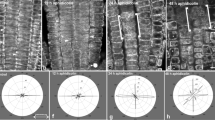
A role for cortical microtubules, cellulose microfibrils and cell wall enzymes in the control of plant cell expansion has been suggested for several decades, but has not yet been proven in any system. Arabidopsis root epidermis is an ideal tissue to test this hypothesis, and consequently is the subject of many recent reports. Microtubules of epidermal cells are transverse to the root axis in the zone of fast elongation, but when cells enter the differentiation zone microtubules switch to a slightly oblique orientation in atrichoblasts and to a disordered distribution in trichoblasts. Factors that severely affect root elongation do so by acting on the process of fast elongation. Ethylene, auxin, water stress as well as reduced cellulose synthesis induce a similar response. Within minutes a new and much shorter final cell size is defined, and cells do not grow beyond this length. Eventually, fast cell elongation is totally inhibited. In cells that are longer than this new final cell size, cortical microtubules change their orientation in the time frame of 10–15 min and adopt orientations typical for the differentiation zone, while the orientation of cellulose fibrils is not changed in the walls of the affected cells. Microtubules, however, are not the causal agents: as fast elongation and its inhibition are insensitive to microtubule disrupting or stabilizing drugs. Control of cell size seems to be located at the level of cell wall metabolism. The profile of xyloglucan endotransglucosylase action changes only slowly, but a significant callose deposition in the walls of the underlying cortex cells accompanies the inhibition of elongation.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
T. Fujino, Y. Sone, Y. Mitsuishi, and T. Itoh, Characterization of cross-links between cellulose microfibrils, and their occurrence during elongation growth in pea epicotyl,Plant Cell Physiol. 41, 486 – 494 (2000).
K. J. Niklas,Plant Biomechanics: An Engineering Approach to Plant Form and Function(University of Chicago Press, Chicago, IL, 1992).
S. Kerstens, F. W. S. Decraemer, and J.-P. Verbelen, Cell walls at the surface behave mechanically like fiber-reinforced composite materials,Plant Physiol. 127, 281 – 285 (2001).
T. I. Baskin, On the alignment of cellulose microfibrils by cortical microtubules: a review and a model,Protoplasma215, 150 – 171 (2001).
T. H. Giddings and L. A. Staehelin, in:The Cytoskeletal Basis of Plant Growth and Form, edited by C. W. Lloyd (Academic Press, San Diego, CA, 1991), pp. 85 – 100.
T. I. Baskin, J. E. Wilson, A. Cork, and R. E. Williamson, Morphology and microtubule organization in Arabidopsis roots exposed to oryzalin or taxol,Plant Cell Physiol. 35, 935 – 942 (1994).
L. Taiz and E. Zeiger,Plant Physiology(Sinauer, Sunderland, MA, 1998).
T. Itoh, Microfibrillar orientation of radially enlarged cells of coumarin- and colchicine-treated pine seedlings,Plant Cell Physiol. 17, 385 – 398 (1976).
S. C. Mueller and R. M. Brown, The control of cellulose microfibril deposition in the cell wall of higher plants. II. Freeze-fracture microfibril patterns in maize seedling tissues following experimental alteration with colchicine and ethylene,Planta154, 501 – 515 (1982).
F. H. A. Wilms, A. M. C. Wolters-Arts, and J. Derksen, Orientation of cellulose microfibrils in cortical cells of tobacco explants. Effects of microtubule-depolymerizing drugs,Planta182, 1 – 8 (1990).
J.-P. Verbelen, K. Vissenberg, S. Kerstens, and J. Le, Cell expansion in the epidermis: microtubules, cellulose orientation and wall loosening enzymes,J. Plant Physiol. 158, 537 – 543 (2001).
A. M. D. Wiedemeier, J. E. Judy-March, C. H. Hocart, G. O. Wasteneys, R. E. Williamson, and T. I. Baskin, Mutant alleles of Arabidopsis RADIALLY SWOLLEN 4 and 7 reduce growth anisotropy without altering the transverse orientation of cortical microtubules or cellulose microfibrils,Development129, 4821 – 4830 (2002).
K. Sugimoto, R. Himmelspach, R. E. Williamson, and G. O. Wasteneys, Mutation or drug-dependent microtubule disruption causes radial swelling without altering parallel cellulose microfibril deposition in Arabidopsis root cells,Plant Cell15, 1414 – 1429 (2003).
J. Le, F. Vandenbussche, D. Van Der Straeten, and J.-P. Verbelen, In the early response of Arabidopsis roots to ethylene, cell elongation is up- and down regulated and uncoupled from differentiation,Plant Physiol. 125, 519 – 522 (2001).
J. L. Mullen, H. Ishikawa, and M. L. Evans, Analysis of changes in relative elemental growth rate in the elongation zone of Arabidopsis roots upon gravistimulation,Planta206, 598 – 603 (1998).
K. Sugimoto, R. E. Williamson, and G. O. Wasteneys, New techniques enable comparative analysis of microtubule orientation, wall texture, and growth rate in intact roots of Arabidopsis, Plant Physiol. 124, 1493 – 1506 (2000).
