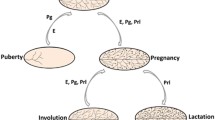
Abstract
The mammary gland is an apocrine organ that undergoes multiple periods of robust change marked with proliferation, differentiation and apoptosis. The profound regenerative potential observed in the mammary gland implies the presence of a population of mammary stem cells (MaSCs) with the capacity to both self-renew and give rise to all mammary lineages. Furthermore, a single mammary epithelial cell enriched for specific cell surface markers has been shown to reconstitute an entire, functional mammary gland in vivo, thereby demonstrating multipotent stem cell potential. The purpose of this chapter is to briefly outline the current state of knowledge on the identity and location of the MaSC, as well as provide a critical overview of the assays utilized to examine MaSC potential.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- FACS:
-
Fluorescence-activated cell sorting
- FCS:
-
Fetal calf serum
- GFP:
-
Green fluorescent protein
- LRC:
-
Label-retaining cells
- MaSC:
-
Mammary stem cell
- MRU:
-
Mammary repopulating unit
- PI-MEC:
-
Parity-induced mammary epithelial cells
- SP:
-
Side population
- TDLU:
-
Terminal ductal lobular units
- TEB:
-
Terminal end buds
- YFP:
-
Yellow fluorescent protein
References
Cardiff RD, Wellings SR (1999) The comparative pathology of human and mouse mammary glands. J Mammary Gland Biol Neoplasia 4(1):105–122
Cardiff RD (1998) Are the TDLU of the human the same as the LA of mice? J Mammary Gland Biol Neoplasia 3(1):3–5
Russo J et al (1990) Comparative study of human and rat mammary tumorigenesis. Lab Invest 62(3):244–278
Murrell TG (1995) The potential for oxytocin (OT) to prevent breast cancer: a hypothesis. Breast Cancer Res Treat 35(2):225–229
Ronnov-Jessen L, Petersen OW, Bissell MJ (1996) Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev 76(1):69–125
Wiseman BS, Werb Z (2002) Stromal effects on mammary gland development and breast cancer. Science 296(5570):1046–1049
Schedin P et al (2004) Mammary ECM composition and function are altered by reproductive state. Mol Carcinog 41(4):207–220
Muschler J, Streuli CH (2010) Cell-matrix interactions in mammary gland development and breast cancer. Cold Spring Harb Perspect Biol 2(10):a003202
Hovey RC, McFadden TB, Akers RM (1999) Regulation of mammary gland growth and morphogenesis by the mammary fat pad: a species comparison. J Mammary Gland Biol Neoplasia 4(1):53–68
Weaver VM et al (1996) The importance of the microenvironment in breast cancer progression: recapitulation of mammary tumorigenesis using a unique human mammary epithelial cell model and a three-dimensional culture assay. Biochem Cell Biol 74(6):833–851
Lim E et al (2010) Transcriptome analyses of mouse and human mammary cell subpopulations reveal multiple conserved genes and pathways. Breast Cancer Res 12(2):R21
Veltmaat JM et al (2003) Mouse embryonic mammogenesis as a model for the molecular regulation of pattern formation. Differentiation 71(1):1–17
Russo J, Russo IH (2004) Development of the human breast. Maturitas 49(1):2–15
Hovey RC, Trott JF, Vonderhaar BK (2002) Establishing a framework for the functional mammary gland: from endocrinology to morphology. J Mammary Gland Biol Neoplasia 7(1):17–38
Mintz B, Slemmer G (1969) Gene control of neoplasia. I. Genotypic mosaicism in normal and preneoplastic mammary glands of allophenic mice. J Natl Cancer Inst 43(1):87–109
Spike BT et al (2012) A mammary stem cell population identified and characterized in late embryogenesis reveals similarities to human breast cancer. Cell Stem Cell 10(2):183–197
Williams JM, Daniel CW (1983) Mammary ductal elongation: differentiation of myoepithelium and basal lamina during branching morphogenesis. Dev Biol 97(2):274–290
Smith GH (2005) Label-retaining epithelial cells in mouse mammary gland divide asymmetrically and retain their template DNA strands. Development 132(4):681–687
Zeps N et al (1998) Estrogen receptor-negative epithelial cells in mouse mammary gland development and growth. Differentiation 62(5):221–226
Welm BE et al (2002) Sca-1(pos) cells in the mouse mammary gland represent an enriched progenitor cell population. Dev Biol 245(1):42–56
Shackleton M et al (2006) Generation of a functional mammary gland from a single stem cell. Nature 439(7072):84–88
Bai L, Rohrschneider LR (2010) s-SHIP promoter expression marks activated stem cells in developing mouse mammary tissue. Genes Dev 24(17):1882–1892
