Abstract
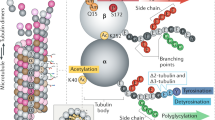
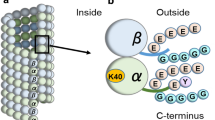
The tubulin molecule is unusual because of the number and nature of post-translational modifications that it undergoes. These modifications may be involved in regulating microtubule stability and interactions with microtubule-associated proteins, but they also may have as yet undiscovered functions. Certain of these modifications are found in many other proteins; these include phosphorylation of a serine residue in β-tubulin and acetylation of a lysine residue in α-tubulin. Other modifications occur exclusively, or almost exclusively in tubulin. Among these are the removal and addition of a tyrosine at the C-terminus of α and the addition of several glutamate or glycine residues to the γ-carboxyl group of glutamate residues in the C-terminal regions of both α and β. The identification of the mechanisms by which these modifications occur and of their roles in microtubule assembly and function are currently very active topics of research; they will be addressed in this chapter.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Ludueña RF. The multiple forms of tubulin: different gene products and covalent modifications. Int Rev Cytol 1998;178:207–275.
MacRae TH. Tubulin post-translational modifications-enzymes and their mechanisms of action. Eur J Biochem 1997;244:265–278.
Westermann S, Weber K. Post-translational modifications regulate microtubule function. Nat Rev Mol Cell Biol 2003;4:938–947.
Erck C, Frank R, Wehland J. Tubulin-tyrosine ligase, a long-lasting enigma. Neurochem Res 2000;25:5–10.
Lafenechére L, Job D. The third tubulin pool. Neurochem Res 2000;25:1–8.
Barra HS, Arce CA, Rodríguez JA, Caputto R. Some common properties of the protein that incorporates tyrosine as a single unit and the microtubule proteins. Biochem Biophys Res Commun 1974;60: 1384–1390.
Gu W, Lewis SA, Cowan NJ. Generation of antisera that discriminate among mammalian α-tubulins. Introduction of specialized isotypes into cultured cells results in their coassembly without disruption of normal microtubule function. J Cell Biol 1988;106:2011–2022.
Raybin D, Flavin M. Enzyme which specifically adds tyrosine to the alpha chain of tubulin. Biochemistry 1977;16:2189–2194.
Ersfeld K, Wehland J, Plessmann Dodermont H, Gerke V, Weber K. Characterization of the tubulin-tyrosine ligase. J Cell Biol 1993; 120:725–732.
Argaraña CE, Barra HS, Caputto R. Tubulinyl-tyrosine carboxypeptidase from chicken brain: properties and partial purification. J Neurochem 1980;34:114–118.
Kumar N, Flavin M. Preferential action of a brain detyrosinolating carboxypeptidase on polymerized tubulin. J Biol Chem 1981;256:7678–7686.
Arce CA, Barra HS. Association of tubulinyl-carboxypeptidase with microtubules. FEBS Lett 1983; 157:75–78.
Wehland J, Weber K. Tubulin-tyrosine ligase has a binding site on β-tubulin: a two domain structure of the enzyme. J Cell Biol 1987; 104:1059–1067.
Webster DR, Gundersen GG, Bulinski JC, Borisy GG. Assembly and turnover of detyrosinated tubulin in vivo. J Cell Biol 1987;105:265–276.
Gundersen GG, Kalnoski MH, Bulinski JC. Distinct populations of microtubules: tyrosinated and nontyrosinated α-tubulin are distributed differently in vivo. Cell 1984;38:779–789.
Kreis TE. Microtubules containing detyrosinated tubulin are less dynamic. EMBO J 1987;6: 2597–2606.
Khawaja S, Gundersen GG, Bulinski JC. Enhanced stability of microtubules enriched in detyrosinated tubulin is not a direct function of detyrosination level. J Cell Biol 1988; 106:141–149.
Kierszenbaum AL. Intramanchette transport (IMT): managing the making of the spermatid head, centrosome, and tail. Mol Reprod Devel 2002;63:1–4.
