Abstract
Aqueous two-phase systems (ATPS) have been widely and successfully used in the purification of various biological macromolecules such as proteins, nucleic acids, antibiotics, and cell components. Interfacial precipitation of the product often results in lower recovery and selectivity of ATPS. Efficient resolubilization of the interfacial precipitate offers a way to improve the recovery as well as selectivity of ATPS systems.
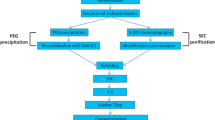
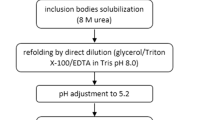
In this protocol, we describe a method for aqueous two-phase-assisted precipitation and resolubilization of the recombinant human Granulocyte Colony Stimulating Factor (GCSF) for its selective isolation from E. coli host cell proteins as well as nucleic acids. This platform purification can be applied to other cytokines as well as most of the hydrophobic proteins that partition into the hydrophobic PEG-rich top phase. Recoveries of up to 100% of the product along with reduction of levels of E. coli host cell proteins (from 250–500 to 10–15 ppm) and of nucleic acids (from 15–20 to 5–15 ng/mL) were observed.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Albertsson PA (1986) Partition of cell particles and macromolecules. Wiley, New York, NY
Diamond AD, Hsu JT (1992) Aqueous two-phase systems for biomolecule separation. Adv Biochem Eng Biotechnol 47:89–135
Haraguchi LH, Mohamed RS, Loh W et al (2004) Phase equilibrium and insulin partitioning in aqueous two-phase systems containing block copolymers and potassium phosphate. Fluid Phase Equilibr 215:1–15
Reh G, Nerli B, Pico G (2002) Isolation of alpha-1 antitrypsin from human plasma by partitioning in aqueous biphasic systems of polyethyleneglycol-phosphate. J Chromatogr B 780:389–396
Cavalcanti MTH, Porto TS, Neto BB et al (2006) Aqueous two-phase systems extraction of α-toxin from Clostridium perfigens type A. J Chromatogr B 833:135–140
Rosa PAJ, Azevedo AM, Ferreira IF et al (2007) Affinity partitioning of human antibodies in aqueous two-phase systems. J Chromatogr A 1162:103–113
Chethana S, Nayak CA, Raghavarao KSMS (2007) Aqueous two-phase extraction for purification and concentration of betalains. J Food Eng 81:679–687
Patil G, Raghavarao KSMS (2007) Aqueous two-phase extraction for purification of C-phycocyanin. Biochem Eng J 34:156–164
Johansson H, Ishii M, Minaguti M et al (2008) Separation and partitioning of green fluorescent protein from Escherichia coli homogenate in poly(ethylene glycol)/sodium-poly(acrylate) aqueous two-phase systems. Sep Purif Technol 62:166–174
Soares RR, Azevedo AM, Van Alstine JM, Aires-Barros MR (2015) Partitioning in aqueous two-phase systems: analysis of strengths, weaknesses, opportunities and threats. Biotechnol J 10:1158–1169
Phong WN, Le CF, Show PL, Chang JS, Ling TC (2017) Extractive disruption process integration using ultrasonication and an aqueous two-phase system for protein recovery from Chlorella sorokiniana. Eng Life Sci 17:357–369
Iqbal M et al (2016) Aqueous two-phase system (ATPS): an overview and advances in its applications. Biol Proced Online 18:18
Lee JW, Forciniti D (2010) Purification of human antibodies from transgenic corn using aqueous two-phase systems. Biotechnol Prog 26:159–167
Singh N, Sibylle H (2017) Downstream processing technologies/capturing and final purification. In: Ghose TK, Fiechter A, Blakebrough N (eds) Advances in biochemical engineering. Springer, Berlin, pp 1–64
Tran R, Titchener-Hooker NJ, Lacki K (2011) Aqueous two phase extraction augmented precipitation process for purification of therapeutic proteins Patent application number: 20110257378
Bhambure R, Sharma R, Gupta D et al (2013) A novel aqueous two phase assisted platform for efficient removal of process related impurities associated with E. coli based biotherapeutic protein products. J Chromatogr A 1307:49–57
Rathore A (2012) A process for purification of Recombinant granulocyte colony stimulating factor. Patent application number: 1880/DEL 2012
GuanY LTH, Garcia-Lisbona MN et al (1995) New approaches to aqueous polymer systems: theory, thermodynamics and applications to biomolecular separations. Pure Appl Chem 67:955–962
Mokhtarani B, Karimzadeh R, Amini MH et al (2008) Partitioning of Ciprofloxacin in aqueous two-phase system of poly(ethylene glycol) and sodium sulphate. Biochem Eng J 38:241–247
Zaslavsky BS (1995) Aqueous two-phase partitioning: physical chemistry and bioanalytical applications. Marcel Dekker Inc., New York, NY
Kaul A (2000) Phase diagram. In: Kaul A (ed) Methods in biotechnology, vol. 11: aqueous two-phase systems: methods and protocols. Humana, Totowa, NJ
Ferreira GB, Evangelista AF, Junio JBS et al (2007) Partitioning optimization of proteins from Zea mays malt in ATPS PEG 6000/CaCl2. Braz Arch Biol Technol 50:557–564
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Rathore, A.S., Bhambure, R. (2021). Aqueous Two-Phase-Assisted Precipitation of Proteins: A Platform for Isolation of Process-Related Impurities from Therapeutic Proteins. In: Labrou, N.E. (eds) Protein Downstream Processing. Methods in Molecular Biology, vol 2178. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-0775-6_8
Download citation
DOI: https://doi.org/10.1007/978-1-0716-0775-6_8
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-0774-9
Online ISBN: 978-1-0716-0775-6
eBook Packages: Springer Protocols