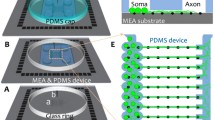
Abstract
The present chapter describes fabrication and utilization of neurite-isolation microelectrode arrays (NI-MEAs). The neurite-isolation device makes it possible to functionally isolate individual neurites from neuronal cell bodies as the neurites are forced to grow into narrow microchannels. Being microfluidic setups, the NI devices can be conveniently employed to carry out laser axotomy, study retrograde/anterograde transport in single axons, investigate the mutual effects of soluble factors (e.g., amyloid-β peptides) secreted by different neuronal populations (each “housed” in a different macrochannel), or to create the so-called axonal diodes imposing unidirectional axonal connectivity, to name but a few options. A separately made MEA is assembled with the NI device so that each of its microchannels is connected to one microelectrode. Unlike in patch-clamp, electrophysiology performed with the aid of NI-MEA is “massively parallel” as many neurites can be recorded simultaneously, and the data acquisition is noninvasive as the electrodes are not forced into a physical contact with the neurites. The capability of such setup is demonstrated here by recording the effects of different concentrations of nerve growth factor (NGF) on the electrical activity of individual neurites of superior cervical ganglions in primary culture, during their outgrowth into the microchannels. Simultaneous microscopic (e.g., immunofluorescence) observation of multiple neurites and their somata is an inherent extra option permitting to correlate their electrical activity with other physiological parameters, recorded by optical means.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- AID:
-
Axon isolation device
- Ig:
-
Immunoglobulin
- iPSC:
-
Induced pluripotent stem cells
- MEA:
-
Micro/Multielectrode array
- NGF:
-
Nerve growth factor
- NI-MEA:
-
Neurite-isolation MEA
- PDMS:
-
Polydimethylsiloxane
- PBS:
-
Phosphate buffered saline
- SCG:
-
Superior cervical ganglion
References
Fattahi P, Yang G, Kim G, Abidian MR (2014) A review of organic and inorganic biomaterials for neural interfaces. Adv Mater 26(12):1846–1885. https://doi.org/10.1002/adma.201304496
Giordano G, Costa L (2011) Primary neurons in culture and neuronal cell lines for in vitro neurotoxicological studies. In: Costa LG, Giordano G, Guizzetti M (eds) In vitro neurotoxicology. (Meth Mol Biol, vol 758. Humana Press, Totowa, NJ, pp 13–27. https://doi.org/10.1007/978-1-61779-170-3_2
Biedler JL, Helson L, Spengler BA (1973) Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res 33(11):2643–2652. https://www.ncbi.nlm.nih.gov/pubmed/4748425
Biedler JL, Roffler-Tarlov S, Schachner M, Freedman LS (1978) Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res 38(11):3751–3757. https://www.ncbi.nlm.nih.gov/pubmed/29704
Greene LA, Tischler AS (1976) Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A 73(7):2424–2428. https://doi.org/10.1073/pnas.73.7.2424
Hilgenberg LG, Smith MA (2007) Preparation of dissociated mouse cortical neuron cultures. J Vis Exp 10:562. https://doi.org/10.3791/562
Kramer D, Minichiello L (2010) Cell culture of primary cerebellar granule cells. Meth Mol Biol 633:233–239. https://doi.org/10.1007/978-1-59745-019-5_17
Mao L, Wang J (2003) Primary striatal neuronal culture. In: Wang J (ed) Drugs of abuse. (Meth Mol Med, vol 79. Humana Press, Totowa, NJ, pp 379–386. https://doi.org/10.1385/1-59259-358-5:379