Geimer S, Teltenkötter A, Plessmann U, Weber K, Lechtreck KF. Purification and characterization of basal apparatuses from a flagellate green alga. Cell Motil Cytoskeleton 1997;37:72–85.
Mansir A, Justine JL. The microtubular system and posttranslationally modified tubulin during spermatogenesis in a parasitic nematode with amoeboid and aflagellate spermatozoa. Mol Reprod Devel 1998;49:150–167.
Mencarelli C, Bré MH, Levilliers N, Dallai R. Accessory tubules and axonemal microtubules of Apis mellifera sperm flagellum differ in their tubulin isoform content. Cell Motil Cytoskeleton 2000;47:1–12.
Poole CA, Zhang ZJ, Ross JM. The differential distribution of acetylated and detyrosinated alpha-tubulin in the microtubular cytoskeleton and primary cilia of hyaline cartilage chondrocytes. J Anat 2001; 199:393–405.
Tannenbaum J, Slepecky NB. Localization of microtubules containing posttranslationally modified tubulin in cochlear epithelial cells during development. Cell Motil Cytoskeleton 1997;38:146–162.
Saha S, Slepecky NB. Age-related changes in microtubules in the guinea pig organ of Corti. Tubulin isoform shifts with increasing age suggest changes in micromechanical properties of the sensory epithelium. Cell Tissue Res 2000;300:29–46.
Gurland G, Gundersen GG. Stable, detyrosinated microtubules function to localize vimentin intermediate filaments in fibroblasts. J Cell Biol 1995;131:1264–1290.
Liao G, Gundersen GG. Kinesin is a candidate for cross-bridging microtubules and intermediate filaments. Selective binding of kinesin to detyrosinated tubulin and vimentin. J Biol Chem 1998;273: 9797–9803.
Kreitzer FG, Liao G, Gundersen GG. Detyrosination of tubulin regulates the interaction of intermediate filaments with microtubules in vivo via a kinesin-dependent mechanism. Mol Biol Cell 1999;10:1105–1138.
Pechart I, Kann MKL, Levilliers N, Bré MH, Fouquet JP. Composition and organization of tubulin isoforms reveals a variety of axonemal models. Biol Cell 1999;91:685–697.
Day R, Criel GRJ, Walling MA, MacRae TH. Posttranslationally modified tubulins and microtubule organization in hemocytes of the brine shrimp, Artemia franciscana. J Morphol 2000;244:153–166.
Wang W, Vignani R, Scali M, Sensi E, Cresti M. Post-translational modification of α-tubulin in Zea mays L are highly tissue specific. Planta 2004;218:460–465.
Gilmer S, Clay P, MacRae TH, Fowke LC. Tyrosinated, but not detyrosinated, α-tubulin is present in root tip cells. Protoplasma 1999;210:92–98.
Idriss HT. Man to Trypanosome: the tubulin tyrosination/detyrosination cycle revisited. Cell Motil Cytoskeleton 2000;45:173–184.
Infante AS, Stein MS, Zhai Y, Borisy GG, Gundersen GG. Detyrosinated (Glu) microtubules are stabilized by an ATP-sensitive plus-end cap. J Cell Sci 2000;113:3907–3919.
Webster DR, Wehland J, Weber K, Borisy GG. Detyrosination of α tubulin does not stabilize microtubules in vivo. J Cell Biol 1990;141:175–185.
Correa LM, Miller MG. Microtubule depolymerization in rat seminiferous epithelium is associated with diminished tyrosination of α-tubulin. Biol Reprod 2001;64:1644–1652.
Ponstingl H, Little M, Krauhs E, Kempf T. Carboxy-terminal amino acid sequence of α-tubulin from porcine brain. Nature 1979;282:423–424.
Kalisz HM, Erck C, Plessmann U, Wehland J. Incorporation of nitrotyrosine into α-tubulin by recombinant mammalian tubulin-tyrosine ligase. Biochim Biophys Acta 2000;148:131–138.
