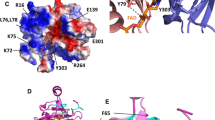
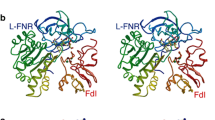
Abstract
An overview is presented of structure/function relationships in the interactions between the small electron transfer proteins ferredoxin (Fd) and flavodoxin (Fld) and the flavoprotein enzyme ferredoxin:NADP+ reductase (FNR), primarily emphasizing the proteins from the cyanobacterium, Anabaena, and the higher plant, spinach. Results are summarized from experiments utilizing rapid-reaction kinetic methods (stopped-flow spectrophotometry and laser flash photolysis) involving wild-type and site-specific mutants of these proteins, redox potential determinations, and X-ray crystallography, including the crystal structure of a Fd/FNR complex. These have provided detailed insights into the protein–protein recognition and electron transfer mechanisms utilized by these systems. Fd and Fld bind to FNR within a concave region of the FNR surface that contains the exposed dimethylbenzene ring of the FAD cofactor. In the Fd case, electron transfer between the iron–sulfur and flavin centers proceeds with a maximum rate constant of 5,500 sec−1 via a direct outer-sphere mechanism. Both electrostatic and hydrophobic interactions occur between the proteins, resulting in a precise surface complementarity.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Alam J, Whitaker RA, Krogman DW and Curtis SE (1986) Isolation and sequence of the gene for ferredoxin I from the cyanobacterium Anabaena sp. strain PCC 7120. J Bact 168: 1265–1271
Aliverti A and Zanetti G (1997) A three-domain iron-sulfur flavoprotein obtained through gene fusion of ferredoxin and ferredoxin NADP+ reductase from spinach leaves. Biochemistry 36: 14771–14777
Aliverti A, Piubelli L, Zanetti G, Lübberstedt T, Herrmann RG and Curti B (1993) The role of cysteine residues of spinach ferredoxin reductase as assessed by site-directed mutagenesis. Biochemistry 32: 6374–6380
Aliverti A, Corrado ME and Zanetti G (1994) Involvement of lysine-88 of spinach ferredoxin:NADP+ reductase in the interaction with ferredoxin. FEBS Lett 343: 247–250
Aliverti A, Hagen WR and Zanetti G (1995a) Direct electrochemistry and EPR spectroscopy of spinach ferredoxin mutants with modified electron transfer proteins. FEBS Lett 368: 220–224
Aliverti A, Bruns CM, Pandini VE, Karplus PA, Vanoni MA, Curti B and Zanetti G (1995b) Involvement of serine 96 in the catalytic mechanism of ferredoxin:NADP+ reductase: structure-function relationship as studied by site-directed mutagenesis and x-ray crystallography. Biochemistry 34: 8371–8379
Aliverti A, Livraghi A, Piubelli L and Zanetti G (1997) On the role of the acidic cluster Glu 92-94 of spinach ferredoxin I. Biochim Biophys Acta 1342: 45–50
Aliverti A, Deng Z, Ravasi D, Piubelli L, Karplus PA and Zanetti G (1998) Probing the function of the invariant glutamyl residue 312 in spinach ferredoxin:NADP+ reductase. J Biol Chem 273: 34008–34015
Batie CJ and Kamin H (1981) The relation of pH and oxidation-reduction potential to the association state of the ferredoxin:ferredoxin:NADP+ reductase complex. J Biol Chem 256: 7756–7763
Batie CJ and Kamin H (1984a) Ferredoxin:NADP+ oxidoreductase. Equilibria in binary and ternary complexes with NADP+ and ferredoxin. J Biol Chem 259: 8832–8839
Batie CJ and Kamin H (1984b) Electron transfer by ferredoxin:NADP+ reductase. Rapid-reaction evidence for participation of a ternary complex. J Biol Chem 259: 11976–11985
Beinert H and Kiley PJ (1999) Fe-S proteins in sensing and regulatory functions. Curr Opin Chem Biol 3: 152–157
Beinert H, Holm RH and Münck E (1997) Iron-sulfur clusters: nature’s modular, multipurpose structures. Science 277: 653–659