Joshi PA et al (2010) Progesterone induces adult mammary stem cell expansion. Nature 465(7299):803–807
Andres AC, Strange R (1999) Apoptosis in the estrous and menstrual cycles. J Mammary Gland Biol Neoplasia 4(2):221–228
Richert MM et al (2000) An atlas of mouse mammary gland development. J Mammary Gland Biol Neoplasia 5(2):227–241
Russo J et al (2005) The protective role of pregnancy in breast cancer. Breast Cancer Res 7(3):131–142
Russo J, Rivera R, Russo IH (1992) Influence of age and parity on the development of the human breast. Breast Cancer Res Treat 23(3):211–218
Matulka LA, Triplett AA, Wagner KU (2007) Parity-induced mammary epithelial cells are multipotent and express cell surface markers associated with stem cells. Dev Biol 303(1):29–44
Deome KB et al (1959) Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res 19(5):515–520
Faulkin LJ Jr, Deome KB (1960) Regulation of growth and spacing of gland elements in the mammary fat pad of the C3H mouse. J Natl Cancer Inst 24:953–969
Daniel CW, Deome KB (1965) Growth of mouse mammary glands in vivo after monolayer culture. Science 149(3684):634–636
Daniel CW et al (1975) Unlimited division potential of precancerous mouse mammary cells after spontaneous or carcinogen-induced transformation. Fed Proc 34(1):64–67
Chepko G, Smith GH (1997) Three division-competent, structurally-distinct cell populations contribute to murine mammary epithelial renewal. Tissue Cell 29(2):239–253
Smith GH, Medina D (1988) A morphologically distinct candidate for an epithelial stem cell in mouse mammary gland. J Cell Sci 90(Pt 1):173–183
Young LJ et al (1971) The influence of host and tissue age on life span and growth rate of serially transplanted mouse mammary gland. Exp Gerontol 6(1):49–56
Kordon EC, Smith GH (1998) An entire functional mammary gland may comprise the progeny from a single cell. Development 125(10):1921–1930
Goodell MA et al (1996) Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med 183(4):1797–1806
Zhou S et al (2002) Bcrp1 gene expression is required for normal numbers of side population stem cells in mice, and confers relative protection to mitoxantrone in hematopoietic cells in vivo. Proc Natl Acad Sci U S A 99(19):12339–12344
Jonker JW et al (2005) Contribution of the ABC transporters Bcrp1 and Mdr1a/1b to the side population phenotype in mammary gland and bone marrow of mice. Stem Cells 23(8):1059–1065
Alvi AJ et al (2003) Functional and molecular characterisation of mammary side population cells. Breast Cancer Res 5(1):R1–R8
Stingl J et al (2006) Purification and unique properties of mammary epithelial stem cells. Nature 439(7079):993–997
Van Keymeulen A et al (2011) Distinct stem cells contribute to mammary gland development and maintenance. Nature 479(7372):189–193
van Amerongen R, Bowman AN, Nusse R (2012) Developmental stage and time dictate the fate of Wnt/beta-catenin-responsive stem cells in the mammary gland. Cell Stem Cell 11(3):387–400
Tsai YC et al (1996) Contiguous patches of normal human mammary epithelium derived from a single stem cell: implications for breast carcinogenesis. Cancer Res 56(2):402–404
Lakhani SR et al (1999) Genetic alterations in ‘normal’ luminal and myoepithelial cells of the breast. J Pathol 189(4):496–503
Deng G et al (1996) Loss of heterozygosity in normal tissue adjacent to breast carcinomas. Science 274(5295):2057–2059
Stingl J et al (2001) Characterization of bipotent mammary epithelial progenitor cells in normal adult human breast tissue. Breast Cancer Res Treat 67(2):93–109
Gudjonsson T et al (2002) Isolation, immortalization, and characterization of a human breast epithelial cell line with stem cell properties. Genes Dev 16(6):693–706
Dontu G et al (2003) In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev 17(10):1253–1270
Villadsen R et al (2007) Evidence for a stem cell hierarchy in the adult human breast. J Cell Biol 177(1):87–101
Kuperwasser C et al (2004) Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci U S A 101(14):4966–4971
Ginestier C et al (2007) ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 1(5):555–567
Parmar H et al (2002) A novel method for growing human breast epithelium in vivo using mouse and human mammary fibroblasts. Endocrinology 143(12):4886–4896
Eirew P et al (2008) A method for quantifying normal human mammary epithelial stem cells with in vivo regenerative ability. Nat Med 14(12):1384–1389
