publisher Name: Humana Press
Print ISBN: 978-1-934115-55-8
Online ISBN: 978-1-59745-507-7
eBook Packages: MedicineMedicine (R0)