Bhattacharyya AK, Meyer TE and Tollin G (1986) Reduction kinetics of the ferredoxin-ferredoxin-NADP+ reductase complex. Biochemistry 25: 4655–4661
Binda C, Coda A, Aliverti A, Zanetti G and Mattevi A (1998) Structure of the mutant E92K of [2Fe-2S] ferredoxin I from Spinacia oleracea at 1.7 Å resolution. Acta Cryst D54: 1353–1358
Böhme H and Haselkorn R (1988) Molecular cloning and nucleotide sequence analysis of the gene coding for the heterocyst ferredoxin from the cyanobacterium Anabaena sp. strain PCC 7120. Mol Gen Genet 214: 278–285
Böhme H and Haselkorn R (1989) Expression of Anabaena ferredoxin genes in Escherichia coli. Plant Mol Biol 12: 667–672
Böhme H and Schrautemeier B (1987) Comparative characterization of ferredoxin from heterocysts and vegetative cells of Anabaena variabilis. Biochim Biophys Acta 891: 1–7
Bruns CM and Karplus PA (1995) Refined crystal structure of spinach ferredoxin reductase at 1.7 Å resolution: oxidized, reduced and 2′-phospho-5′-AMP bound states. J Mol Biol 247: 125–145
Cammack R, Rao KK, Bargeron CP, Hutson KG, Andrew PW and Rogers LJ (1977) Midpoint redox potentials of plant and algal ferredoxins. Biochem J 168: 205–209
Carrillo N and Ceccarelli EA (2003) Open questions in ferredoxin-NADP+ reductase catalytic mechanism. Eur J Biochem 270: 1900–1915
Casaus JL, Navarro JA, Hervas M, Lostao A, De la Rosa MA, Gomez-Moreno C, Sancho J and Medina M (2002) Ana- baena sp. PCC 7119 flavodoxin as electron carrier from photosystem I to ferredoxin-NADP+ reductase. Role of Trp(57) and Tyr(94). J Biol Chem 277: 22338–22344
Cheng H, Xia B, Reed GH and Markley JL (1994) Optical, EPR, and 1H NMR spectroscopy of serine-ligated [2Fe-2S] ferredoxins produced by site-directed mutagenesis of cysteine residues in recombinant Anabaena 7120 vegetative ferredoxin. Biochemistry 33: 3155–3164
Cheng H, Westler WM, Oh B-H and Markley JL (1995) Protein expression, selective isotopic labeling, and analysis of hyperfine-shifted NMR signals of Anabaena 7120 vegetative [2Fe-2S] ferredoxin. Arch Biochem Biophys 316: 619–634
Corrado ME, Aliverti A, Zanetti G and Mayhew SG (1996) Analysis of the oxidation-reduction potentials of recombinant ferredoxin-NADP+ reductase from spinach chloroplasts. Eur J Biochem 239: 662–667
De Pascalis AR, Jelesarov I, Ackermann F, Koppenol WH, Hirasawa M, Knaff DB and Bosshard HR (1993) Binding of ferredoxin to ferredoxin:NADP+ reductase: the role of carboxyl groups, electrostatic surface potential, and molecular dipole moment. Protein Sci 2: 1126–1135
Drennan CL, Pattridge KA, Weber CH, Metzger AL, Hoover DM and Ludwig ML (1999) Refined structures of oxidized flavodoxin from Anacystis nidulans. J Mol Biol 294: 711–724
Dugard LB, La Mar GN, Banci L and Bertine L (1990) Identification of localized redox states in plant-type two-iron ferredoxins using the nuclear overhauser effect. Biochemistry 29: 2263–2271
Faro M, Gómez-Moreno C, Stankovich M and Medina M (2002a) Role of critical charged residues in reduction potential modulation of ferredoxin-NADP+ reductase. Differential stabilization of FAD redox forms. Eur J Biochem 269: 2656–2661
Faro M, Frago S, Mayoral T, Hermoso JA, Sanz-Aparicio J, Gómez-Moreno C and Medina M (2002b) Probing the role of the glutamic 139 residue in Anabaena ferredoxin-NADP+ reductase in its interaction with substrates. Eur J Biochem 269: 4938–4947
Fillat MF, Borrias WE and Weisbeek PJ (1991) Cloning of the ferredoxin-NADP+-oxidoreductase gene and overexpression of a synthetic flavodoxin gene from the cyanobacteria Anabaena PCC 7119. In: Curti B, Ronchi S and Zanetti G (eds) Flavins and Flavoproteins 1990, pp 445–448. Walter de Gruyter & Co., Berlin
Fillat MF, Pacheco MC, Peleato ML and Gómez-Moreno C (1994) Overexpression of ferredoxin-NADP+ reductase from Anabaena sp. PCC 7119 in E. coli. In: Yagi K (ed) Flavins and Flavoproteins 1993, pp 447–450. Walter de Gruyter & Co., Berlin