Zeitelhofer M, Vessey JP, Xie Y, Tubing F, Thomas S, Kiebler M, Dahm R (2007) High-efficiency transfection of mammalian neurons via nucleofection. Nat Protoc 2(7):1692–1704. https://doi.org/10.1038/nprot.2007.226
De Biasi M (2002) Nicotinic receptor mutant mice in the study of autonomic function. Curr Drug Targets CNS Neurol Disord 1(4):331–336. https://doi.org/10.2174/1568007023339148
Fischer H, Orr-Urtreger A, Role LW, Huck S (2005) Selective deletion of the α5 subunit differentially affects somatic-dendritic versus axonally targeted nicotinic ACh receptors in mouse. J Physiol 563(Pt 1):119–137. https://doi.org/10.1113/jphysiol.2004.075788
Putz G, Kristufek D, Orr-Urtreger A, Changeux JP, Huck S, Scholze P (2008) Nicotinic acetylcholine receptor-subunit mRNAs in the mouse superior cervical ganglion are regulated by development but not by deletion of distinct subunit genes. J Neurosci Res 86(5):972–981. https://doi.org/10.1002/jnr.21559
David R, Ciuraszkiewicz A, Simeone X, Orr-Urtreger A, Papke RL, McIntosh JM, Huck S, Scholze P (2010) Biochemical and functional properties of distinct nicotinic acetylcholine receptors in the superior cervical ganglion of mice with targeted deletions of nAChR subunit genes. Eur J Neurosci 31(6):978–993. https://doi.org/10.1111/j.1460-9568.2010.07133.x
Scholze P, Ciuraszkiewicz A, Groessl F, Orr-Urtreger A, McIntosh JM, Huck S (2011) α4β2 nicotinic acetylcholine receptors in the early postnatal mouse superior cervical ganglion. Dev Neurobiol 71(5):390–399. https://doi.org/10.1002/dneu.20870
Ciuraszkiewicz A, Schreibmayer W, Platzer D, Orr-Urtreger A, Scholze P, Huck S (2013) Single-channel properties of α3β4, α3β4α5 and α3β4β2 nicotinic acetylcholine receptors in mice lacking specific nicotinic acetylcholine receptor subunits. J Physiol 591(Pt13):3271–3288. https://doi.org/10.1113/jphysiol.2012.246595
Blumenfeld H (2003) From molecules to networks: cortical/subcortical interactions in the pathophysiology of idiopathic generalized epilepsy. Epilepsia 44:7–15. https://doi.org/10.1046/j.1528-1157.44.s.2.2.x
Luna LG (1968) Manual of histologic staining methods of the Armed Forces Institute of Pathology. McGraw-Hill (Blakiston Div.), New York. https://archive.org/details/ManualHistologicalStaining
Coons AH, Creech HJ, Jones RN (1941) Immunological properties of an antibody containing a fluorescent Group. Exp Biol Med (Maywood) 47(2):200–202. https://doi.org/10.3181/00379727-47-13084p
Volgyi B, Bloomfield SA (2002) Axonal neurofilament-H immunolabeling in the rabbit retina. J Comp Neurol 453(3):269–279. https://doi.org/10.1002/cne.10392
Seifert U, Hartig W, Grosche J, Bruckner G, Riedel A, Brauer K (1998) Axonal expression sites of tyrosine hydroxylase, calretinin- and calbindin-immunoreactivity in striato-pallidal and septal nuclei of the rat brain: a double-immunolabelling study. Brain Res 795(1–2):227–246. https://doi.org/10.1016/S0006-8993(98)00298-4
Haykal-Coates N, O'Callaghan JP, Reinhard JF Jr, Jensen KF (1991) Pargyline and γbutyrolactone enhance tyrosine hydroxylase immunostaining of nigrostriatal axons. Brain Res 556(2):353–357. https://doi.org/10.1016/0006-8993(91)90330-X
Hechler D, Nitsch R, Hendrix S (2006) Green-fluorescent-protein-expressing mice as models for the study of axonal growth and regeneration in vitro. Brain Res Rev 52(1):160–169. https://doi.org/10.1016/j.brainresrev.2006.01.005
