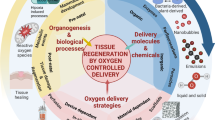
Abstract
Adequate oxygen transport is vital to the success of a tissue-engineered construct. Modulating oxygen tension within tissue-engineered constructs is necessary for the creation of devices with optimal functionality. Oxygen tension significantly influences cellular behavior through mechanisms which promote both cell proliferation and apoptosis. Given the negative consequences of low oxygen tension for most grafted tissues, many investigators have worked to improve oxygen tension within tissue engineered scaffolds through the use of synthetic oxygen carriers, natural or artificial heme, and polymeric oxygen generating thin films, or by inducing blood vessel growth into the matrix. Cellular oxygen consumption and transport within a scaffold can be calculated and predicted using diffusion models to improve device design. This section explores the interplay between fundamental engineering and biological processes used to modulate oxygen tension for the creation of functional tissue-engineered devices.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Khattak, S.F., Chin, K.S., Bhatia, S.R., Roberts, S.C.: Enhancing oxygen tension and cellular function in alginate cell encapsulation devices through the use of perfluorocarbons. Biotechnol. Bioeng. 96(1), 156–166 (2007)
Arifin, D.R., Palmer, A.F.: Polymersome encapsulated hemoglobin: a novel type of oxygen carrier. Biomacromolecules 6(4), 2172–2181 (2005)
Harrison, B.S., Eberli, D., Lee, S.J., Atala, A., Yoo, J.J.: Oxygen producing biomaterials for tissue regeneration. Biomaterials 28(31), 4628–4634 (2007)
Oh, S.H., Ward, C.L., Atala, A., Yoo, J.J., Harrison, B.S.: Oxygen generating scaffolds for enhancing engineered tissue survival. Biomaterials 30(5), 757–762 (2009)
Laschke, M.W., Harder, Y., Amon, M., Martin, I., Farhadi, J., Ring, A., et al.: Angiogenesis in tissue engineering: Breathing life into constructed tissue substitutes. Tissue Eng. 12(8), 2093–2104 (2006)
Santos, M.I., Reis, R.L.: Vascularization in bone tissue engineering: physiology, current strategies, major hurdles and future challenges. Macromol. Biosci. 10(1), 12–27 (2010)
Semenza, G.L.: Regulation of mamalian O2 homeostasis by hypoxia-inducible factor 1. Annu. Rev. Cell Dev. Biol. 15(1), 551–578 (1999)
Jiang, B.H., Semenza, G.L., Bauer, C., Marti, H.H.: Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. AJP - Cell Physiol. 271(4), C1172–C1180 (1996)
Iyer, N.V., Kotch, L.E., Agani, F., Leung, S.W., Laughner, E., Wenger, R.H., et al.: Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev. 12(2), 149–162 (1998)
Wang, G.L., Semenza, G.L.: General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc. Natl. Acad. Sci. USA 90(9), 4304–4308 (1993)
Reynolds, T.Y., Rockwell, S., Glazer, P.M.: Genetic instability induced by the tumor microenvironment. Cancer Res. 56(24), 5754–5757 (1996)
Malda, J., Rouwkema, J., Martens, D.E., le Comte, E.P., Kooy, F.K., Tramper, J., et al.: Oxygen gradients in tissue-engineered PEGT/PBT cartilaginous constructs: Measurement and modeling. Biotechnol. Bioeng. 86(1), 9–18 (2004)
Radisic, M., Malda, J., Epping, E., Geng, W., Langer, R., Vunjak-Novakovic, G.: Oxygen gradients correlate with cell density and cell viability in engineered cardiac tissue. Biotechnol. Bioeng. 93(2), 332–343 (2006)
Malda, J., Klein, T.J., Upton, Z.: The roles of hypoxia in the in vitro engineering of tissues. Tissue Eng. 13(9), 2153–2162 (2007)
Kellner, K., Liebsch, G., Klimant, I., Wolfbeis, O.S., Blunk, T., Schulz, M.B., et al.: Determination of oxygen gradients in engineered tissue using a fluorescent sensor. Biotechnol. Bioeng. 80(1), 73–83 (2002)
Galban, C.J., Locke, B.R.: Effects of spatial variation of cells and nutrient and product concentrations coupled with product inhibition on cell growth in a polymer scaffold. Biotechnol. Bioeng. 64(6), 633–643 (1999)
Johnson, A.S., Fisher, R.J., Weir, G.C., Colton, C.K.: Oxygen consumption and diffusion in assemblages of respiring spheres: Performance enhancement of a bioartificial pancreas. Chem. Eng. Sci. 64(22), 4470–4487 (2009)
Croll, T.I., Gentz, S., Mueller, K., Davidson, M., O’Connor, A.J., Stevens, G.W., et al.: Modelling oxygen diffusion and cell growth in a porous, vascularising scaffold for soft tissue engineering applications. Chem. Eng. Sci. 60(17), 4924–4934 (2005)
Landman, K., Cai, A.: Cell proliferation and oxygen diffusion in a vascularising scaffold. Bull. Math. Biol. 69(7), 2405–2428 (2007)
Griffith, L.G., Naughton, G.: Tissue engineering – Current challenges and expanding opportunities. Science 295(5557), 1009–1014 (2002)
Taylor, C., Pouyssegur, J.: Oxygen, hypoxia, and stress. Ann. N. Y. Acad. Sci. 1113(1), 87–94 (2007)
Semenza, G.L.: Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 3(10), 721–732 (2003)
Greijer, A., Van der Wall, E.: The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J. Clin. Pathol. 57(10), 1009–1014 (2004)
Bunn, H.F., Poyton, R.O.: Oxygen sensing and molecular adaptation to hypoxia. Physiol. Rev. 76(3), 839–885 (1996)
Huang, L.E., Gu, J., Schau, M., Bunn, H.F.: Regulation of hypoxia-inducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitin–proteasome pathway. Proc. Natl Acad. Sci. USA 95(14), 7987–7992 (1998)
Krieg, M., Haas, R., Brauch, H., Acker, T., Flamme, I., Plate, K.: Up-regulation of hypoxia-inducible factors HIF-1 and HIF-2 under normoxic conditions in renal carcinoma cells by von Hippel-Lindau tumor suppressor gene loss of function. Oncogene 19(48), 5435–5443 (2000)
Bruick, R.K., McKnight, S.L.: A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294(5545), 1337–1340 (2001)
Schipani, E., Ryan, H.E., Didrickson, S., Kobayashi, T., Knight, M., Johnson, R.S.: Hypoxia in cartilage: HIF-1α is essential for chondrocyte growth arrest and survival. Genes Dev. 15(21), 2865–2876 (2001)
Guillemin, K., Krasnow, M.A.: The hypoxic response: Huffing and HIFing. Cell 89(1), 9–12 (1997)
Raval, R.R., Lau, K.W., Tran, M.G.B., Sowter, H.M., Mandriota, S.J., Li, J.-L., et al.: Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol. Cell. Biol. 25(13), 5675–5686 (2005)
Carmeliet, P., Dor, Y., Herbert, J.-M., Fukumura, D., Brusselmans, K., Dewerchin, M., et al.: Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394(6692), 485–490 (1998)
Moritz, W., Meier, F., Stroka, D., Giuliani, M., Kugelmeier, P., Nett, P.C., et al.: Apoptosis in hypoxic human pancreatic islets correlates with HIF-1α expression. FASEB J. 16, 745–747 (2002). doi:01-0403fje
Chen, J., Zhao, S., Nakada, K., Kuge, Y., Tamaki, N., Okada, F., et al.: Dominant-negative hypoxia-inducible factor-1α reduces tumorigenicity of pancreatic cancer cells through the suppression of glucose metabolism. Am. J. Pathol. 162(4), 1283–1291 (2003)