Foust GP and Massey V (1967) Studies of ferredoxin-TPN reductase and ferredoxin from spinach. Fed Proc 26: 732
Foust GP, Mayhew SG and Massey V (1969) Complex formation between ferredoxin triphosphopyridine nucleotide reductase and electron transfer proteins. J Biol Chem 244: 964–970
Freigang J, Diederichs K, Schaefer KP, Welte W and Paul R (2002) Crystal structure of oxidized flavodoxin, an essential protein in Helicobacter pylori. Protein Sci 11: 253–261
Fritz J, Müller F and Mayhew SG (1973) Electron-nuclear double resonance study of flavodoxin from Peptostreptococcus elsdenii. Helv Chim Acta 56: 2250–2254
Fu W, Drozdzewski PM, Davies MD, Sligar SG and Johnson MK (1992) Resonance Raman and magnetic circular dichroism studies of reduced [2Fe-2S] proteins. J Biol Chem 267: 15502–15510
Fukuyama K, Matsubara H and Rogers LJ (1992) Crystal structure of oxidized flavodoxin from a red alga Chondrus crispus refined at 1.8 A resolution. Description of the flavin mononucleotide binding site. J Mol Biol 225: 775–789
Fukuyama K, Ueki N, Nakamura H, Tsukihara T and Matsubara H (1995) Tertiary structure of [2Fe-2S] ferredoxin from Spirulina platensis refined at 2.5 Å resolution: structural comparisons of plant-type ferredoxins and electrostatic potential analysis. J Biochem 117: 1017–1023
Gómez-Moreno C, Choy M and Edmondson DE (1979) Purification and properties of the bacterial flavoenzyme thiamine dehydrogenase. J Biol Chem 254: 7630–7635
Gómez-Moreno C, Martínez-Júlvez M, Fillat MF, Hurley JK and Tollin G (1995) Molecular recognition in electron transfer proteins. In: Mathis P (ed) Photosynthesis: From Light to Biosphere, Vol II, pp 627–632. Kluwer Academic Publishers, The Netherlands
Hermoso JA, Mayoral T, Faro M, Gómez-Moreno C, Sanz-Aparicio J and Medina M (2002) Mechanism of coenzyme recognition and binding revealed by crystal structure analysis of ferredoxin-NADP+ reductase complexed with NADP+. J Mol Biol 319: 1133–1142
Holden HM, Jacobson BL, Hurley JK, Tollin G, Oh B-H, Skjeldal L, Chae YK, Cheng H, Xia B and Markley JL (1994) Structure-function studies of [2Fe-2S] ferredoxins. J Bioenerg Biomembr 26: 67–88
Hurley JK, Salamon Z, Meyer TE, Fitch JC, Cusanovich MA, Markley JL, Cheng H, Xia B, Chae YK, Medina M, Gómez-Moreno C and Tollin G (1993a) Amino acid residues in Anabaena ferredoxin crucial to interaction with ferredoxin-NADP+ reductase: site-directed mutagenesis and laser flash photolysis. Biochemistry 32: 9346–9354
Hurley JK, Cheng H, Xia B, Markley JL, Medina M, Gómez-Moreno C and Tollin G (1993b) An aromatic amino acid is required at position 65 in Anabaena ferredoxin for rapid electron transfer to ferredoxin:NADP+ reductase. J Am Chem Soc 115: 11698–11701
Hurley JK, Medina M, Gómez-Moreno C and Tollin G (1994) Further characterization by site-directed mutagenesis of the protein–protein interface in the ferredoxin/ferredoxin: NADP+ system from Anabaena: requirement for a negative charge at position 94 in ferredoxin for rapid electron transfer. Arch Biochem Biophys 312: 480–486
Hurley JK, Fillat MF, Gómez-Moreno C and Tollin G (1996a) Electrostatic and hydrophobic interactions during complex formation and electron transfer in the ferredoxin/ ferredoxin:NADP+ reductase system from Anabaena. J Am Chem Soc 118: 5526–5531
Hurley JK, Schmeits JL, Genzor C, Gómez-Moreno C and Tollin G (1996b) Charge reversal mutations in a conserved acidic patch in Anabaena ferredoxin can attenuate or enhance electron transfer to ferredoxin:NADP+ reductase by altering protein/protein orientation within the intermediate complex. Arch Biochem Biophys 333: 243–250