Cappelletti G, Maggioni MG, Tedeschi G, Maci R. Protein tyrosine nitration is triggered by nerve growth factor during neuronal differentiation of PC12 cells. Exp Cell Res 2003;288:9–20.
Chang W, Webster DR, Salam AA, et al. Alteration of the C-terminal amino acid of tubulin specifically inhibits myogenic differentiation. J Biol Chem 2002;277:30,690–30,698.
Eiserich JP, Estévez AG, Bamberg TV, et al. Microtubule dysfunction by posttranslational nitrotyrosination of α-tubulin: a nitric oxide-dependent mechanism of cellular injury. Proc Nat Acad Sci USA 1999;96:6365–6370.
Paturle-Lafanechère L, Eddé B, Denoulet P, Van Dorsselaer A, Mazarguil H, Le Caer JP. Characterization of a major brain tubulin variant which cannot be tyrosinated. Biochemistry 1991;30:10,523–10,528.
Mary J, Redeker V, Le Caer JP, Promé JC, Rossier J. Class I and IVa β-tubulin isotypes expressed in adult mouse brain are glutamylated. FEBS Lett 1994;353:89–94.
Belmadani S, Poüs C, Ventura-Clapier R, Fischmeister R, Méry PF. Post-translational modifications of cardiac tubulin during chronic heart failure in the rat. Mol Cell Biochem 2002;237:39–46.
Banerjee A, Kasmala LT. Differential assembly kinetics of α-tubulin isoforms in the presence of paclitaxel. Biochem Biophys Res Commun 1998;245:349–351.
Bane BC, MacRae TH, Xiang H, Bateman, Slepecky NB. Microtubule cold stability in supporting cells of the gerbil auditory sensory epithelium: correlation with tubulin post-translational modifications. Cell Tissue Res 2002;307:57–67.
Banerjee A. Coordination of posttranslational modifications of bovine brain α-tubulin. Polyglycylation of Δ2-tubulin. J Biol Chem 2002;277:46,140–46,144.
L’Hernault SW, Rosenbaum JL. Chlamydomonas α-tubulin is posttranslationally modified by acetylation on the ɛ-amino group of a lysine. Biochemistry 1985;24:473–478.
LeDizet M, Piperno G. Identification of an acetylation site of Chlamydomonas α-tubulin. Proc Nat Acad Sci USA 1987;84:5720–5724.
Piperno G, Fuller MT. Monoclonal antibodies specific for an acetylated form of α-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J Cell Biol 1985; 101:2085–2094.
Gallo JM, Precigout E. Tubulin expression in trypanosomes. Biol Cell 1988;64:137–143.
Wolf KW, Regan CL, Fuller MT. Temporal and spatial pattern of differences in microtubule behaviour during Drosophila embryogenesis revealed by distribution of a tubulin isoform. Development 1988;102:311–324.
Siddiqui SS, Aamodt E, Rastinejad F, Culotti J. Anti-tubulin monoclonal antibodies that bind to specific neurons in Caenorhabditis elegans. J Neurosci 1989;9:2963–2972.
Wilson PJ, Forer A. Acetylated α-tubulin in spermatogenic cells of the crane fly Nephrotoma suturalis: Kinetochore microtubules are selectively acetylated. Cell Motil Cytoskeleton 1989; 14:237–250.
Souto-Padron T, Cunha e Silva NL, de Souza W. Acetylated alpha-tubulin in Trypanosoma cruzi: Immunocytochemical localization. Mem Inst Oswaldo Cruz 1993;88:517–528.
Delgado-Viscogliosi P, Brugerolle G, Viscogliosi E. Tubulin post-translational modifications in the primitive protist Trichomonas vaginalis. Cell Motil Cytoskeleton 1996;33:288–297.