Kvietikova, I., Wenger, R.H., Marti, H.H., Gassmann, M.: The transcription factors ATF-1 and CREB-1 bind constitutively to the hypoxia-inducible factor-1 (HIF-1) DNA recognition site. Nucleic Acids Res. 23(22), 4542–4550 (1995)
Wang, G.L., Semenza, G.L.: Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J. Biol. Chem. 268(29), 21513–21518 (1993)
Gassmann, M., Fandrey, J., Bichet, S., Wartenberg, M., Marti, H.H., Bauer, C., et al.: Oxygen supply and oxygen-dependent gene expression in differentiating embryonic stem cells. Proc. Natl Acad. Sci. USA 93(7), 2867–2872 (1996)
Lim, J.-H., Chun, Y.-S., Park, J.-W.: Hypoxia-inducible factor-1α obstructs a Wnt signaling pathway by inhibiting the hARD1-mediated activation of β-catenin. Cancer Res. 68(13), 5177–5184 (2008)
Kaufman, D.S.: HIF hits Wnt in the stem cell niche. Nat. Cell Biol. 12(10), 926–927 (2010)
Bijlsma, M.F., Groot, A.P., Oduro, J.P., Franken, R.J., Schoenmakers, S.H.H.F., Peppelenbosch, M.P., et al.: Hypoxia induces a hedgehog response mediated by HIF-1α. J. Cell. Mol. Med. 13(8(b)), 2053–2060 (2009)
Covello, K.L., Kehler, J., Yu, H., Gordan, J.D., Arsham, A.M., Hu, C.-J., et al.: HIF-2α regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 20(5), 557–570 (2006)
Chen, R., Silva, E., Yuen, W., Mooney, D.: Spatio-temporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharm. Res. 24(2), 258–264 (2007)
Pugh, C., Ratcliffe, P.: Regulation of angiogenesis by hypoxia: role of the HIF system. Nat. Med. 9(6), 677–684 (2003)
Lee, K., Mooney, D.: Hydrogels for tissue engineering. Chem. Rev. 101(7), 1869–1880 (2001)
Ennett, A.B., Mooney, D.J.: Tissue engineering strategies for in vivo neovascularisation. Expert Opin. Biol. Ther. 2(8), 805–818 (2002)
Mieszawska, A., Kaplan, D.: Smart biomaterials – regulating cell behavior through signaling molecules. BMC Biol. 8(1), 59 (2010)
Halterman, M.W., Miller, C.C., Federoff, H.J.: Hypoxia-inducible factor-1α mediates hypoxia-induced delayed neuronal death that involves p53. J. Neurosci. 19(16), 6818–6824 (1999)
Kothari, S., Cizeau, J., McMillan-Ward, E., Israels, S.J., Bailes, M., Ens, K., et al.: BNIP3 plays a role in hypoxic cell death in human epithelial cells that is inhibited by growth factors EGF and IGF. Oncogene 22(30), 4734–4744 (2000)
Young, S.D., Marshall, R.S., Hill, R.P.: Hypoxia induces DNA overreplication and enhances metastatic potential of murine tumor cells. Proc. Natl Acad. Sci. USA 85(24), 9533–9537 (1988)
Coquelle, A., Toledo, F., Stern, S., Bieth, A., Debatisse, M.: A new role for hypoxia in tumor progression: induction of fragile site triggering genomic rearrangements and formation of complex DMs and HSRs. Mol. Cell 2(2), 259–265 (1998)
Bencokova, Z., Kaufmann, M.R., Pires, I.M., Lecane, P.S., Giaccia, A.J., Hammond, E.M.: ATM activation and signaling under hypoxic conditions. Mol. Cell. Biol. 29(2), 526–537 (2009)
Zhu, W., Chen, J., Cong, X., Hu, S., Chen, X.: Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells. Stem Cells 24(2), 416–425 (2006)
Saikumar, P., Dong, Z., Patel, Y., Hall, K., Hopfer, U., Weinberg, J., et al.: Role of hypoxia-induced Bax translocation and cytochrome c release in reoxygenation injury. Oncogene 17(26), 3401–3415 (1998)
Boutilier, R.G., St-Pierre, J.: Surviving hypoxia without really dying. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 126(4), 481–490 (2000)
Henrotin, Y.E., Bruckner, P., Pujol, J.P.L.: The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis Cartilage 11(10), 747–755 (2003)
Kim, J.-Y., Park, J.-H.: ROS-dependent caspase-9 activation in hypoxic cell death. FEBS Lett. 549(1–3), 94–98 (2003)
Kunz, M., Ibrahim, S., Koczan, D., Thiesen, H., Köhler, H., Acker, T., et al.: Activation of c-Jun NH2-terminal kinase/stress-activated protein kinase (JNK/SAPK) is critical for hypoxia-induced apoptosis of human malignant melanoma. Cell Growth Differ. 12(3), 137–145 (2001)
Tibbles, L.A., Woodgett, J.R.: The stress-activated protein kinase pathways. Cell. Mol. Life Sci. 55(10), 1230–1254 (1999)
Lovett, M., Lee, K., Edwards, A., Kaplan, D.: Vascularization strategies for tissue engineering. Tissue Eng. Part B Rev. 15(3), 353–370 (2009)
Goosen, M.F.A.: Fundamentals of Animal Cell Encapsulation and Immobilization. CRC, Boca Raton (1993)
Poncelet, D., Bugarski, B., Amsden, B.G., Zhu, J., Neufeld, R., Goosen, M.F.A.: A parallel-plate electrostatic droplet generator – parameters affecting microbead size. Appl. Microbiol. Biotechnol. 42(2–3), 251–255 (1994)
Strand, B.L., Gaserod, O., Kulseng, B., Espevik, T., Skjak-Braek, G.: Alginate-polylysine-alginate microcapsules: effect of size reduction on capsule properties. J. Microencapsul. 19(5), 615–630 (2002)
Klokk, T.I., Melvik, J.E.: Controlling the size of alginate gel beads by use of a high electrostatic potential. J. Microencapsul. 19(4), 415–424 (2002)
Sakai, S., Hashimoto, I., Kawakami, K.: Development of alginate-agarose subsieve-size capsules for subsequent modification with a polyelectrolyte complex membrane. Biochem. Eng. J. 30(1), 76–81 (2006)
Agrawal, C.M., Ray, R.B.: Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J. Biomed. Mater. Res. 55(2), 141–150 (2001)
Lim, F., Sun, A.M.: Microencapsulated islets as bioartificial endocrine pancreas. Science 210(4472), 908–910 (1980)
Fu, X.W., Sun, A.M.: Microencapsulated parathyroid cells as a bioartificial parathyroid- in vivo studies. Transplantation 47(3), 432–435 (1989)
Cieslinski, D.A., Humes, H.D.: Tissue engineering of a bioartificial kidney. Biotechnol. Bioeng. 43(7), 678–681 (1994)
de Vos, P., Bucko, M., Gemeiner, P., Navratil, M., Svitel, J., Faas, M., et al.: Multiscale requirements for bioencapsulation in medicine and biotechnology. Biomaterials 30(13), 2559–2570 (2009)
Hernández, R.M., Orive, G., Murua, A., Pedraz, J.L.: Microcapsules and microcarriers for in situ cell delivery. Adv. Drug Deliv. Rev. 62(7–8), 711–730 (2010)
Hay, P.D., Veitch, A.R., Smith, M.D., Cousins, R.B., Gaylor, J.D.S.: Oxygen transfer in a diffusion-limited hollow fiber bioartificialâ liver. Artif. Organs 24(4), 278–288 (2000)
Yu, P., Zeng, Y., Lee, T.S., Low, H.T.: A numerical analysis of cell density effect on oxygen transport in a micro-bioreactor with a tissue engineering scaffold. Int. Commun. Heat Mass Transfer 36(6), 569–573 (2009)
Chin, K., Khattak, S.F., Bhatia, S.R., Roberts, S.C.: Hydrogel-perfluorocarbon composite scaffold promotes oxygen transport to immobilized cells. Biotechnol. Prog. 24(2), 358–366 (2008)
Valentin, J.E., Freytes, D.O., Grasman, J.M., Pesyna, C., Freund, J., Gilbert, T.W., et al.: Oxygen diffusivity of biologic and synthetic scaffold materials for tissue engineering. J. Biomed. Mater. Res. A 91A(4), 1010–1017 (2009)