Lim E et al (2009) Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med 15(8):907–913
Pece S et al (2010) Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell 140(1):62–73
Smith GH (1996) Experimental mammary epithelial morphogenesis in an in vivo model: evidence for distinct cellular progenitors of the ductal and lobular phenotype. Breast Cancer Res Treat 39(1):21–31
Stingl J et al (1998) Phenotypic and functional characterization in vitro of a multipotent epithelial cell present in the normal adult human breast. Differentiation 63(4):201–213
Sleeman KE et al (2006) CD24 staining of mouse mammary gland cells defines luminal epithelial, myoepithelial/basal and non-epithelial cells. Breast Cancer Res 8(1):R7
Taddei I et al (2008) Beta1 integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nat Cell Biol 10(6):716–722
Zeng YA, Nusse R (2010) Wnt proteins are self-renewal factors for mammary stem cells and promote their long-term expansion in culture. Cell Stem Cell 6(6):568–577
Plaks V et al (2013) Lgr5-expressing cells are sufficient and necessary for postnatal mammary gland organogenesis. Cell Rep 3(1):70–78
Asselin-Labat ML et al (2006) Steroid hormone receptor status of mouse mammary stem cells. J Natl Cancer Inst 98(14):1011–1014
Asselin-Labat ML et al (2007) Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol 9(2):201–209
Sleeman KE et al (2007) Dissociation of estrogen receptor expression and in vivo stem cell activity in the mammary gland. J Cell Biol 176(1):19–26
Kendrick H et al (2008) Transcriptome analysis of mammary epithelial subpopulations identifies novel determinants of lineage commitment and cell fate. BMC Genomics 9:591
Regan JL et al (2012) c-Kit is required for growth and survival of the cells of origin of Brca1-mutation-associated breast cancer. Oncogene 31(7):869–883
Shehata M et al (2012) Phenotypic and functional characterization of the luminal cell hierarchy of the mammary gland. Breast Cancer Res 14(5):R134
Taylor-Papadimitriou J et al (2002) MUC1 and the immunobiology of cancer. J Mammary Gland Biol Neoplasia 7(2):209–221
Gusterson BA et al (1986) Identification of myoepithelial cells in human and rat breasts by anti-common acute lymphoblastic leukemia antigen antibody A12. J Natl Cancer Inst 77(2):343–349
Latza U et al (1990) Ber-EP4: new monoclonal antibody which distinguishes epithelia from mesothelial. J Clin Pathol 43(3):213–219
Koukoulis GK et al (1991) Immunohistochemical localization of integrins in the normal, hyperplastic, and neoplastic breast. Correlations with their functions as receptors and cell adhesion molecules. Am J Pathol 139(4):787–799
Raouf A et al (2008) Transcriptome analysis of the normal human mammary cell commitment and differentiation process. Cell Stem Cell 3(1):109–118
Clayton H, Titley I, Vivanco M (2004) Growth and differentiation of progenitor/stem cells derived from the human mammary gland. Exp Cell Res 297(2):444–460
Skalli O et al (1986) A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol 103(6 Pt 2):2787–2796
Smalley MJ et al (2012) Isolation of mouse mammary epithelial subpopulations: a comparison of leading methods. J Mammary Gland Biol Neoplasia 17(2):91–97
Joshi PA, Di Grappa MA, Khokha R (2012) Active allies: hormones, stem cells and the niche in adult mammopoiesis. Trends Endocrinol Metab 23(6):299–309
Acknowledgments
The authors would like to thank Mr. Hartland Jackson for providing the images of colonies from various colony-forming assays and for sample flow cytometry plots. The authors would also like to thank Dr. Alison Casey, Mr. Hartland Jackson, and Dr. Purna Joshi for their critical reading of the manuscript. This work was supported by funding to the Khokha lab from the Canadian Institutes of Health Research (CIHR), the Canadian Cancer Society Research Institute (CCSRI), and the Canadian Breast Cancer Foundation (CBCF). Pirashaanthy Tharmapalan is supported by a CBCF studentship.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Tharmapalan, P., Khokha, R. (2014). Adult Mammary Stem Cells: Identity, Location, and Functional Assays. In: Turksen, K. (eds) Adult Stem Cells. Stem Cell Biology and Regenerative Medicine. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4614-9569-7_9
Download citation
DOI: https://doi.org/10.1007/978-1-4614-9569-7_9
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4614-9568-0
Online ISBN: 978-1-4614-9569-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)