Wolf KW. Cytology of Lepidoptera VIII. Acetylation of α-tubulin in mitotic and meiotic spindles of two Lepidoptera species, Ephesia kuehniella (Pyralidae) and Pieris brassicae (Pieridae). Protoplasma 1996;190:88–98.
Wolf KW. Acetylation of α-tubulin in male meiotic spindles of Pyrrhocoris apterus, an insect with holokinetic chromosomes. Protoplasma 1996; 191:148–157.
Huang RF, Lloyd CW. Gibberellic acid stabilises microtubules in maize suspension cells to cold and stimulates acetylation of α-tubulin. FEBS Lett 1999;443:317–320.
Noël C, Gerbod D, Fast NM, et al. Tubulins in Trichomonas vaginalis: molecular characterization of α-tubulin genes, posttranslational modifications, and homology modeling of the tubulin dimer. J Eukaryot Microbiol 2001;48:647–654.
Lazareva EM, Polyakov VY, Chentsov YS, Smirnova EA. Time and cell cycle dependent formation of heterogeneous tubulin arrays induced by colchicine in Triticum aestivum root meristem. Cell Biol Int 2003;27:633–646.
Campanati L, Bré MH, Levilliers N, de Souza W. Expression of tubulin polyglycylation in Giardia lamblia. Biol Cell 1999;91:499–506.
Piperno G, LeDizet M, Chang XJ. Microtubules containing acetylated α-tubulin in mammalian cells in culture. J Cell Biol 1987; 104:289–302.
Robson SJ, Burgoyne RD. Differential localisation of tyrosinated, detyrosinated, and acetylated α-tubulins in neurites and growth cones of dorsal root ganglion neurons. Cell Motil Cytoskeleton 1989; 12:273–282.
Greer K, Maruta H, L’Hernault SW, Rosenbaum JL. α-Tubulin acetylase activity in isolated Chlamydomonas flagella. J Cell Biol 1985;101:2081–2084.
Maruta H, Greer K, Rosenbaum JL. The acetylation of alpha-tubulin and its relationship to the assembly and disassembly of microtubules. J Cell Biol 1986;103:571–579.
Hubbert C, Guardiola A, Shao R, et al. HDAC6 is a microtubule-associated deacetylase. Nature 2002;417:455–458.
Zhang Y, Caron C, Matthias G, Hess D, Khochbin S, Matthias P. HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo. EMBO J 2003;22:1168–1179.
Boggild AK, Sundermann CA, Estridge BH. Localization of post-translationally modified α-tubulin and pseudocyst formation in tritrichomonads. Parasitol Res 2002;88:468–474.
Pisano C, Battistoni A, Antoccia A, Degrassi F, Tanzarella C. Changes in microtubule organization after exposure to a benzimidazole derivative in Chinese hamster cells. Mutagenesis 2000;15: 507–515.
Palazzo A, Ackerman B, Gundersen GG. Tubulin acetylation and cell motility. Nature 2003;421:230.
Matsuyama A, Shimazu T, Sumida Y, Saito A, Yoshimatsu Y, Seigneurin-Berny D. In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J 2002;21:6820–6831.
Haggarty SJ, Koeller KM, Wong JC, Butcher RA, Schreiber SL. Multidimensional chemical genetic analysis of diversity-oriented synthesis-derived deacetylase inhibitors using cell-based assays. Chem Biol 2003; 10:383–396.
Haggarty SJ, Koeller KM, WOng JC, Grozinger CM, Schreiber SL. Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDA6)-mediated tubulin deacetylation. Proc Nat Acad Sci USA 2003; 100:4389–4394.
Casale CH, Alonso AC, Barra HS. Brain plasma membrane Na+, K+-ATPase is inhibited by acetylated tubulin. Mol Cell Biochem 2001;216:85–92.
Pucciarelli S, Ballarini P, Miceli C. Cold-adapted microtubules: Characterization of tubulin posttranslational modifications in the Antarctic ciliate Euplotes focardii. Cell Motil Cytoskeleton 1997;38: 329–340.