Radisic, M., Deen, W., Langer, R., Vunjak-Novakovic, G.: Mathematical model of oxygen distribution in engineered cardiac tissue with parallel channel array perfused with culture medium containing oxygen carriers. Am. J. Physiol. Heart Circ. Physiol. 288(3), H1278–H1289 (2005)
Acosta, M.A., Ymele-Leki, P., Kostov, Y.V., Leach, J.B.: Fluorescent microparticles for sensing cell microenvironment oxygen levels within 3D scaffolds. Biomaterials 30(17), 3068–3074 (2009)
Mishra, A., Starly, B.: Real time in vitro measurement of oxygen uptake rates for HEPG2 liver cells encapsulated in alginate matrices. Microfluid. Nanofluidics 6(3), 373–381 (2009)
Kino-oka, M., Kagita, S., Nadzir, M.M., Inoue, H., Sugawara, K., Taya, M.: Direct measurement of oxygen concentration inside cultured cartilage for relating to spatial growth of rabbit chondrocytes. J. Biosci. Bioeng. 110(3), 363–366 (2010)
Terai, S., Tsujimura, T., Li, S., Hori, Y., Toyama, H., Shinzeki, M., et al.: Effect of oxygenated perfluorocarbon on isolated islets during transportation. J. Surg. Res. 162(2), 284–289 (2010)
Chen, G., Palmer, A.F.: Mixtures of hemoglobin-based oxygen carriers and perfluorocarbons exhibit a synergistic effect in oxygenating hepatic hollow fiber bioreactors. Biotechnol. Bioeng. 105(3), 534–542 (2010)
Brown, D.A., MacLellan, W.R., Laks, H., Dunn, J.C.Y., Wu, B.M., Beygui, R.E.: Analysis of oxygen transport in a diffusion-limited model of engineered heart tissue. Biotechnol. Bioeng. 97(4), 962–975 (2007)
Kimelman-Bleich, N., Pelled, G., Sheyn, D., Kallai, I., Zilberman, Y., Mizrahi, O., et al.: The use of a synthetic oxygen carrier-enriched hydrogel to enhance mesenchymal stem cell-based bone formation in vivo. Biomaterials [Article] 30(27), 4639–4648 (2009)
Lowe, K.C., Davey, M.R., Power, J.B.: Perfluorochemicals: their applications and benefits to cell culture. Trends Biotechnol. 16(6), 272–277 (1998)
Peng, C.C., Kim, J., Chauhan, A.: Extended delivery of hydrophilic drugs from silicone-hydrogel contact lenses containing Vitamin E diffusion barriers. Biomaterials 31(14), 4032–4047 (2010)
Stoppel, W.L., White, J.C., Horava, S.D., Bhatia, S.R., Roberts, S.C.: Transport of biological molecules in surfactant-alginate composite hydrogels. Acta Biomaterialia. In Press, Accepted Manuscript
Demol, J., Lambrechts, D., Geris, L., Schrooten, J., Van Oosterwyck, H.: Towards a quantitative understanding of oxygen tension and cell density evolution in fibrin hydrogels. Biomaterials 32(1), 107–118 (2011)
Wood, B.D., Quintard, M., Whitaker, S.: Calculation of effective diffusivities for biofilms and tissues. Biotechnol. Bioeng. 77(5), 495–516 (2002)
Rumsey, W.L., Schlosser, C., Nuutinen, E.M., Robiolio, M., Wilson, D.F.: Cellular energetics and the oxygen dependence of respiration in cardiac myocytes isolated from adult rat. J. Biol. Chem. 265(26), 15392–15402 (1990)
Xiong, Y., Xu, J., Zhu, D-q, Duan, C-f, Guan, Y-f: Fiber-optic fluorescence sensor for dissolved oxygen detection based on fluorinated xerogel immobilized with ruthenium (II) complex. J. Sol-Gel Sci. Technol. 53(2), 441–447 (2010)
Bellucci, J.J., Hamaker, K.H.: Evaluation of oxygen transfer rates in stirred-tank bioreactors for clinical manufacturing. Biotechnol. Prog. 27(2), 368–376 (2011)
Suresh, S., Srivastava, V.C., Mishra, I.M.: Techniques for oxygen transfer measurement in bioreactors: a review. J. Chem. Technol. Biotechnol. 84(8), 1091–1103 (2009)
Niebruegge, S., Bauwens, C.L., Peerani, R., Thavandiran, N., Masse, S., Sevaptisidis, E., et al.: Generation of human embryonic stem cell-derived mesoderm and cardiac cells using size-specified aggregates in an oxygen-controlled bioreactor. Biotechnol. Bioeng. 102(2), 493–507 (2009)
Volkmer, E., Drosse, I., Otto, S., Stangelmayer, A., Stengele, M., Kallukalam, B.C., et al.: Hypoxia in static and dynamic 3D culture systems for tissue engineering of bone. Tissue Eng. Part A 14(8), 1331–1340 (2008)
Janssen, F.W., Oostra, J., Oorschot, Av, van Blitterswijk, C.A.: A perfusion bioreactor system capable of producing clinically relevant volumes of tissue-engineered bone: In vivo bone formation showing proof of concept. Biomaterials 27(3), 315–323 (2006)
Picioreanu, C., van Loosdrecht, M.C.M., Heijnen, J.J.: A new combined differential-discrete cellular automaton approach for biofilm modeling: Application for growth in gel beads. Biotechnol. Bioeng. 57(6), 718–731 (1998)
Shuler, M., Kargi, F.: Bioprocess Engineering. Prentice Hall Upper Saddle River, NJ (2002)
Cassell, O.C.S., Morrison, W.A., Messina, A., Penington, A.J., Thompson, E.W., Stevens, G.W., et al.: The influence of extracellular matrix on the generation of vascularized, engineered, transplantable tissue. Ann. N. Y. Acad. Sci. 944(1), 429–442 (2001)
Vunjak-Novakovic, G., Obradovic, B., Martin, I., Bursac, P.M., Langer, R., Freed, L.E.: Dynamic cell seeding of polymer scaffolds for cartilage tissue engineering. Biotechnol. Prog. 14(2), 193–202 (1998)
Discher, D.E., Mooney, D.J., Zandstra, P.W.: Growth factors, matrices, and forces combine and control stem cells. Science 324(5935), 1673–1677 (2009)
Clark, L.C.J.R., Wolf, R., Granger, D., Taylor, Z.: Continuous recording of blood oxygen tensions by polarography. J. Appl. Physiol. 6(3), 189–193 (1953)
Androjna, C., Gatica, J., Belovich, J., Derwin, K.: Oxygen diffusion through natural extracellular matrices: Implications for estimating critical thickness” values in tendon tissue engineering. Tissue Eng. Part A 14(4), 559–569 (2008)
Strickland, J.D.H., Parsons, T.R., Fisheries Research Board of Canada: A Practical Handbook of Seawater Analysis, 2nd edn. Fisheries Research Board of Canada, Ottawa (1972)
Koo, Y.-E.L., Cao, Y., Kopelman, R., Koo, S.M., Brasuel, M., Philbert, M.A.: Real-time measurements of dissolved oxygen inside live cells by organically modified silicate fluorescent nanosensors. Anal. Chem. 76(9), 2498–2505 (2004)
Choi, N.W., Cabodi, M., Held, B., Gleghorn, J.P., Bonassar, L.J., Stroock, A.D.: Microfluidic scaffolds for tissue engineering. Nat. Mater. 6(11), 908–915 (2007)
Oppegard, S.C., Blake, A.J., Williams, J.C., Eddington, D.T.: Precise control over the oxygen conditions within the Boyden chamber using a microfabricated insert. Lab Chip 10(18), 2366–2373 (2010)
Ishaug, S.L., Crane, G.M., Miller, M.J., Yasko, A.W., Yaszemski, M.J., Mikos, A.G.: Bone formation by three-dimensional stromal osteoblast culture in biodegradable polymer scaffolds. J. Biomed. Mater. Res. 36(1), 17–28 (1997)
Laurencin, C.T., Attawia, M.A., Elgendy, H.E., Herbert, K.M.: Tissue engineered bone-regeneration using degradable polymers: The formation of mineralized matrices. Bone 19(1 Suppl), S93–S99 (1996)