C. M. Van Der Weele, H. Jiang, K. K. Palaniappan, V. B. Ivanov, K. Palaniappan, and T. I. Baskin, A new algorithm for computational image analysis of deformable motion at high spatial and temporal resolution applied to root growth. Roughly uniform elongation in the meristem and also, after an abrupt acceleration, in the elongation zone,Plant Physiol. 132, 1 – 11 (2003).
F. Vandenbusche, J. Smalle, J. Le, N. J. Madeira Saibo, A. De Paepe, L. Chaerle, O. Tietz, R. Smets, L. J. J. Laarhoven, F. J. M. Harren, H. Van Onckelen, K. Palme, J.-P. Verbelen, and D. Van Der Straeten, TheArabidopsismutant alh1 illustrates a cross talk between ethylene and auxin,Plant Physiol. 131, 1228 – 1238 (2003).
A. B. Bleecker, M. A. Estelle, C. Somerville, and H. Kende, Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana, Science 241, 1086 – 1089 (1988).
T. Arioli, L. Peng, A. S. Betzner, J. Burn, W. Wittke, W. Herth, C. Camilleri, H. Hé fte, J. Plazinski, R. Birch, A. Cork, J. Glover, J. Redmond, and R. E. Williamson, Molecular analysis of cellulose biosynthesis in Arabidopsis, Science 279, 717 – 720 (1998).
M. Fagard, T. Desnos, T. Desprez, F. Goubet, G. Refregier, G. Mouille, M. McCann, C. Rayon, S. Vernhettes, and H. Hé fte, PROCUSTE1 encodes a cellulose synthase required for normal cell elongation specifically in roots and dark-grown hypocotyls of Arabidopsis, Plant Cell 12, 2409 – 2423 (2000).
K. Ueda, T. Matsuyama, and T. Hashimoto, Visualization of microtubules in living cells of transgenic Arabidopsis thaliana, Protoplasma 206, 210 – 206 (1999).
J.-P. Verbelen and D. Stickens,In vivodetermination of fibril orientation in plant cell walls with polarization CSLM,J. Microsc. 177, 1 – 6 (1995).
J.-P. Verbelen and S. Kerstens, Polarization confocal microscopy and Congo Red fluorescence: a simple and rapid method to determine the mean cellulose fibril orientation in plants,J. Microsc. 198, 101 – 107 (2000).
F. Baluška, D. Volkmann, and P. W. Barlow, A polarity crossroad in the transition growth zone of maize root apices: cytoskeletal and developmental implications,J. Plant Growth Regul. 20, 170 – 181 (2001).
N. C. Carpita and D. M. Gibeaut, Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth,Plant J. 3, 1 – 30 (1993).
S. E. C. Whitney, M. G. E. Gothard, J. T. Mitchell, and M. J. Gidley, Roles of cellulose and xyloglucan in determining the mechanical properties of primary plant cell walls,Nature411, 610 – 613 (1999).
C. Andeme-Onzighi, M. Sivaguru, J. Judy-March, T. I. Baskin, and A. Driouich, The reb1-1 mutation ofArabidopsisalters the morphology of trichoblasts, the expression of arabinogalactan proteins and the organization of cortical microtubules,Planta215, 949 – 958 (2002).
G. J. Seifert, C. Barber, B. Wells, L. Dolan, and K. Roberts, Galactose biosynthesis inArabidopsis: genetic evidence for substrate chanelling from UDP-D-galactose into cell wall polymers,Curr. Biol. 12, 1840 – 1845 (2002).
K. Vissenberg, I. M. Martinez-Vilchez, J. G. Miller, S. C. Fry, and J.-P. Verbelen,In vivocolocalization of xyloglucan endotransglucosylase activity and its donor substrate in the elongation zone ofArabidopsisroots,Plant Cell12, 1229 – 1237 (2000).
T. C. Nickle and D. W. Meinke, A cytokinesis-defective mutant ofArabidopsis(cyt1) characterized by embryonic lethality, incomplete cell walls, and excessive callose accumulation,Plant J. 15, 321 – 332 (1998).
R. S. Beffa, R. M. Hofer, M. Thomas, and F. Meins, Decreased susceptibility to viral disease of beta-1,3-glucanase-deficient plants generated by antisense transformation,Plant Cell8, 1001 – 1011 (1996).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2008 Springer Science + Business Media B.V.
About this paper
Cite this paper
Verbelen, JP. et al. (2008). Microtubules And The Control Of Cell Elongation In Arabidopsis Roots. In: Blume, Y.B., Baird, W.V., Yemets, A.I., Breviario, D. (eds) The Plant Cytoskeleton: a Key Tool for Agro-Biotechnology. NATO Science for Peace and Security Series C: Environmental Security. Springer, Dordrecht. https://doi.org/10.1007/978-1-4020-8843-8_4
Download citation
DOI: https://doi.org/10.1007/978-1-4020-8843-8_4
Publisher Name: Springer, Dordrecht
Print ISBN: 978-1-4020-8842-1
Online ISBN: 978-1-4020-8843-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)