Bobinnec Y, Marcaillou C, Debec A. Microtubule polyglutamylation in Drosophila melanogaster brain and testis. Eur J Cell Biol 1999;78:671–674.
Huitorel P, White D, Fouquet JP, Kann ML, Cosson J, Gagnon C. Differential distribution of glutamylated tubulin isoforms along the sea urchin sperm axoneme. Mol Reprod Devel 2002;62:139–148.
Boggild AK, Sundermann CA, Estridge BH. Post-translational glutamylation and tyrosination in tubulin of tritrichomonads and the diplomonad Giardia intestinalis. Parasitol Res 2002;88:58–62.
Mencarelli C, Caroti D, Bré MH, et al. Glutamylated and glycylated tubulin isoforms in the aberrant sperm axoneme of the gall-midge fly, Asphondylia ruebsaameni. Cell Motil Cytoskeleton 2004;58: 160–174.
Fouquet JP, Kann ML, Péchart I, Prigent Y. Expression of tubulin isoforms during the differentiation of mammalian spermatozoa. Tissue Cell 1997;29:573–583.
Bonnet C, Denarier E, Bosc C, Lazereg S, Denoulet P, Larcher JC. Interaction of STOP with neuronal tubulin is independent of polyglutamylation. Biochem Biophys Res Commun 2002;297:787–793.
Million K, Larcher JC, Laoukili J, Bourguignon D, Marano F, Tournier F. Polyglutamylation and polyglycylation of α-and β-tubulins during in vitro ciliated cell differentiation of human respiratory epithelial cells. J Cell Sci 1999;112:4357–4366.
Kann ML, Soues S, Levilliers N, Fouquet JP. Glutamylated tubulin: diversity of expression and distribution of isoforms. Cell Motil Cytoskeleton 2003;55:14–25.
Wolff A, de Néchaud B, Chillet D, et al. Distribution of glutamylated α-and β-tubulin in mouse tissues using a specific monoclonal antibody, GT335. Eur J Cell Biol 1992;59:425–432.
Verdier-Pinard P, Wang F, Martello L, Burd B, Orr GA, Horwitz SB. Analysis of tubulin isotypes and mutations from taxol-resistant cells by combined isoelectrofocusing and mass spectrometry. Biochemistry 2003;42:5349–5357.
Audebert S, Desbruyères E, Gruszczynski C, et al. Reversible polyglutamylation of α-and β-tubulin and microtubule dynamics in mouse brain neurons. Mol Biol Cell 1993;4:615–626.
Rao S, Åberg F, Nieves E, Horwitz SB, Orr GA. Identification by mass spectrometry of a new α-tubulin isotype expressed in human breast and lung carcinoma cell lines. Biochemistry 2001;40: 2096–2103.
Westermann S, Plessmann U, Weber K. Synthetic peptides identify the minimal substrate requirements of tubulin polyglutamylase in side chain elongation. FEBS Lett 1999;459:90–94.
Westermann S, Schneider A, Horn EK, Weber K. Isolation of tubulin polyglutamylase from Crithidia; binding to microtubules and tubulin, and glutamylation of mammalian brain α-and β-tubulins. J Cell Sci 1999;112:2185–2193.
Regnard C, Desbruyères E, Denoulet P, Eddé B. Tubulin polyglutamylase: isozymic variants and regulation during the cell cycle in HeLa cells. J Cell Sci 1999;112:4281–4289.
Boucher D, Larcher JC, Gros F, Denoulet P. Polyglutamylation of tubulin as a progressive regulator of in vitro interactions between the microtubule-associated protein tau and tubulin. Biochemistry 1994;33:12,471–12,477.
Larcher JC, Boucher D, Lazereg S, Gros F, Denoulet P. Interaction of kinesin motor domains with α-and β-tubulin subunits at a tau-independent binding site. Regulation of polyglutamylation. J Biol Chem 1996;271:22,117–22,124.