Zhou XY, Ji M, Liu JY, Tan MQ (2007) GFP gene transfection of dendritic cells mediated by recombinated adeno-associated virus. J Centr South Univ (Med Sci) 32(5):742–746. (in Chinese). https://www.ncbi.nlm.nih.gov/pubmed/18007063
van den Pol AN, Spencer DD (2000) Differential neurite growth on astrocyte substrates: interspecies facilitation in green fluorescent protein-transfected rat and human neurons. Neuroscience 95(2):603–616. https://doi.org/10.1016/s0306-4522(99)00430-3
Cassereau J, Nicolas G, Lonchampt P, Pinier M, Barthelaix A, Eyer J, Letournel F (2013) Axonal regeneration is compromised in NFH-LacZ transgenic mice but not in NFH-GFP mice. Neuroscience 228:101–108. https://doi.org/10.1016/j.neuroscience.2012.10.011
Kameda H, Furuta T, Matsuda W, Ohira K, Nakamura K, Hioki H, Kaneko T (2008) Targeting green fluorescent protein to dendritic membrane in central neurons. Neurosci Res 61(1):79–91. https://doi.org/10.1016/j.neures.2008.01.014
Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI (1998) Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci 18(7):2697–2708. https://www.ncbi.nlm.nih.gov/pubmed/9502827
Hartline FF (1979) Biological applications for voltage sensitive dyes. Science 203(4384):992–994. https://doi.org/10.1126/science.424731
Ross WN, Reichardt LF (1979) Species-specific effects on the optical signals of voltage-sensitive dyes. J Membr Biol 48(4):343–356. https://doi.org/10.1007/BF01869445
Baker BJ, Kosmidis EK, Vucinic D, Falk CX, Cohen LB, Djurisic M, Zecevic D (2005) Imaging brain activity with voltage- and calcium-sensitive dyes. Cell Mol Neurobiol 25(2):245–282. https://doi.org/10.1007/s10571-005-3059-6
Zochowski M, Wachowiak M, Falk CX, Cohen LB, Lam YW, Antic S, Zecevic D (2000) Imaging membrane potential with voltage-sensitive dyes. Biol Bull 198(1):1–21. https://doi.org/10.2307/1542798
Foust A, Popovic M, Zecevic D, McCormick DA (2010) Action potentials initiate in the axon initial segment and propagate through axon collaterals reliably in cerebellar Purkinje neurons. J Neurosci 30(20):6891–6902. https://doi.org/10.1523/jneurosci.0552-10.2010
Popovic M, Gao X, Zecevic D (2012) Voltage-sensitive dye recording from axons, dendrites and dendritic spines of individual neurons in brain slices. J Vis Exp 69:e4261. https://doi.org/10.3791/4261
Grinvald A, Ross WN, Farber I (1981) Simultaneous optical measurements of electrical activity from multiple sites on processes of cultured neurons. Proc Natl Acad Sci U S A 78(5):3245–3249. https://doi.org/10.1073/pnas.78.5.3245
Kinoshita M, Ueda R, Kojima S, Sato K, Watanabe M, Urano A, Ito E (2002) Multiple-site optical recording for characterization of functional synaptic organization of the optic tectum of rainbow trout. Eur J Neurosci 16(5):868–876. https://doi.org/10.1046/j.1460-9568.2002.02160.x
Tsytsarev V, Liao LD, Kong KV, Liu YH, Erzurumlu RS, Olivo M, Thakor NV (2014) Recent progress in voltage-sensitive dye imaging for neuroscience. J Nanosci Nanotechnol 14(7):4733–4744. https://doi.org/10.1166/jnn.2014.9531
Mao C, Kisaalita WS (2004) Determination of resting membrane potential of individual neuroblastoma cells (IMR-32) using a potentiometric dye (TMRM) and confocal microscopy. J Fluoresc 14(6):739–743. https://doi.org/10.1023/B:JOFL.0000047224.41328.f8