Burg, K.J.L., Porter, S., Kellam, J.F.: Biomaterial developments for bone tissue engineering. Biomaterials 21(23), 2347–2359 (2000)
Ma, P.X., Zhang, R., Xiao, G., Franceschi, R.: Engineering new bone tissue in vitro on highly porous poly(α-hydroxyl acids)/hydroxyapatite composite scaffolds. J. Biomed. Mater. Res. 54(2), 284–293 (2001)
Pisu, M., Lai, N., Cincotti, A., Concas, A., Cao, G.: Modeling of engineered cartilage growth in rotating bioreactors. Chem. Eng. Sci. 59(22–23), 5035–5040 (2004)
Grande, D.A., Halberstadt, C., Naughton, G., Schwartz, R., Manji, R.: Evaluation of matrix scaffolds for tissue engineering of articular cartilage grafts. J. Biomed. Mater. Res. 34(2), 211–220 (1997)
Chromiak, J., Shansky, J., Perrone, C., Vandenburgh, H.: Bioreactor perfusion system for the long-term maintenance of tissue-engineered skeletal muscle organoids. In Vitro Cell. Dev. Biol. Anim. 34(9), 694–703 (1998)
Jeong, S.I., Kwon, J.H., Lim, J.I., Cho, S.-W., Jung, Y., Sung, W.J., et al.: Mechano-active tissue engineering of vascular smooth muscle using pulsatile perfusion bioreactors and elastic PLCL scaffolds. Biomaterials 26(12), 1405–1411 (2005)
Carrier, R.L., Rupnick, M., Langer, R., Schoen, F.J., Freed, L.E., Vunjak-Novakovic, G.: Effects of oxygen on engineered cardiac muscle. Biotechnol. Bioeng. 78(6), 617–625 (2002)
Brown, D.A., MacLellan, W.R., Dunn, J.C.Y., Wu, B.M., Beygui, R.E.: Hypoxic cell death is reduced by pH buffering in a model of engineered heart tissue. Artif. Cells Blood Substit. Immobil. Biotechnol. 36(2), 94–113 (2008)
Nieuwoudt, M.J., Moolman, S.F., Van Wyk, K.J., Kreft, E., Olivier, B., Laurens, J.B., et al.: Hepatocyte function in a radial-flow bioreactor using a perfluorocarbon oxygen carrier. Artif. Organs 29(11), 915–918 (2005)
Nieuwoudt, M., Wiggett, S., Malfeld, S., van der Merwe, S.: Imaging glucose metabolism in perfluorocarbon-perfused hepatocyte bioreactors using positron emission tomography. J. Artif. Organs 12(4), 247–257 (2009)
Gordon, J.E., Dare, M.R., Palmer, A.F.: Engineering select physical properties of cross-linked red blood cells and a simple a priori estimation of their efficacy as an oxygen delivery vehicle within the context of a hepatic hollow fiber bioreactor. Biotechnol. Prog. 21(6), 1700–1707 (2005)
Zhao, F., Chella, R., Ma, T.: Effects of shear stress on 3-D human mesenchymal stem cell construct development in a perfusion bioreactor system: Experiments and hydrodynamic modeling. Biotechnol. Bioeng. 96(3), 584–595 (2007)
Datta, N., Pham, Q.P., Sharma, U., Sikavitsas, V.I., Jansen, J.A., Mikos, A.G.: In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblastic differentiation. Proc. Natl Acad. Sci. USA 103(8), 2488–2493 (2006)
Holtorf, H.L., Jansen, J.A., Mikos, A.G.: Flow perfusion culture induces the osteoblastic differentiation of marrow stromal cell-scaffold constructs in the absence of dexamethasone. J. Biomed. Mater. Res. A 72A(3), 326–334 (2005)
Riess, J.G.: Perfluorocarbon-based oxygen delivery. Artif. Cells Blood Substit. Immobil.Biotechnol. 34(6), 567–580 (2006)
Riess, J.G.: Oxygen carriers (“blood substitutes”) – Raison d’Etre, chemistry, and some physiology. Chem. Rev. 101(9), 2797–2919 (2001)
Phillips, W.T., Goins, B., Klipper, R., Cook, B.G., Martin, C., Lemen, L., et al.: Tissue oxygen delivery and tissue distribution of liposome encapsulated hemoglobin. In: Eishun, T. (ed.) Blood Substitutes, Present and Future Perspectives, pp. 147–160. Elsevier Science, Lausanne (1998)
Sakai, H., Tomiyama, K-i, Sou, K., Takeoka, S., Tsuchida, E.: Poly(ethylene glycol)-conjugation and deoxygenation enable long-term preservation of hemoglobin-vesicles as oxygen carriers in a liquid state. Bioconjug. Chem. 11(3), 425–432 (2000)
Phillips, W.T., Klipper, R.W., Awasthi, V.D., Rudolph, A.S., Cliff, R., Kwasiborski, V., et al.: Polyethylene glycol-modified liposome-encapsulated hemoglobin: A long circulating red cell substitute. J. Pharmacol. Exp. Ther. 288(2), 665–670 (1999)
Sakai, H., Takeoka, S., Park, S.I., Kose, T., Nishide, H., Izumi, Y., et al.: Surface modification of hemoglobin vesicles with poly(ethylene glycol) and effects on aggregation, viscosity, and blood flow during 90 exchange transfusion in anesthetized rats. Bioconjug. Chem. 8(1), 23–30 (1997)
Usuba, A., Osuka, F., Kimura, T., Sato, R., Ogata, Y., Gotoh, H., et al.: Effect of liposome-encapsulated hemoglobin, neo red cells, on hemorrhagic shock. Surg. Today 28(10), 1027–1035 (1998)
Takahashi, A.: Characterization of neo red cells (NRCs), their function and safety in vivo tests. Artif. Cells Blood Substit. Immobil.Biotechnol. 23(3), 347–354 (1995)
Li, S., Nickels, J., Palmer, A.F.: Liposome-encapsulated actin-hemoglobin (LEAcHb) artificial blood substitutes. Biomaterials 26(17), 3759–3769 (2005)
Discher, B.M., Bermudez, H., Hammer, D.A., Discher, D.E., Won, Y.-Y., Bates, F.S.: Cross-linked polymersome membranes: vesicles with broadly adjustable properties. J. Phys. Chem. B 106(11), 2848–2854 (2002)
Discher, B.M., Won, Y.-Y., Ege, D.S., Lee, J.C.M., Bates, F.S., Discher, D.E., et al.: Polymersomes: Tough vesicles made from diblock copolymers. Science 284(5417), 1143–1146 (1999)
Discher, D.E., Ahmed, F.: Polymersomes. Annu. Rev. Biomed. Eng. 8(1), 323–341 (2006)
Rameez, S., Alosta, H., Palmer, A.F.: Biocompatible and biodegradable polymersome encapsulated hemoglobin: A potential oxygen carrier. Bioconjug. Chem. 19(5), 1025–1032 (2008)
Riess, J.G.: Understanding the fundamentals of perfluorocarbons and perfluorocarbon emulsions relevant to in vivo oxygen delivery. Artif. Cells Blood Substit. Immobil. Biotechnol. 33(1), 47–63 (2005)
Krafft, M.P., Riess, J.G.: Highly fluorinated amphiphiles and colloidal systems, and their applications in the biomedical field. A contribution. Biochimie 80(5–6), 489–514 (1998)
Kuznetsova, I.N.: Perfluorocarbon emulsions: Stability in vitro and in vivo (A Review). Pharm. Chem. J. 37(8), 415–420 (2003)
Fijan, R., Sostar-Turk, S., Lapasin, R.: Rheological study of interactions between non-ionic surfactants and polysaccharide thickeners used in textile printing. Carbohydr. Polym. 68(4), 708–717 (2007)
Krafft, M.P., Riess, J.G.: Perfluorocarbons: Life sciences and biomedical uses dedicated to the memory of Professor Guy Ourisson, a true Renaissance man. J. Polym. Sci. A: Polym. Chem. 45(7), 1185–1198 (2007)
Gauger, P.G., Pranikoff, T., Schreiner, R.J., Moler, F.W., Hirschl, R.B.: Initial experience with partial liquid ventilation in pediatric patients with the acute respiratory distress syndrome. Crit. Care Med. 24(1), 16–22 (1996)
Kin, T., Mirbolooki, M., Salehi, P., Tsukada, M., O’Gorman, D., Imes, S., et al.: Islet isolation and transplantation outcomes of pancreas preserved with University of Wisconsin solution versus two-layer method using preoxygenated perfluorocarbon. Transplantation 82(10), 1286–1290 (2006)