Bonnet C, Boucher D, Lazereg S, et al. Differential binding regulation of microtubule-associated proteins MAP1A, MAP1B, and MAP2 by tubulin polyglutamylation. J Biol Chem 2001;276:12,839–12,848.
Klotz A, Rutberg M, Denoulet P, Wallin M. Polyglutamylation of Atlantic cod tubulin: immunochemical localization and possible role in pigment granule transport. Cell Motil Cytoskeleton 1999; 44:263–273.
Redeker V, Frankfurter A, Parker SK, Rossier J, Detrich HW 3rd. Posttranslational modification of brain tubulins from the Antarctic fish Notothenia coriiceps: reduced C-terminal glutamylation correlates with efficient microtubule assembly at low temperature. Biochemistry 2004;43:12,265–12,274.
Redeker V, Levilliers N, Schmitter JM, et al. Polyglycylation of tubulin: A posttranslational modification in axonemal microtubules. Science 1992;266:1688–1691.
Rüdiger M, Plessman U, Rüdiger AH, Weber K. β tubulin of bull sperm is polyglycylated. FEBS Lett 1995;264:147–151.
Weber K, Schneider A, Müller N, Plessman U. Polyglycylation of tubulin in the diplomonad Giardia lamblia, one of the oldest eukaryotes. FEBS Lett 1996;393:27–30.
Mary J, Redeker V, Le Caer JP, Rossier J, Schmitter JM. Posttranslational modifications in the C-terminal tail of axonemal tubulin from sea urchin sperm. J Biol Chem 1994;271:9928–9933.
Vinh J, Langridge JI, Bré MH, et al. Structural characterization by tandem mass spectrometry of the posttranslational polyglycylation of tubulin. Biochemistry 1999;38:3133–3139.
Raff EC, Fackenthal JD, Hutchens JA, Hoyle HD, Turner FR. Microtubule architecture specified by a β-tubulin isoform. Science 1997;275:70–73.
Bressac C, Bré MH, Darmanaden-Delorme J, Laurent M, Levilliers N, Fleury A. A massive new posttranslational modification occurs on axonemal tubulin at the final step of spermatogenesis in Drosophila. Eur J Cell Biol 1995;67:346–355.
Bré MH, Redeker V, Quibell M, et al. Axonemal tubulin polyglycylation probes with two monoclonal antibodies: Widespread evolutionary distribution, appearance during spermatozoan maturation and possible function in motility. J Cell Sci 1996; 109:727–738.
Levilliers N, Fleury A, Hill AM. Monoclonal and polyclonal antibodies detect a new type of posttranslational modification in axonemal tubulin. J Cell Sci 1995;108:3013–3028.
Ittode F, Clérot JC, Levilliers N, Bré MH. Tubulin polyglycylation: a morphogenetic marker in ciliates. Biol Cell 2000;92:615–628.
Bré MH, Redeker V, Vinh J, Rossier J, Levilliers N. Tubulin polyglycylation: differential posttranslational modification of dynamic cytoplasmic and stable axonemal microtubules in Paramecium. Mol Biol Cell 1998;9:2655–2665.
Xia L, Hai B, Gao Y, et al. Polyglycylation of tubulin is essential and affects cell motility and division in Tetrahymena thermophila. J Cell Biol 2000;149:1097–1106.
Thazhath R, Liu C, Gaertig J. Polyglycylation domain of β-tubulin maintains axonemal architecture and affects cytokinesis in Tetrahymena. Nat Cell Biol 2002;4:256–259.
Bergen LG, Borisy GG. Head-to-tail polymerization of microtubules in vitro. Electron microscope analysis of seeded assembly. J Cell Biol 1980;84:141–150.
Cibert C. Entropy and information in flagellar axoneme cybernetics: a radial spokes integrative function. Cell Motil Cytoskeleton 2003;54:296–316.
Dahl HA. Fine structure of cilia in rat cerebral cortex. Z Zellforsch Mikr Anat 1963;60:369–386.