Maric D, Maric I, Smith SV, Serafini R, Hu Q, Barker JL (1998) Potentiometric study of resting potential, contributing K+ channels and the onset of Na+ channel excitability in embryonic rat cortical cells. Eur J Neurosci 10(8):2532–2546. https://doi.org/10.1046/j.1460-9568.1998.00284.x
Bullen A, Saggau P (1999) High-speed, random-access fluorescence microscopy: II. Fast quantitative measurements with voltage-sensitive dyes. Biophys J 76(4):2272–2287. https://doi.org/10.1016/s0006-3495(99)77383-2
Mohajerani MH, McVea DA, Fingas M, Murphy TH (2010) Mirrored bilateral slow-wave cortical activity within local circuits revealed by fast bihemispheric voltage-sensitive dye imaging in anesthetized and awake mice. J Neurosci 30(10):3745–3751. https://doi.org/10.1523/jneurosci.6437-09.2010
Kibler AB, Jamieson BG, Durand DM (2012) A high aspect ratio microelectrode array for mapping neural activity in vitro. J Neurosci Methods 204(2):296–305. https://doi.org/10.1016/j.jneumeth.2011.11.027
Johansson F, Wallman L, Danielsen N, Schouenborg J, Kanje M (2009) Porous silicon as a potential electrode material in a nerve repair setting: Tissue reactions. Acta Biomater 5(6):2230–2237. https://doi.org/10.1016/j.actbio.2009.02.010
Renshaw S (ed) (2007) Immunohistochemistry. Scion Publishing, Bloxham
Herzog W, Weber K (1978) Fractionation of brain microtubule-associated proteins. Isolation of two different proteins which stimulate tubulin polymerization in vitro. Eur J Biochem 92(1):1–8. https://doi.org/10.1111/j.1432-1033.1978.tb12716.x
Bébarová M (2012) Advances in patch clamp technique: towards higher quality and quantity. Gen Physiol Biophys 31(2):131–140. https://doi.org/10.4149/gpb_2012_016
Hodgkin AL, Huxley AF (1952) A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol 117(4):500–544. https://www.ncbi.nlm.nih.gov/pubmed/12991237
Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 391(2):85–100. https://doi.org/10.1007/BF00656997
Neher E, Sakmann B, Steinbach JH (1978) The extracellular patch clamp: a method for resolving currents through individual open channels in biological membranes. Pflugers Arch 375(2):219–228. https://doi.org/10.1007/BF00584247
Neher E, Sakmann B (1992) The patch clamp technique. Sci Am 266(3):44–51. https://doi.org/10.1038/scientificamerican0392-44
Caldwelland JH, Milton RL (1995) The loose patch voltage clamp technique. In: Boulton A, Baker G, Walz W (eds) Patch-clamp applications and protocols, Neuromethods, vol 26. Humana Press, Totowa, NJ, pp 173–192. https://doi.org/10.1385/0-89603-311-2:173
Messerli MA, Collis LP, Smith PJS (2009) Ion trapping with fast-response ion-selective microelectrodes enhances detection of extracellular ion channel gradients. Biophys J 96(4):1597–1605. https://doi.org/10.1016/j.bpj.2008.11.025
Spira ME, Hai A (2013) Multi-electrode array technologies for neuroscience and cardiology. Nat Nanotechnol 8(2):83–94. https://doi.org/10.1038/nnano.2012.265
Thomas CA Jr, Springer PA, Loeb GE, Berwald-Netter Y, Okun LM (1972) A miniature microelectrode array to monitor the bioelectric activity of cultured cells. Exp Cell Res 74(1):61–66. https://doi.org/10.1016/0014-4827(72)90481-8
Liu M-G, Chen X-F, He T, Li Z, Chen J (2012) Use of multi-electrode array recordings in studies of network synaptic plasticity in both time and space. Neurosci Bull 28(4):409–422. https://doi.org/10.1007/s12264-012-1251-5