Fraker, C.A., Alvarez, S., Papadopoulos, P., Giraldo, J., Gu, W.Y., Ricordi, C., et al.: Enhanced oxygenation promotes beta-cell differentiation in vitro. Stem Cells 25(12), 3155–3164 (2007)
Tan, Q., El-Badry, A.M., Contaldo, C., Steiner, R., Hillinger, S., Welti, M., et al.: The effect of perfluorocarbon-based artificial oxygen carriers on tissue-engineered trachea. Tissue Eng. Part A 15(9), 2471–2480 (2009)
MathyHartert, M., Krafft, M.P., Deby, C., DebyDupont, G., Meurisse, M., Lamy, M., et al.: Effects of perfluorocarbon emulsions on cultured human endothelial cells. Artif. Cells Blood Substit. Immobil. Biotechnol. 25(6), 563–575 (1997)
Ricordi, C., Fraker, C., Szust, J., Al-Abdullah, I., Poggioli, R., Kirlew, T., et al.: Improved human islet isolation outcome from marginal donors following addition of oxygenated perfluorocarbon to the cold-storage solution. Transplantation 75(9), 1524–1527 (2003)
Centis, V., Doillon, C.J., Vermette, P.: (eds). Perfluorocarbon emulsions cytotoxic effects on human fibroblasts and effect of aging on particle size distribution. 9th Vienna International Workshop on Functional Electrical Stimulation; pp. 19–22. Krems, Austria (2007)
Ward, C., Haines, N., Patel, R., Eisele, E., Harrison, B.: Assessing angiogenic activity of cells in the presence of oxygen generating biomaterials. FASEB J. 23((1_MeetingAbstracts)), 9518 (2009)
West, J., Moon, J.: Vascularization of engineered tissues: approaches to promote angiogenesis in biomaterials. Curr. Top. Med. Chem. 8(4), 300–310 (2008)
Hirschi, K., Skalak, T., Peirce, S., Little, C.: Vascular assembly in natural and engineered tissues. Ann. N. Y. Acad. Sci. 961(Reparative medicine: Growing tissues and organs), 223–242 (2002)
Jay, S., Shepherd, B., Andrejecsk, J., Kyriakides, T., Pober, J., Saltzman, W.: Dual delivery of VEGF and MCP-1 to support endothelial cell transplantation for therapeutic vascularization. Biomaterials 31(11), 3054–3062 (2010)
Carmeliet, P.: Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 6(4), 389–395 (2000)
Sun, G., Kusuma, S., Gerecht, S.: The integrated role of biomaterials and stem cells in vascular regeneration. In: Roy, K. (ed.) Biomaterials as Stem Cell Niche, pp. 195–223. Springer, Berlin, Heidelberg (2010)
Jain, R.: Molecular regulation of vessel maturation. Nat. Med. 9(6), 685–693 (2003)
West, J.L., Hubbell, J.A.: Polymeric biomaterials with degradation sites for proteases involved in cell migration. Macromolecules 32(1), 241–244 (1998)
Lutolf, M.P., Hubbell, J.A.: Synthesis and physicochemical characterization of end-linked poly(ethylene glycol)-co-peptide hydrogels formed by Michael-type addition. Biomacromolecules 4(3), 713–722 (2003)
Zisch, A.H., Lutolf, M.P., Ehrbar, M., Raeber, G.P., Rizzi, S.C., Davies, N., et al.: Cell-demanded release of VEGF from synthetic, biointeractive cell-ingrowth matrices for vascularized tissue growth. FASEB J. 17(13), 2260–2262 (2003)
Kamei, M., Saunders, W.B., Bayless, K.J., Dye, L., Davis, G.E., Weinstein, B.M.: Endothelial tubes assemble from intracellular vacuoles in vivo. Nature 442(7101), 453–456 (2006)
Moon, J.J., Saik, J.E., Poche, R.A., Leslie-Barbick, J.E., Lee, S.H., Smith, A.A., et al.: Biomimetic hydrogels with pro-angiogenic properties. Biomaterials 31(14), 3840–3847 (2010)
Leslie-Barbick, J., Moon, J., West, J.: Covalently-immobilized vascular endothelial growth factor promotes endothelial cell tubulogenesis in poly (ethylene glycol) diacrylate hydrogels. J. Biomater. Sci. Polym. Ed. 20(12), 1763–1779 (2009)
Leslie-Barbick, J.E., Shen, C., Chen, C., West, J.L.: Micron-scale spatially patterned, covalently immobilized vascular endothelial growth factor on hydrogels accelerates endothelial tubulogenesis and increases cellular angiogenic responses. Tissue Eng. Part A 17(1–2), 221–229 (2011)
Richardson, T.P., Peters, M.C., Ennett, A.B., Mooney, D.J.: Polymeric system for dual growth factor delivery. Nat. Biotechnol. 19(11), 1029–1034 (2001)
Pike, D.B., Cai, S., Pomraning, K.R., Firpo, M.A., Fisher, R.J., Shu, X.Z., et al.: Heparin-regulated release of growth factors in vitro and angiogenic response in vivo to implanted hyaluronan hydrogels containing VEGF and bFGF. Biomaterials 27(30), 5242–5251 (2006)
Freeman, I., Cohen, S.: The influence of the sequential delivery of angiogenic factors from affinity-binding alginate scaffolds on vascularization. Biomaterials 30(11), 2122–2131 (2009)
Carmeliet, P.: Angiogenesis in life, disease and medicine. Nature 438(7070), 932–936 (2005)
Lutolf, M.P., Hubbell, J.A.: Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 23(1), 47–55 (2005)
Carmeliet, P.: Angiogenesis in health and disease. Nat. Med. 9(6), 653–660 (2003)
Jay, S.M., Shepherd, B.R., Bertram, J.P., Pober, J.S., Saltzman, W.M.: Engineering of multifunctional gels integrating highly efficient growth factor delivery with endothelial cell transplantation. FASEB J. 22(8), 2949–2956 (2008)
Ozawa, C.R., Banfi, A., Glazer, N.L., Thurston, G., Springer, M.L., Kraft, P.E., et al.: Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J. Clin. Invest. 113(4), 516–527 (2004)
Wei, G., Jin, Q., Giannobile, W.V., Ma, P.X.: Nano-fibrous scaffold for controlled delivery of recombinant human PDGF-BB. J. Control. Release 112(1), 103–110 (2006)
Sahni, A., Sporn, L.A., Francis, C.W.: Potentiation of endothelial cell proliferation by fibrin(ogen)-bound fibroblast growth factor-2. J. Biol. Chem. 274(21), 14936–14941 (1999)
DeLong, S.A., Moon, J.J., West, J.L.: Covalently immobilized gradients of bFGF on hydrogel scaffolds for directed cell migration. Biomaterials 26(16), 3227–3234 (2005)
Refojo, M.F.: Ophthalmological Applications. In: Rattner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E. (eds.) Biomaterials science: An introduction to materials in medicine, 2nd edn, pp. 583–591. Elsevier Academic, San Diego (2004)
Holden, B.A., Newton-Howes, J., Winterton, L., Fatt, I., Hamano, H., Hood, D.L., et al.: The Dk project: An interlaboratory comparison of DK/L measurements. Optom. Vis. Sci. 67(6), 476–481 (1990)
Peppas, N.A., Yang, W.-H.M.: Properties-based optimization of the structure of polymers for contact lens applications. Eye Contact Lens 7(4), 300–314 (1981)
Hong, X.I.N., Himebaugh, N., Thibos, L.N.: On-eye evaluation of optical performance of rigid and soft contact lenses. Optom. Vis. Sci. 78(12), 872–880 (2001)
Alvord, L., Court, J., Davis, T., Morgan, C.F., Schindhelm, K., Vogt, J., et al.: Oxygen permeability of a new type of high Dk soft contact lens material. Optom. Vis. Sci. 75(1), 30–36 (1998)
Wichterle, O., Lim, D.: Hydrophilic gels for biological use. Nature 185(4706), 117–118 (1960)