Händel M, Schulz S, Stanarius A, et al. Selective targeting of somatostatin receptor 3 to neuronal cilia. Neurosci 1999;89:909–926.
Jensen-Smith HC, Ludueña RF, Hallworth R. Requirement for the βI and βIV tubulin isotypes in mammalian cilia. Cell Motil Cytoskeleton 2003;55:213–220.
Eipper BA. Rat brain microtubule protein. Purification and determination of covalently bound phosphate and carbohydrate. Proc Nat Acad Sci USA 1972;69:2283–2287.
Alexander JE, Hunt DF, Lee MK, et al. Characterization of posttranslational modifications in neuron-specific class III β-tubulin by mass spectrometry. Proc Nat Acad Sci USA 1991;88:4685–4689.
Ludueña RF, Zimmermann HP, Little M. Identification of the phosphorylated β-tubulin isotype in differentiated neuroblastoma cells. FEBS Lett 1988;230:142–146.
Díaz-Nido J, Serrano L, López-Otin C, Vandekerckhove J, Ávila J. Phosphorylation of a neuronal-specific β-tubulin isotype. J Biol Chem 1990;265:13,949–13,954.
Lee MK, Tuttle JB, Rebhun LI, Cleveland DW, Frankfurter A. The expression and posttranslational modification of neuron-specific β-tubulin isotype during chick embryogenesis. Cell Motil Cytoskeleton 1990;17:118–132.
Rüdiger M, Weber K. Characterization of the post-translational modifications in tubulin from the marginal band of avian erythrocytes. Eur J Biochem 1993;218:107–116.
Stephens RE. Structural chemistry of the axoneme: Evidence for chemically and functionally unique tubulin dimers in outer fibers. Soc Gen Physiol Ser 1975;30:181–204.
Piperno G, Luck DJ. Phosphorylation of axonemal proteins in Chlamydomonas reinhardtii. J Biol Chem 1976;251:2161–2167.
Koontz DA, Choi JH. Evidence of phosphorylation of tubulin in carrot suspension cells. Physiol Plant 1993;87:576–583.
Peters JD, Furlong MT, Asai DJ, Harrison ML Geahlen RL, Syk, activated by cross-linking the B-cell antigen receptor, localizes to the cytosol where it interacts with and phosphorylates α-tubulin on tyrosine. J Biol Chem 1996;271:4755–4762.
Matten WJ, Aubry M, West J, Maness PF. Tubulin is phosphorylated at tyrosine by pp60c-sec in nerve growth cone membranes. J Cell Biol 1990;111: 1959–1970.
Atashi JR, Klinz SG, Ingraham CA, Matten WT, Schacher M, Maness PF. Neural cell adhesion molecules modulate tyrosine phosphorylation of tubulin in nerve growth cone membranes. Neuron 1992;8:831–842.
Joseph MK, Fernström MA, Soloff MS. Switching of β-to α-tubulin phosphorylation in uterine smooth muscle of parturient rats. J Biol Chem 1982;257:11,728–11,733.
Serrano L, Díaz-Nido J, Wandosell F, Ávila J. Tubulin phosphorylation by casein kinase II is similar to that found in vivo. J Cell Biol 1987;105:1731–1739.
Crute BE, Van Buskirk RG. A casein kinase-like kinase phosphorylates β-tubulin and may be a microtubule-associated protein. J Neurochem 1992;59:2017–2023.
Takahashi M, Tomizawa K, Sato K, Ohtake A, Omori A. A novel tau-tubulin kinase from bovine brain. FEBS Lett 1995;372:59–64.
Khan IA, Ludueña RF. Phosphorylation of βIII-tubulin. Biochemistry 1996;35:3704–3711.
Littauer UZ, Giveon D, Thierauf M, Ginzburg I, Ponstingl H. Common and distinct tubulin binding sites for microtubule-associated proteins. Proc Nat Acad Sci USA 1986;83:7162–7166.