Hutzler M, Lambacher A, Eversmann B, Jenkner M, Thewes R, Fromherz P (2006) High-resolution multitransistor array recording of electrical field potentials in cultured brain slices. J Neurophysiol 96(3):1638–1645. https://doi.org/10.1152/jn.00347.2006
Frey U, Egert U, Heer F, Hafizovic S, Hierlemann A (2009) Microelectronic system for high-resolution mapping of extracellular electric fields applied to brain slices. Biosens Bioelectron 24(7):2191–2198. https://doi.org/10.1016/j.bios.2008.11.028
Berdondini L, Imfeld K, Maccione A, Tedesco M, Neukom S, Koudelka-Hep M, Martinoia S (2009) Active pixel sensor array for high spatio-temporal resolution electrophysiological recordings from single cell to large scale neuronal networks. Lab Chip 9(18):2644–2651. https://doi.org/10.1039/b907394a
Huys R, Braeken D, Jans D, Stassen A, Collaert N, Wouters J, Loo J, Severi S, Vleugels F, Callewaert G, Verstreken K, Bartic C, Eberle W (2012) Single-cell recording and stimulation with a 16k micro-nail electrode array integrated on a 0.18 μm CMOS chip. Lab Chip 12(7):1274–1280. https://doi.org/10.1039/c2lc21037a
Lewicki MS (1998) A review of methods for spike sorting: the detection and classification of neural action potentials. Network 9(4):R53–R78. https://doi.org/10.1088/0954-898X_9_4_001
Brown EN, Kass RE, Mitra PP (2004) Multiple neural spike train data analysis: state-of-the-art and future challenges. Nat Neurosci 7(5):456–461. https://doi.org/10.1038/nn1228
Offenhäusser A, Böcker-Meffert S, Decker T, Helpenstein R, Gasteier P, Groll J, Möller M, Reska A, Schäfer S, Schulte P, Vogt-Eisele A (2007) Microcontact printing of proteins for neuronal cell guidance. Soft Matter 3(3):290–298. https://doi.org/10.1039/B607615G
Pushparaj VL, Shaijumon MM, Kumar A, Murugesan S, Ci L, Vajtai R, Linhardt RJ, Nalamasu O, Ajayan PM (2007) Flexible energy storage devices based on nanocomposite paper. Proc Natl Acad Sci U S A 104(34):13574–13577. https://doi.org/10.1073/pnas.0706508104
Klemic KG, Klemic JF, Reed MA, Sigworth FJ (2002) Micromolded PDMS planar electrode allows patch clamp electrical recordings from cells. Biosens Bioelectron 17(6–7):597–604. https://doi.org/10.1016/S0956-5663(02)00015-5
Andersson H, van den Berg A (2004) Microtechnologies and nanotechnologies for single-cell analysis. Curr Opin Biotechnol 15(1):44–49. https://doi.org/10.1016/j.copbio.2004.01.004
Bruhn BR, Liu H, Schuhladen S, Hunt AJ, Mordovanakis A, Mayer M (2014) Dual-pore glass chips for cell-attached single-channel recordings. Lab Chip 14(14):2410–2417. https://doi.org/10.1039/c4lc00370e
Fertig N, Blick RH, Behrends JC (2002) Whole cell patch clamp recording performed on a planar glass chip. Biophys J 82(6):3056–3062. https://doi.org/10.1016/s0006-3495(02)75646-4
Py C, Denhoff MW, Sabourin N, Weber J, Shiu M, Zhao P (2014) Priming and testing silicon patch-clamp neurochips. N Biotechnol 31(5):430–435. https://doi.org/10.1016/j.nbt.2014.04.003
Py C, Martina M, Diaz-Quijada GA, Luk CC, Martinez D, Denhoff MW, Charrier A, Comas T, Monette R, Krantis A, Syed NI, Mealing GA (2011) From understanding cellular function to novel drug discovery: the role of planar patch-clamp array chip technology. Front Pharmacol 2:51. https://doi.org/10.3389/fphar.2011.00051
Hierlemann A, Frey U, Hafizovic S, Heer F (2011) Growing cells atop microelectronic chips: Interfacing electrogenic cells in vitro with CMOS-based microelectrode arrays. Proc IEEE 99(2):252–284. https://doi.org/10.1109/JPROC.2010.2066532