Hurley JK, Weber-Main AM, Stankovich MT, Benning MM, Thoden JB, Vanhooke JL, Holden HM, Chae YK, Xia B, Cheng H, Markley JL, Martínez-Júlvez M, Gómez-Moreno C, Schmeits JL and Tollin G (1997a) Structure-function relationships in Anabaena ferredoxin: correlations between x-ray crystal structures, reduction potentials, and rate constants for electron transfer to ferredoxin:NADP+ reductase for site-specific ferredoxin mutants. Biochemistry 36: 11100–11117
Hurley JK, Weber-Main AM, Hodges AE, Stankovich MT, Benning MM, Holden HM, Cheng H, Xia B, Markley JL, Genzor C, Gómez-Moreno C, Hafezi R and Tollin G (1997b) Iron-sulfur cluster cysteine-to-serine mutants of Anabaena [2Fe-2S] ferredoxin exhibit unexpected redox properties and are competent in electron transfer to ferredoxin:NADP+ reductase. Biochemistry 36: 15109–15117
Hurley JK, Hazzard JT, Martínez-Júlvez M, Medina M, Gómez-Moreno C and Tollin G (1999) Electrostatic forces involved in orienting Anabaena ferredoxin during binding to Anabaena ferredoxin:NADP+ reductase: site-specific mutagenesis, transient kinetic measurements, and electrostatic surface potentials. Protein Sci 8: 1614–1622
Hurley JK, Faro M, Brodie TB, Hazzard JT, Medina M, Gómez-Moreno C and Tollin G (2000) Highly nonproductive complexes with Anabaena ferredoxin at low ionic strength are induced by nonconservative amino acid substitutions at Glu 139 in Anabaena ferredoxin:NADP+ reductase. Biochemistry 39: 13695–13702
Hurley JK, Morales R, Martínez-Júlvez M, Brody TB, Medina M, Gómez-Moreno C and Tollin G (2002) Structure-function relationships in Anabaena ferredoxin/ferredoxin:NADP+ electron transfer: insights from site-directed mutagenesis, transient absorption measurements and X-ray crystallography. Biochim Biophys Acta 1554: 5–21
Jacobson BL, Chae YK, Markley JL, Rayment I and Holden HM (1993) Molecular structure of the oxidized, recombinant, heterocyst [2Fe-2S] ferredoxin from Anabaena 7120 determined to 1.7-Å resolution. Biochemistry 32: 6788–6793
Jelesarov I and Bosshard HR (1994) Thermodynamics of ferredoxin binding to ferredoxin:NADP+ reductase and the role of water at the complex interface. Biochemistry 33: 13321–13328
Jelesarov I, De Pascalis AR, Koppenol WH, Hirasawa M, Knaff DB and Bosshard HR (1993) Ferredoxin binding site on ferredoxin:NADP+ reductase. Differential chemical modification of free and ferredoxin-bound enzyme. Eur J Biochem 216: 57–66
Jenkins CM, Genzor, CG, Fillat MF, Waterman MR and Gómez-Moreno M (1997) Negatively charged Anabaena flavodoxin residues (Asp144 and Glu145) are important for reconstitution of cytochrome P450 17 alpha-hydroxylase activity. J Biol Chem 36: 22509–22513
Johnson MK (1994) Iron-sulfur proteins. In: King RB (ed) Encyclopedia of inorganic chemistry, pp 1896–1915. John Wiley and Sons, New York
Kang CH, Ferguson-Miller S and Margoliash E (1977) Steady state kinetics and binding of eukaryotic cytochromes c with yeast cytochrome c peroxidase. Biochemistry 252: 919–926
Karplus PA (1991) Structure/function of spinach ferredoxin:NADP+ oxidoreductase. In: Curti B, Ronchi S and Zanetti G (eds) Flavins and Flavoproteins 1990, pp 449–455. Walter de Gruyter & Co., Berlin
Karplus PA, Daniels MJ and Herriot JR (1991) Atomic structure of ferredoxin-NADP+ reductase: prototype for a structurally novel flavoenzyme family. Science 251: 60–66
Keirns JJ and Wang JH (1972) Studies on nicotinamide adenine dinucleotide phosphate reductase from spinach chloroplasts. J Biol Chem 22: 7374–7382
Knaff DB (1996) Ferredoxin and ferredoxin-dependent enzymes. In: Ort DR and Yocum CF (eds) Oxygenic Photosynthesis: The Light Reactions, pp 333–361. Kluwer Academic Publishers, Dordrecht
Knauf MA, Lohr F, Curley GP, O’Farrell P, Mayhew SG, Muller F and Ruterjans H (1993) Homonuclear and heteronuclear NMR studies of oxidized Desulfovibrio vulgaris flavodoxin. Sequential assignments and identification of secondary structure elements. Eur J Biochem 213: 167–184