Ehlers, W., Donshik, P.C.: Update on lotrafilcon A contact lenses. Expert Rev. Ophthalmol. 5, 19–25 (2010)
Dillehay, S.M., Miller, M.B.: Performance of lotrafilcon B silicone hydrogel contact lenses in experienced low-Dk/t daily lens wearers. Eye Contact Lens 33(6, Part 1 of 2), 272–277 (2007)
Teramura, Y., Iwata, H.: Islet encapsulation with living cells for improvement of biocompatibility. Biomaterials 30(12), 2270–2275 (2009)
Schneider, S., Feilen, P.J., Slotty, V., Kampfner, D., Preuss, S., Berger, S., et al.: Multilayer capsules: a promising microencapsulation system for transplantation of pancreatic islets. Biomaterials 22(14), 1961–1970 (2001)
de Vos, P., Marchetti, P.: Encapsulation of pancreatic islets for transplantation in diabetes: the untouchable islets. Trends Mol. Med. 8(8), 363–366 (2002)
Zimmermann, H., Hillgartner, M., Manz, B., Feilen, P., Brunnenmeier, F., Leinfelder, U., et al.: Fabrication of homogeneously cross-linked, functional alginate microcapsules validated by NMR-, CLSM- and AFM-imaging. Biomaterials 24(12), 2083–2096 (2003)
Cheung, C.Y., Anseth, K.S.: Synthesis of immunoisolation barriers that provide localized immunosuppression for encapsulated pancreatic islets. Bioconjug. Chem. 17(4), 1036–1042 (2006)
Weber, L.M., Cheung, C.Y., Anseth, K.S.: Multifunctional pancreatic islet encapsulation barriers achieved via multilayer PEG hydrogels. Cell Transplant. 16(10), 1049–1057 (2007)
Abalovich, A.G., Bacque, M.C., Grana, D., Milei, J. (eds). Pig pancreatic islet transplantation into spontaneously diabetic dogs. 22nd International Congress of the Transplantation-Society, Sydney, Australia, 10–14 Aug 2008
Koo, S.K., Kim, S.C., Wee, Y.M., Kim, Y.H., Jung, E.J., Choi, M.Y., et al.: Experimental microencapsulation of porcine and rat pancreatic islet cells with air-driven droplet generator and alginate. Transplant. Proc. 40(8), 2578–2580 (2008)
Qi, M., Strand, B.L., Morch, Y., Lacik, I., Wang, Y., Salehi, P., et al.: Encapsulation of human islets in novel inhomogeneous alginate-Ca2+/Ba2+ microbeads: In vitro and in vivo function. Artif. Cells Blood Substit.Immobil. Biotechnol. 36(5), 403–420 (2008)
de Groot, M., Schuurs, T.A., van Schilfgaarde, R.: Causes of limited survival of microencapsulated pancreatic islet grafts. J. Surg. Res. 121(1), 141–150 (2004)
de Groot, M., Schuurs, T., Keizer, P., Fekken, S., Leuvenink, H., van Schilfgaarde, R.: Response of encapsulated rat pancreatic islets to hypoxia. Cell Transplant. 12(8), 867–875 (2003)
Zimmermann, H., Shirley, S., Zimmermann, U.: Alginate-based encapsulation of cells: Past, present, and future. Curr. Diab. Rep. 7(4), 314–320 (2007)
Strand, B.L., Ryan, L., Veld, P.I., Kulseng, B., Rokstad, A.M., Skjak-Braek, G., et al.: Poly-L-lysine induces fibrosis on alginate microcapsules via the induction of cytokines. Cell Transplant. 10(3), 263–275 (2001)
Dionne, K.E., Colton, C.K., Yarmush, M.L.: Effect of hypoxia on insulin-secretion by isolated rate and canine islets of Langerhans. Diabetes 42(1), 12–21 (1993)
Matsumoto, S., Qualley, S.A., Rigley, T.H., Marsh, C.L., Stevens, R.B.: Prolonged preservation of the human pancreas prior to islet isolation using the two-layer (University of Wisconsin solution [UW]/perfluorocarbon) method. Transplantation 69(8), 384 (2000)
Maillard, E., Sanchez-Dominguez, M., Kleiss, C., Langlois, A., Sencier, M.C., Vodouhe, C., et al.: Perfluorocarbons: New tool for islets preservation in vitro. Transplant. Proc. 40(2), 372–374 (2008)
Sigrist, S., Mechine-Neuville, A., Mandes, K., Calenda, V., Braun, S., Legeay, G., et al.: Influence of VEGF on the viability of encapsulated pancreatic rat islets after transplantation in diabetic mice. Cell Transplant. 12(6), 627–635 (2003)
Sigrist, S., Mechine-Neuville, A., Mandes, K., Calenda, V., Legeay, G., Bellocq, J.P., et al.: Induction of angiogenesis in omentum with vascular endothelial growth factor: Influence on the viability of encapsulated rat pancreatic islets during transplantation. J. Vasc. Res. 40(4), 359–367 (2003)
Lai, Y., Schneider, D., Kidszun, A., Hauck-Schmalenberger, I., Breier, G., Brandhorst, D., et al.: Vascular endothelial growth factor increases functional beta-cell mass by improvement of angiogenesis of isolated human and murine pancreatic islets. Transplantation 79(11), 1530–1536 (2005)
Lembert, N., Wesche, J., Petersen, P., Doser, M., Zschocke, P., Becker, H.D., et al.: Encapsulation of islets in rough surface, hydroxymethylated polysulfone capillaries stimulates VEGF release and promotes vascularization after transplantation. Cell Transplant. 14(2–3), 97–108 (2005)
Juang, J., Hsu, B., Kuo, C., Ueng, S.: Beneficial effects of hyperbaric oxygen therapy on islet transplantation. Cell Transplant. 11(2), 95–101 (2002)
Lakey, J.R.T., Mirbolooki, M., Shapiro, A.M.J.: Current status of clinical islet cell transplantation. Transplant. Immunol. 333, 47–103 (2006)
Salcedo, R., Ponce, M.L., Young, H.A., Wasserman, K., Ward, J.M., Kleinman, H.K., et al.: Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood 96(1), 34–40 (2000)
Paule, M.F., McColl, S.R., Simeonovic, C.J.: Murine chemokine gene expression in rejecting pig proislet xenografts. Transplant. Proc. 32(5), 1062 (2000)
de Vos, P., Hillebrands, J.-L., De Haan, B.J., Strubbe, J.H., Van Schilfgaarde, R.: Efficacy of a prevascularized expanded polytetrafluoroethylene solid support system as a transplantation site for pancreatic islets 1. Transplantation 63(6), 824–830 (1997)
Sen, C.: Wound healing essentials: let there be oxygen. Wound Repair Regen. 17(1), 1–18 (2009)
Tandara, A.A., Mustoe, T.A.: Oxygen in wound healing – More than a nutrient. World J. Surg. 28(3), 294–300 (2004)
Greif, R., Akça, O., Horn, E.-P., Kurz, A., Sessler, D.I.: Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. N. Engl. J. Med. 342(3), 161–167 (2000)
Kumari, R., Willing, L.B., Krady, J.K., Vannucci, S.J., Simpson, I.A.: Impaired wound healing after cerebral hypoxia-ischemia in the diabetic mouse. J. Cereb. Blood Flow Metab. 27(4), 710–718 (2006)
Distler, O., Distler, J.H.W., Scheid, A., Acker, T., Hirth, A., Rethage, J., et al.: Uncontrolled expression of vascular endothelial growth factor and its receptors leads to insufficient skin angiogenesis in patients with systemic sclerosis. Circ. Res. 95(1), 109–116 (2004)
Salaman, R.A., Harding, K.G.: The aetiology and healing rates of chronic leg ulcers. J. Wound Care 4(7), 320–323 (1995)
Shai, A., Maibach, H.I.: Wound healing and ulcers of the skin diagnosis and therapy – The practical approach. Springer, Berlin, Heidelberg (2005)
McGill, M., Collins, P., Bolton, T., Yue, D.K.: Management of neuropathic ulceration. J. Wound Care 5(2), 52–54 (1996)
Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011.