Fanarraga ML, Avila J, Zabala JC. Expression of unphosphorylated class III β-tubulin isotype in neuroepithelial cells demonstrates neuroblast commitment and differentiation. Eur J Neurosci 1999;11:517–527.
Gard DL, Kirschner MW. A polymer-dependent increase in phosphorylation of β-tubulin accompanies differentiation of a mouse neuroblastoma cell line. J Cell Biol 1985; 100:764–774.
Aletta JM. Phosphorylation of type III β-tubulin in PC12 cell neurites, during NGF-induced process outgrowth. J Neurobiol 1996;31:461–475.
Caron JM. Posttranslational modification of tubulin by palmitoylation. I. In vivo and cell-free studies. Mol Biol Cell 1997;8:621–636.
Ozols J, Caron JM. Posttranslational modification of tubulin by palmitoylation. II. Identification of sites of palmitoylation. Mol Biol Cell 1997;8:637–645.
Caron JM, Vega LR, Fleming J, Bishop R, Solomon F. Single site α-tubulin mutation affects astral microtubules and nuclear positioning during anaphase in Saccharomyces cerevisiae: possible role of palmitoylation of α-tubulin. Mol Biol Cell 2001;12:2672–2687.
Terashima M, Yamamori C, Tsuchiya M, Shimoyama M. ADP-Ribosylation of tubulin by chicken NAD-arginine ADP-ribosyltransferase suppresses microtubule formation. J Nutr Sci Vitaminol 1999;45:393–400.
Najbauer J, Orpiszerski J, Aswad DW. Molecular aging of tubulin: Accumulation of isoaspartyl sites in vitro and in vivo. Biochemistry 1996;35:5183–5190.
Correia JJ, Lipscomb LD, Lobert S. Nondisulfide crosslinking and chemical cleavage of tubulin subunits: pH and temperature dependence. Arch Biochem Biophys 1993;300:105–114.
Stanchi F, Corso V, Scannapieco P, et al. TUBA8: a new tissue-specific isoform of α-tubulin that is highly conserved in human and mouse. Biochem Biophys Res Commun 2000;270:1111–1118.
Polevoda B, Sherman F. The diversity of acetylated proteins. Genome Biol 2002;3/5/reviews/0006.1 (on-line).
Naryzhny SN, Lee H. The post-translational modifications of proliferating cell nuclear antigen: acetylation, not phosphorylation, plays an important role in the regulation of its function. J Biol Chem 2004;279:20,194–20,199.
Odintsova TI, Muller EC, Ivanov AV, et al. Characterization and analysis of posttranslational modifications of the human large cytoplasmic ribosomal subunit proteins by mass spectrometry and Edman sequencing. J Protein Chem 2003;22:249–258.
Wardleworth BN, Russell RJ, Bell SD, Taylor GL, White MF. Structure of Alba: an archaeal protein modulated by acetylation. EMBO J 2002;21:4654–4662.
Williams RC, Shah C, Sackett D. Separation of tubulin isoforms by isoelectric focusing in immobilized pH gradient gels. Anal Biochem 1999;275:265–267.
Shah C, Xu CZQ, Vickers J, Williams R. Properties of microtubules assembled from mammalian tubulin synthesized in Escherichia coli. Biochemistry 2001;40:4844–4852.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2008 Humana Press, Totowa, NJ
About this chapter
Cite this chapter
Ludueña, R.F., Banerjee, A. (2008). The Post-Translational Modifications of Tubulin. In: Fojo, T. (eds) The Role of Microtubules in Cell Biology, Neurobiology, and Oncology. Cancer Drug Discovery and Development. Humana Press. https://doi.org/10.1007/978-1-59745-336-3_5
Download citation
DOI: https://doi.org/10.1007/978-1-59745-336-3_5
Publisher Name: Humana Press
Print ISBN: 978-1-58829-294-0
Online ISBN: 978-1-59745-336-3
eBook Packages: MedicineMedicine (R0)