Kurisu G, Kusunoki M, Katoh E, Yamazaki T, Teshima K, Onda Y, Kimata-Ariga Y and Hase T (2001) Structure of the electron transfer complex between ferredoxin and ferredoxin-NADP+ reductase. Nat Struct Biol 8: 117–121
Lostao A, Gómez-Moreno C, Mayhew SG and Sancho J (1997) Differential stabilization of the three FMN redox forms by tyrosine 94 and tryptophan 57 in flavodoxin from Anabaena and its influence on the redox potentials. Biochemistry 36: 14334–14344
Lovenberg W (ed) (1973) (1974) (1977) Iron-Sulfur Proteins, Vol. I, II, III. Academic Press, New York
Ludwig ML, Pattridge KA, Metzger AL, Dixon MM, Eren M, Feng Y and Swenson RP (1997) Control of oxidation-reduction potentials in flavodoxin from Clostridium beijerinckii: the role of conformation changes. Biochemistry 36: 1259–1280
Malkin R (1996) Photosystem I electron transfer reactions –components and kinetics. In: Ort DR and Yocum CF (eds) Oxygenic Photosynthesis: The Light Reactions, pp 333–361. Kluwer Academic Publishers, Dordrecht
Martínez-Júlvez M, Medina M, Hurley JK, Hafezi R, Brodie TB, Tollin G and Gómez-Moreno C (1998a) Lys 75 of Anabaena ferredoxin-NADP+ reductase is a critical residue for binding ferredoxin and flavodoxin during electron transfer. Biochemistry 37: 13604–13613
Martínez-Júlvez M, Hermoso J, Hurley JK, Mayoral T, Sanz-Aparicio J, Tollin G, Gómez-Moreno C and Medina M (1998b) Role of Arg100 and Arg264 from Anabaena PCC 7119 ferredoxin-NADP+ reductase: binding and electron transfer. Biochemistry 37: 17680–17691
Martínez-Júlvez M, Medina M and Gómez-Moreno C (1999) Ferredoxin-NADP+ reductase uses the same site for the interaction with ferredoxin and flavodoxin. J Biol Inorg Chem 4: 568–578
Martínez-Júlvez M, Nogués I, Faro M, Hurley JK, Brodie TB, Mayoral T, Sanz-Aparicio J, Hermoso JA, Stankovich MT, Medina M, Tollin G and Gómez-Moreno C (2001) Role of a cluster of hydrophobic residues near the FAD cofactor in Anabaena PCC 7119 ferrredoxin-NADP+ reductase for optimal complex formation and electron transfer to ferredoxin. J Biol Chem 276: 27498–27510
Masaki R, Yoshikawa S and Matsubara H (1982a) Steady-state kinetics of oxidation of reduced ferredoxin with ferredoxin-NADP+ reductase. Biochim Biophys Acta 700: 101–109
Masaki R, Matsumoto M, Yoshikawa S and Matsubara H (1982b) Steady state and transient kinetics of reduced ferredoxin with ferredoxin-NADP+ reductase. In: Massey V and Williams CH (eds) Flavins and Flavoproteins, pp 675–678. Elsevier, North Holland
Matsubara H and Hase T (1983) Phylogenetic consideration of ferredoxins sequences in plants, particularly algae. In: Jensen U and Fairbrothers DE (eds) Proteins and Nucleic Acids in Plant Systematics, pp 168–181. Springer-Verlag, Berlin
Matsubara H and Saeki K (1992) Structural and functional diversity of ferredoxins and related proteins. In: Cammack R and Sykes AG (eds) Advances in Inorganic Chemistry, Iron-Sulfur Proteins, Vol 38, pp 223–280. Academic Press, Inc., San Diego, CA
Mauk MR, Ferrer JC and Mauk AG (1994) Proton linkage in formation of the cytochrome c-cytochrome c peroxidase complex: electrostatic properties of the high- and low-affinity cytochrome binding sites on the peroxidase. Biochemistry 33: 12609–12614
Mayoral T, Medina M, Sanz-Aparicio J, Gómez-Moreno C and Hermoso JA (2000) Structural basis of the catalytic role of Glu301 in Anabaena PCC 7119 ferredoxin-NADP+ reductase revealed by X-ray crystallography. Proteins: Struct Funct Gen 38: 60–69
Medina M and Gómez-Moreno C (2004) Interaction of ferredoxin-NADP+ reductase with its substrates: optimal interaction for efficient electron transfer. Photosynth Res 79: 113–131.