Hafner, J., Schaad, I., Schneider, E., Seifert, B., Burg, G., Cassina, P.C.: Leg ulcers in peripheral arterial disease (arterial leg ulcers): Impaired wound healing above the threshold of chronic critical limb ischemia. J. Am. Acad. Dermatol. 43(6), 1001–1008 (2000)
Salaman, J.H., Miller, L., Harding, K.G.: Management of foot ulceration in a patient with diabetes mellitus. J. Wound Care 4(10), 443–444 (1995)
Lait, M.E., Smith, L.N.: Wound management: a literature review. J. Clin. Nurs. 7(1), 11–17 (1998)
Gordillo, G., Roy, S., Khanna, S., Schlanger, R., Khandelwal, S., Phillips, G., et al.: Topical oxygen therapy induces vascular endothelial growth factor expression and improves closure of clinically presented chronic wounds. Clin. Exp. Pharmacol. Physiol. 35(8), 957–964 (2008)
Kalliainen, L., Gordillo, G., Schlanger, R., Sen, C.: Topical oxygen as an adjunct to wound healing: a clinical case series. Pathophysiology 9(2), 81–87 (2003)
Fries, R., Wallace, W., Roy, S., Kuppusamy, P., Bergdall, V., Gordillo, G., et al.: Dermal excisional wound healing in pigs following treatment with topically applied pure oxygen. Mutat. Res. 579(1–2), 172–181 (2005)
Davis, S., Cazzaniga, A., Ricotti, C., Zalesky, P., Hsu, L., Creech, J., et al.: Topical oxygen emulsion: a novel wound therapy. Arch. Dermatol. 143(10), 1252 (2007)
Scadden, D.T.: The stem-cell niche as an entity of action. Nature 441(7097), 1075–1079 (2006)
Mohyeldin, A., Garzon-Muvdi, T., Quinones-Hinojosa, A.: Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell 7(2), 150–161 (2010)
Engler, A.J., Sen, S., Sweeney, H.L., Discher, D.E.: Matrix elasticity directs stem cell lineage specification. Cell 126(4), 677–689 (2006)
Yoshida, Y., Takahashi, K., Okita, K., Ichisaka, T., Yamanaka, S.: Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell 5(3), 237–241 (2009)
Grayson, W.L., Zhao, F., Izadpanah, R., Bunnell, B., Ma, T.: Effects of hypoxia on human mesenchymal stem cell expansion and plasticity in 3D constructs. J. Cell. Physiol. 207(2), 331–339 (2006)
Ma, T., Grayson, W.L., Fröhlich, M., Vunjak-Novakovic, G.: Hypoxia and stem cell-based engineering of mesenchymal tissues. Biotechnol. Prog. 25(1), 32–42 (2009)
Zhao, F., Veldhuis, J.J., Duan, Y., Yang, Y., Christoforou, N., Ma, T., et al.: Low oxygen tension and synthetic nanogratings improve the uniformity and stemness of human mesenchymal stem cell layer. Mol. Ther. 18(5), 1010–1018 (2010)
Simon, M.C., Keith, B.: The role of oxygen availability in embryonic development and stem cell function. Nat. Rev. Mol. Cell Biol. 9(4), 285–296 (2008)
Eliasson, P., Jönsson, J.: The hematopoietic stem cell niche: low in oxygen but a nice place to be. J. Cell. Physiol. 222(1), 17–22 (2010)
dos Santos, F., Andrade, P.Z., Boura, J.S., Abecasis, M.M., dos Silva, C.L., Cabral, J.M.S.: Ex vivo expansion of human mesenchymal stem cells: A more effective cell proliferation kinetics and metabolism under hypoxia. J. Cell. Physiol. 223(1), 27–35 (2010)
Tottey, S., Corselli, M., Jeffries, E.M., Londono, R., Peault, B., Badylak, S.F.: Extracellular matrix degradation products and low-oxygen conditions enhance the regenerative potential of perivascular stem cells. Tissue Eng. Part A 17(1–2), 37–44 (2011)
Cipolleschi, M.G., Dello Sbarba, P., Olivotto, M.: The role of hypoxia in the maintenance of hematopoietic stem cells. Blood 82(7), 2031–2037 (1993)
Chow, D.C., Wenning, L.A., Miller, W.M., Papoutsakis, E.T.: Modeling pO2 distributions in the bone marrow hematopoietic compartment. II. Modified Kroghian Models. Biophys. J. 81(2), 685–696 (2001)
Nilsson, S.K., Johnston, H.M., Coverdale, J.A.: Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood 97(8), 2293–2299 (2001)
Parmar, K., Mauch, P., Vergilio, J.-A., Sackstein, R., Down, J.D.: Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc. Natl Acad. Sci. USA 104(13), 5431–5436 (2007)
Abdollahi, H., Harris, L., Zhang, P., McIlhenny, S., Srinivas, V., Tulenko, T., et al.: The role of hypoxia in stem cell differentiation and therapeutics. J. Surg. Res. 1(6), 1 (2009)
Ivanovic, Z.: Hypoxia or in situ normoxia: The stem cell paradigm. J. Cell. Physiol. 219(2), 271–275 (2009)
Raheja, L.F., Genetos, D.C., Yellowley, C.E.: The effect of oxygen tension on the long-term osteogenic differentiation and MMP/TIMP expression of human mesenchymal stem cells. Cells Tissues Organs 191(3), 175–184 (2010)
Takahashi, K., Tanabe, K., Ohnuki, M., Narita, M., Ichisaka, T., Tomoda, K., et al.: Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5), 861–872 (2007)
Nakagawa, M., Koyanagi, M., Tanabe, K., Takahashi, K., Ichisaka, T., Aoi, T., et al.: Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat. Biotech. 26(1), 101–106 (2008). doi:10.1038/nbt1374
Millman, J.R., Tan, J.H., Colton, C.K.: The effects of low oxygen on self-renewal and differentiation of embryonic stem cells. Curr. Opin. Organ Transplant. 14(6), 694–700 (2009)
Ji, A.R., Ku, S.Y., Cho, M.S., Kim, Y.Y., Kim, Y.J., Oh, S.K., et al.: Reactive oxygen species enhance differentiation of human embryonic stem cells into mesendodermal lineage. Exp. Mol. Med. 42(3), 175–186 (2010)
Robertson, E.J.: Teratocarcinomas and Embryonic Stem Cells: A Practical Approach. IRL, Oxford (1987)
Boiani, M., Eckardt, S., Schöler, H.R., McLaughlin, K.J.: Oct4 distribution and level in mouse clones: consequences for pluripotency. Genes Dev. 16(10), 1209–1219 (2002)
Forristal, C.E., Wright, K.L., Hanley, N.A., Oreffo, R.O.C., Houghton, F.D.: Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction 139(1), 85–97 (2010)
Takahashi, K., Yamanaka, S.: Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4), 663–676 (2006)
Boyer, L.A., Lee, T.I., Cole, M.F., Johnstone, S.E., Levine, S.S., Zucker, J.P., et al.: Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 122(6), 947–956 (2005)
Keith, B., Simon, M.C.: Hypoxia-inducible factors, stem cells, and cancer. Cell 129(3), 465–472 (2007)
Gustafsson, M.V., Zheng, X., Pereira, T., Gradin, K., Jin, S., Lundkvist, J., et al.: Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev. Cell 9(5), 617–628 (2005)
Panchision, D.M.: The role of oxygen in regulating neural stem cells in development and disease. J. Cell. Physiol. 220(3), 562–568 (2009)
Main, H., Lee, K.L., Yang, H., Haapa-Paananen, S., Edgren, H., Jin, S.B., et al.: Interactions between Notch- and hypoxia-induced transcriptomes in embryonic stem cells. Exp. Cell Res. 316(9), 1610–1624 (2010)
Zachar, V., Prasad, S., Weli, S., Gabrielsen, A., Petersen, K., Petersen, M., et al.: The effect of human embryonic stem cells (hESCs) long-term normoxic and hypoxic cultures on the maintenance of pluripotency. In Vitro Cell. Dev. Biol. Animal. 46(3), 276–283 (2010)
Pear, W.S., Simon, M.C.: Lasting longer without oxygen: The influence of hypoxia on Notch signaling. Cancer Cell 8(6), 435–437 (2005)
Kirouac, D.C., Zandstra, P.W.: The systematic production of cells for cell therapies. Cell Stem Cell 3(4), 369–381 (2008)