Medina M, Méndez E and Gómez-Moreno C (1992a) Identification of arginyl residues involved in the binding of ferredoxin-NADP+ reductase from Anabaena sp. 7119 to its substrates. Arch Biochem Biophys 299: 281–286
Medina M, Méndez E and Gómez-Moreno C (1992b) Lysine residues on ferredoxin-NADP+ reductase from Anabaena sp. 7119 involved in substrate binding. FEBS Lett 298: 25–28
Medina M, Peleato ML, Mendez E and Gómez-Moreno C (1992c) Identification of specific carboxyl groups on Anabaena PCC 7119 flavodoxin which are involved in the interaction with ferredoxin-NADP+ reductase. Eur J Biochem 203: 373–379
Medina M, Martínez-Júlvez M, Hurley JK, Tollin G and Gómez-Moreno C (1998) Involvement of glutamic acid 301 in the catalytic mechanism of ferredoxin-NADP+ reductase from Anabaena PCC 7119. Biochemistry 37: 2715–2728
Morales R, Charon M-H, Hudry-Clergeon G, Pétillot Y, Norager S, Medina M and Frey M (1999) Refined X-ray structures of the oxidized, at 1.3 Å, and reduced, at 1.17 Å, [2Fe-2S] ferredoxin from the cyanobacterium Anabaena PCC7119 show redox-linked conformational changes. Biochemistry 38: 15764–15773
Morales R, Charon M-H, Kacholova G, Serre L, Medina M, Gómez-Moreno C and Frey M (2000) A redox-dependent interaction between two electron-transfer partners involved in photosynthesis. EMBO Rep 1: 271–276
Nakamura S and Kimura T (1971a) A possible regulation of activity of ferredoxin-NADP+ reductase and ferredoxin system by ionic strength: catalytic significance of the one to one complex. FEBS Lett 15: 352–354
Nakamura S and Kimura T (1971b) Studies on spinach ferredoxin-nicotinomide adenine dinucleotide phosphate reductase. FEBS Lett 15: 352–354
Navarro JA, Hervas M, Genzor CG, Cheddar G, Fillat MF, de la Rosa MA, Gómez-Moreno C, Cheng H, Xia B, Chae YK, Yan H, Wong B, Straus A, Markley JL, Hurley JK and Tollin G (1995) Site-specific mutagenesis demonstrates that the structural requirements for efficient electron transfer in Anabaena ferredoxin and flavodoxin are highly dependent on the reaction partner: kinetic studies with photosystem I, ferredoxin:NADP+ reductase, and cytochrome c. Arch Biochem Biophys 321: 229–238
Nelson N and Neumann J (1968) Interaction between ferredoxin and ferredoxin-NADP+ reductase from chloroplasts. Biochem Biophys Res Comm 30: 142–147
Nogués I, Martínez-Júlvez M, Navarro JA, Hervás M, Armenteros L, de la Rosa MA, Brodie TB, Hurley JK, Tollin G, Gómez-Moreno C and Medina M (2003) Role of hydrophobic interactions in the flavodoxin mediated electron transfer from Photosystem I to ferredoxin-NADP+ reductase in Anabaena PCC 7119. Biochemistry 42: 2036–2045
Nuevo MR, Chu H-H, Vitello LB and Erman JE (1993) Salt-dependent switch in the pathway of electron transfer from cytochrome c to cytochrome c reductase compound I. J Am Chem Soc 115: 5873–5874
Palmer G (1973) Current insights into the active center of spinach ferredoxin and other iron-sulfur proteins. In: Lovenberg W (ed) Iron-Sulfur Proteins, Vol 2, pp 285–325. Academic Press, New York
Peleato ML, Ayora S, Inda LA and Gómez-Moreno C (1994) Isolation and characterization of two different flavodoxins from the eukaryotic alga Chlorella fusca. Biochem J 302: 807–811
Piubelli L, Aliverti A, Bellintani F and Zanetti G (1996) Mutations of Glu92 in ferredoxin I from spinach leaves produces proteins fully functional in electron transfer but less efficient in supporting NADP+ photoreduction. Eur J Biochem 236: 465–469
Price NT, Smith AJ and Rogers LJ (1992) Relationship of the flavodoxin isoforms from Porphyra umbilicalis. Phytochemistry 30: 2835–2839
Pueyo JJ and Gómez-Moreno C (1991) Purification of ferredoxin-NADP+ reductase, flavodoxin and ferredoxin from a single batch of the cyanobacterium Anabaena PCC7119. Prep Biochem 21: 191–204
Pueyo JJ, Gómez-Moreno C and Mayhew SG (1991) Oxidation-reduction potentials of ferredoxin-NADP+ reductase and flavodoxin from Anabaena PCC 7119 and their electrostatic and covalent complexes. Eur J Biochem 202: 1065–1071
Pueyo JJ, Revilla C, Mayhew SG and Gómez-Moreno C (1992) Complex formation between ferredoxin and ferredoxin-NADP+ reductase from Anabaena PCC 7119: cross-linking studies. Arch Biochem Biophys 294: 367–372
Rao ST, Shaffie F, Yu C, Satyshur KA, Stockman BJ, Markley JL and Sundarlingam M (1992) Structure of the oxidized long-chain flavodoxin from Anabaena 7120 at 2 Å resolution. Protein Sci 1: 1413–1427
Rogers LJ (1987) Ferredoxins, flavodoxins and related proteins: structure, function and evolution. In: Fay P and Van Baalen C (eds) The Cyanobacteria, pp 35–67. Elsevier, Amsterdam
Rypniewski WR, Breiter DR, Benning MM, Wesenberg G, Oh B-H, Markley JL, Rayment I and Holden HM (1991) Crystallization and structure determination to 2.5-Å resolution of the oxidized [2Fe-2S] ferredoxin from Anabaena 7120. Biochemistry 30: 4126–4131
Sancho J, Peleato ML, Gómez-Moreno C and Edmondson DE (1988) Purification and properties of ferredoxin-NADP+ oxidoreductase from the nitrogen-fixing cyanobacteria Anabaena variabilis. Arch Biochem Biophys 260: 200–207
Sancho J, Medina M and Gómez-Moreno C (1990) Arginyl groups involved in the binding of Anabaena ferredoxin-NADP+ reductase to NADP+ and to ferredoxin. Eur J Biochem 187: 39–48
Sands RH and Dunham WR (1975) Spectroscopic studies on two-iron ferredoxins. In: Engström A, Ehrenberg A, Keynes RD and Felsenfeld G (eds) Quarterly Reviews of Biophysics, Vol 7, pp 443–504. Cambridge University Press, Cambridge
Schmitz S and Böhme H (1995) Amino acid residues involved in functional interaction of vegetative cell ferredoxin from the cyanobacterium Anabaena sp. PCC 7120 with ferredoxin:NADP+ reductase, nitrite reductase and nitrate reductase. Biochim Biophys Acta 1231: 335–341
Schmitz S, Martínez-Júlvez M, Gómez-Moreno C and Böhme H (1998) Interaction of positively charged amino acid residues of recombinant, cyanobacterial ferredoxin:NADP+ reductase with ferredoxin probed by site-directed mutagenesis. Biochim Biophys Acta 1363: 85–93
Serre L, Vellieux FMD, Medina M, Gómez-Moreno C, Fontecilla-Camps JC and Frey M (1996) X-ray structure of the ferredoxin:NADP+ reductase from the cyanobacterium Anabaena PCC 7119 at 1.8 Å resolution, and the crystallographic studies of NADP+ binding at 2.25 Å resolution. J Mol Biol 263: 20–39
Shin M and San Pietro A (1968) Complex formation of ferredoxin-NADP reductase with ferredoxin and with NADP. Biochem Biophys Res Comm 33: 38–42
Shin M, Tagawa K and Arnon DI (1963) Crystallization of ferredoxin-TPN reductase and its role in the photosynthetic apparatus of chloroplasts. Biochem Zeitsch 338: 84–96
Skjeldal L, Westler WM, Oh B-H, Krezel AM, Holden HM, Jacobson BL, Rayment I and Markley JL (1991) Two-dimensional magnetization exchange spectroscopy of Anabaena 7120 ferredoxin. Nuclear overhauser effect and electron self-exchange cross peaks from amino acid residues surrounding the 2Fe-2S cluster. Biochemistry 30: 7363–7368
Smillie RM (1965) Isolation of phytoflavin, a flavoprotein with chloroplast ferredoxin activity. Plant Physiol 40: 1124–1165
Smith JM, Smith WH and Knaff DB (1981) Electrochemical titrations of ferredoxin-ferredoxin:NADP+ oxidoreductase complex. Biochim Biophys Acta 635: 405–411
Sticht H and Rösch P (1998) The structure of iron-sulfur proteins. Progr Biophys Mol Biol 70, 95–136
Stombaugh NA, Sundquist JE, Burris RH and Orme-Johnson WH (1976) Oxidation-reduction properties of several low potential iron-sulfur proteins and of methylviologen. Biochemistry 15: 2633–2641
Swenson RP and Krey GD (1994) Site-directed mutagenesis of tyrosine-98 in the flavodoxin from Desulfovibrio vulgaris (Hildenborough): regulation of oxidation-reduction properties of the bound FMN cofactor by aromatic, solvent, and electrostatic interactions. Biochemistry 33: 15298–15308
Tagawa K and Arnon DI (1962) Ferredoxins as electron carriers in photosynthesis and the biological production and consumption of hydrogen gas. Nature 195: 537–543
Tagawa K and Arnon DI (1968) Oxidation reduction potentials and stoichiometry of electron transfer in ferredoxins. Biochim Biophys Acta 153: 602–613