Rosová, I., Dao, M., Capoccia, B., Link, D., Nolta, J.A.: Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells 26(8), 2173–2182 (2008)
Li, Z., Leung, M., Hopper, R., Ellenbogen, R., Zhang, M.: Feeder-free self-renewal of human embryonic stem cells in 3D porous natural polymer scaffolds. Biomaterials 31(3), 404–412 (2010)
Leach, J.B., Powell, E.M.: Understanding hypoxic environments: biomaterials approaches to neural stabilization and regeneration after ischemia. In: Roy, K. (ed.) Biomaterials as Stem Cell Niche, pp. 247–274. Springer, Berlin Heidelberg (2010)
Furth, M.E., Atala, A.: Stem cell sources to treat diabetes. J. Cell. Biochem. 106(4), 507–511 (2009)
Holle, A.W., Engler, A.J.: Cell rheology: Stressed-out stem cells. Nat. Mater. 9(1), 4–6 (2010)
Siti-Ismail, N., Bishop, A.E., Polak, J.M., Mantalaris, A.: The benefit of human embryonic stem cell encapsulation for prolonged feeder-free maintenance. Biomaterials 29(29), 3946–3952 (2008)
Liu, H., Collins, S.F., Suggs, L.J.: Three-dimensional culture for expansion and differentiation of mouse embryonic stem cells. Biomaterials 27(36), 6004–6014 (2006)
Fisher, O.Z., Khademhosseini, A., Langer, R., Peppas, N.A.: Bioinspired materials for controlling stem cell fate. Acc. Chem. Res. 43(3), 419–428 (2009)
Bratt-Leal, A.M., Carpenedo, R.L., Ungrin, M.D., Zandstra, P.W., McDevitt, T.C.: Incorporation of biomaterials in multicellular aggregates modulates pluripotent stem cell differentiation. Biomaterials 32(1), 48–56 (2011)
Hwang, Y.-S., Cho, J., Tay, F., Heng, J.Y.Y., Ho, R., Kazarian, S.G., et al.: The use of murine embryonic stem cells, alginate encapsulation, and rotary microgravity bioreactor in bone tissue engineering. Biomaterials 30(4), 499–507 (2009)
Sengupta, S., Park, S.-H., Patel, A., Carn, J., Lee, K., Kaplan, D.L.: Hypoxia and amino acid supplementation synergistically promote the osteogenesis of human mesenchymal stem cells on silk protein scaffolds. Tissue Eng. Part A 16(12), 3623–3634 (2010)
Fridley, K.M., Fernandez, I., Li, M.-T.A., Kettlewell, R.B., Roy, K.: Unique differentiation profile of mouse embryonic stem cells in rotary and stirred tank bioreactors. Tissue Eng. Part A 16(11), 3285–3298 (2010)
Serra, M., Brito, C., Sousa, M.F.Q., Jensen, J., Tostoes, R., Clemente, J., et al.: Improving expansion of pluripotent human embryonic stem cells in perfused bioreactors through oxygen control. J. Biotechnol. 148(4), 208–215 (2010)
Lovett, M., Rockwood, D., Baryshyan, A., Kaplan, D.L.: Simple modular bioreactors for tissue engineering: a system for characterization of oxygen gradients, human mesenchymal stem cell differentiation, and prevascularization. Tissue Eng. Part C Methods 16(6), 1565–1573 (2010)
Malda, J., Martens, D.E., Tramper, J., van Blitterswijk, C.A., Riesle, J.: Cartilage tissue engineering: controversy in the effect of oxygen. Crit. Rev. Biotechnol. 23(3), 175–194 (2003)
Barbero, A., Grogan, S., Schäfer, D., Heberer, M., Mainil-Varlet, P., Martin, I.: Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity. Osteoarthritis Cartilage 12(6), 476–484 (2004)
Kuo, C.K., Li, W.-J., Mauck, R.L., Tuan, R.S.: Cartilage tissue engineering: its potential and uses. Curr. Opin. Rheumatol. 18(1), 64–73 (2006)
Grimshaw, M.J., Mason, R.M.: Bovine articular chondrocyte function in vitro depends upon oxygen tension. Osteoarthritis Cartilage 8(5), 386–392 (2000)
Chen, J., Wang, C., Lü, S., Wu, J., Guo, X., Duan, C., et al.: In vivo chondrogenesis of adult bone-marrow-derived autologous mesenchymal stem cells. Cell Tissue Res. 319(3), 429–38 (2005)
Xu, Y., Malladi, P., Chiou, M., Bekerman, E., Giaccia, A.J., Longaker, M.T.: In vitro expansion of adipose-derived adult stromal cells in hypoxia enhances early chondrogenesis. Tissue Eng. 13(12), 2981–2993 (2007)
Poole, A.R., Kojima, T., Yasuda, T., Mwale, F., Kobayashi, M., Laverty, S.: Composition and structure of articular cartilage: A template for tissue repair. Clin. Orthop. Relat. Res. 391, S26–S33 (2001)
Schnabel, M., Marlovits, S., Eckhoff, G., Fichtel, I., Gotzen, L., Vécsei, V., et al.: Dedifferentiation-associated changes in morphology and gene expression in primary human articular chondrocytes in cell culture. Osteoarthritis Cartilage 10(1), 62–70 (2002)
Lafont, J.E., Talma, S., Murphy, C.L.: Hypoxia-inducible factor 2α is essential for hypoxic induction of the human articular chondrocyte phenotype. Arthritis Rheum. 56(10), 3297–3306 (2007)
Domm, C., Schünke, M., Christesen, K., Kurz, B.: Redifferentiation of dedifferentiated bovine articular chondrocytes in alginate culture under low oxygen tension. Osteoarthritis Cartilage 10(1), 13–22 (2002)
Domm, C., Schünke, M., Steinhagen, J., Freitag, S., Kurz, B.: Influence of various alginate brands on the redifferentiation of dedifferentiated bovine articular chondrocytes in alginate bead culture under high and low oxygen tension. Tissue Eng. 10(11–12), 1796–1805 (2004)
Vinatier, C., Mrugala, D., Jorgensen, C., Guicheux, J., Noël, D.: Cartilage engineering: a crucial combination of cells, biomaterials and biofactors. Trends Biotechnol. 27(5), 307–314 (2009)
Malladi, P., Xu, Y., Chiou, M., Giaccia, A.J., Longaker, M.T.: Effect of reduced oxygen tension on chondrogenesis and osteogenesis in adipose-derived mesenchymal cells. Am. J. Physiol. Cell Physiol. 290(4), C1139–C1146 (2006)
Zscharnack, M., Poesel, C., Galle, J., Bader, A.: Low oxygen expansion improves subsequent chondrogenesis of ovine bone-marrow-derived mesenchymal stem cells in collagen type I hydrogel. Cells Tissues Organs 190(2), 81–93 (2008)
Mizuno, S., Glowacki, J.: Low oxygen tension enhances chondroinduction by demineralized bone matrix in human dermal fibroblasts in vitro. Cells Tissues Organs 180(3), 151–158 (2005)
Buxton, A.N., Bahney, C.S., Yoo, J.U., Johnstone, B.: Temporal exposure to chondrogenic factors modulates human mesenchymal stem cell chondrogenesis in hydrogels. Tissue Eng. Part A 17(3–4), 371–380 (2011)
Grande, D.A., Breitbart, A.S., Mason, J., Paulino, C., Laser, J., Schwartz, R.E.: Cartilage tissue engineering: Current limitations and solutions. Clin. Orthop. Relat. Res. 367, S176–S185 (1999)
Mostafa, S.S., Miller, W.M., Papoutsakis, E.T.: Oxygen tension influences the differentiation, maturation and apoptosis of human megakaryocytes. Br. J. Haematol. 111(3), 879–889 (2000)
Acknowledgments
W.L.S. acknowledges support from a National Research Service Award T32 GM08515 from the National Institutes of Health. The authors would also like to acknowledge Joseph C. White for assistance in figure design and creation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Stoppel, W.L., Roberts, S.C. (2012). Oxygen Supply for Tissue Engineering. In: Bhatia, S. (eds) Engineering Biomaterials for Regenerative Medicine. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-1080-5_3
Download citation
DOI: https://doi.org/10.1007/978-1-4614-1080-5_3
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-1079-9
Online ISBN: 978-1-4614-1080-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)