Tsukihara T, Kobayashi M, Nakamura M, Katsube Y, Fukuyama K, Hase T and Matsubara H (1982) Structure-function relationship of [2Fe-2S] ferredoxins and design of a model molecule. Biosystems 15: 243–257
Tsukihara T, Fukuyama K, Mizushima M, Harioka T, Kusunoki M, Katsube Y, Hase T and Matsubara H (1990) Structure of the [2Fe-2S] ferredoxin I from blue-green alga Aphanothece sacrum. J Mol Biol 216: 399–410
Ullmann GM, Hauswald M, Jensen A and Knapp EW (2000) Structural alignment of ferredoxin and flavodoxin based on electrostatic potentials: implications for their interactions with photosystem I and ferredoxin-NADP+ reductase. Proteins 38: 301–309
van Mierlo CP, Muller F and Vervoort J (1990) Secondary and tertiary structure characteristics of Megasphaera elsdenii flavodoxin in the reduced state as determined by two-dimensional 1H NMR. Eur J Biochem 189: 589–600
Verhagen MFJM, Link TA and Hagen WR (1995) Electrochemical study of the redox properties of [2Fe-2S] ferredoxins. Evidence of superreduction of the rieske [2Fe-2S] cluster. FEBS Lett 361: 75–78
Vidakovic M, Fraczkiewicz G, Dave BC, Czernuszewicz RS and Germanas J (1995) The environment of [2Fe-2S] clusters in ferredoxins: the role of residue 45 probed by site-directed mutagenesis. Biochemistry 34: 13906–13913
Vieira BJ and Davis DJ (1986) Interaction of ferredoxin with ferredoxin:NADP reductase: effects of chemical modification of ferredoxin. Arch Biochem Biophys 247: 140–146
Vieira BJ, Colvert KK and Davis DJ (1986) Chemical modification and cross-linking as probes of regions on ferredoxin involved in interaction with ferredoxin:NADP reductase. Biochim Biophys Acta 851: 109–122
Walker MC, Pueyo JJ, Navarro JA, Gómez-Moreno C and Tollin G (1991) Laser flash photolysis studies of reduction of ferredoxins and ferredoxin-NADP+ reductases from Anabaena PCC 7119 and spinach: electrostatic effects on intracomplex electron transfer. Arch Biochem Biophys 287: 351–358
Walsh MA, McCarthy A, O’Farrell PA, McArdle P, Cunningham PD, Mayhew SG and Higgins TM (1998) X-ray crystal structure of the Desulfovibrio vulgaris (Hildenborough) apoflavodoxin-riboflavin complex. Eur J Biochem 258: 362–371
Wang M, Roberts DL, Paschke R, Shea TM, Masters BS and Kim JJ (1997) Three-dimensional structure of NADPH-cytochrome P450 reductase: prototype for FMN- and FAD-containing enzymes. Proc Natl Acad Sci USA 94: 8411–8416
Weber-Main AM, Hurley JK, Cheng H, Xia B, Chae YK, Markley JL, Martínez-Júlvez M, Gómez-Moreno C, Stankovich MT and Tollin G (1998) An electrostatic, kinetic and spectroscopic characterization of [2Fe-2S] vegetative and heterocyst ferredoxins from Anabaena 7120 with mutations in the cluster binding loop. Arch Biochem Biophys 355: 181–188
Zanetti G, Aliverti A and Curti B (1984) A cross-linked complex between ferredoxin and ferredoxin-NADP+ reductase. J Biol Chem 259: 6153–6157
Zanetti G, Morelli D, Ronchi S, Negri A, Aliverti A and Curti B (1988) Structural studies on the interaction between ferredoxin and ferredoxin:NADP+ reductase. Biochemistry 27: 3753–3759
Zanetti G, Aliverti A, Ravasi D, Curti B, Deng Z and Karplus PA (1997) On the role of glutamate 312 of spinach ferredoxin:NADP+ reductase. In: Stevenson KJ, Massey V and Williams CH Jr (eds) Flavins and Flavoproteins 1996, pp 509–512. University of Calgary Press, Calgary, Alberta
Zhou J, Nocek JM, DeVan ML and Hoffman BM (1995) Inhibitor-enhanced electron transfer: copper cytochrome c as a redox-inert probe of ternary complexes. Science 269: 204–207
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2006 Springer
About this chapter
Cite this chapter
Hurley, J.K., Tollin, G., Medina, M., Gómez-Moreno, C. (2006). Electron Transfer From Ferredoxin and Flavodoxin to Ferredoxin:NADP+ Reductase. In: Golbeck, J.H. (eds) Photosystem I. Advances in Photosynthesis and Respiration, vol 24. Springer, Dordrecht. https://doi.org/10.1007/978-1-4020-4256-0_27
Download citation
DOI: https://doi.org/10.1007/978-1-4020-4256-0_27
Publisher Name: Springer, Dordrecht
Print ISBN: 978-1-4020-4255-3
Online ISBN: 978-1-4020-4256-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)