Fromherz P, Offenhausser A, Vetter T, Weis J (1991) A neuron-silicon junction: a Retzius cell of the leech on an insulated-gate field-effect transistor. Science 252(5010):1290–1293. https://doi.org/10.1126/science.1925540
Taylor AM, Rhee SW, Tu CH, Cribbs DH, Cotman CW, Jeon NL (2003) Microfluidic multicompartment device for neuroscience research. Langmuir 19(5):1551–1556. https://doi.org/10.1021/la026417v
Peyrin JM, Deleglise B, Saias L, Vignes M, Gougis P, Magnifico S, Betuing S, Pietri M, Caboche J, Vanhoutte P, Viovy JL, Brugg B (2011) Axon diodes for the reconstruction of oriented neuronal networks in microfluidic chambers. Lab Chip 11(21):3663–3673. https://doi.org/10.1039/c1lc20014c
Dinh ND, Chiang YY, Hardelauf H, Baumann J, Jackson E, Waide S, Sisnaiske J, Frimat JP, Thriel CV, Janasek D, Peyrin JM, West J (2013) Microfluidic construction of minimalistic neuronal co-cultures. Lab Chip 13(7):1402–1412. https://doi.org/10.1039/c3lc41224e
Park J, Kim S, Park SI, Choe Y, Li J, Han A (2014) A microchip for quantitative analysis of CNS axon growth under localized biomolecular treatments. J Neurosci Methods 221 (0):166-174. doi:https://doi.org/10.1016/j.jneumeth.2013.09.018
Poon WW, Blurton-Jones M, Tu CH, Feinberg LM, Chabrier MA, Harris JW, Jeon NL, Cotman CW (2011) β-Amyloid impairs axonal BDNF retrograde trafficking. Neurobiol Aging 32(5):821–833. https://doi.org/10.1016/j.neurobiolaging.2009.05.012
Takayama Y, Kotake N, Haga T, Suzuki T, Mabuchi K (2012) Formation of one-way-structured cultured neuronal networks in microfluidic devices combining with micropatterning techniques. J Biosci Bioeng 114(1):92–95. https://doi.org/10.1016/j.jbiosc.2012.02.011
Hulme SE, Shevkoplyas SS, Samuel A (2008) Microfluidics: streamlining discovery in worm biology. Nat Meth 5(7):589–590. https://doi.org/10.1038/nmeth0708-589
Fior R, Maggiolino S, Lazzarino M, Sbaizero O (2011) A new transparent Bio-MEMS for uni-axial single cell stretching. Microsystem Technologies 17(10–11):1581–1587. https://doi.org/10.1007/s00542-011-1325-8
Nguyen TD, Hogue IB, Cung K, Purohit PK, McAlpine MC (2013) Tension-induced neurite growth in microfluidic channels. Lab Chip 13(18):3735–3740. https://doi.org/10.1039/c3lc50681a
Bhattacharjee N, Li N, Keenan TM, Folch A (2010) A neuron-benign microfluidic gradient generator for studying the response of mammalian neurons towards axon guidance factors. Integr Biol 2(11-12):669–679. https://doi.org/10.1039/c0ib00038h
Xiao RR, Zeng WJ, Li YT, Zou W, Wang L, Pei XF, Xie M, Huang WH (2013) Simultaneous generation of gradients with gradually changed slope in a microfluidic device for quantifying axon response. Anal Chem 85(16):7842–7850. https://doi.org/10.1021/ac4022055
Vogt AK, Lauer L, Knoll W, Offenhäusser A (2003) Micropatterned substrates for the growth of functional neuronal networks of defined geometry. Biotechnol Prog 19(5):1562–1568. https://doi.org/10.1021/bp034016f
Gilles S, Winter S, Michael KE, Meffert SH, Li P, Greben K, Simon U, Offenhäusser A, Mayer D (2012) Control of cell adhesion and neurite outgrowth by patterned gold nanoparticles with tunable attractive or repulsive surface properties. Small 8(21):3357–3367. https://doi.org/10.1002/smll.201200465
Taylor AM, Blurton-Jones M, Rhee SW, Cribbs DH, Cotman CW, Jeon NL (2005) A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat Meth 2(8):599–605. https://doi.org/10.1038/Nmeth777
Lee SW, Lee SS (2008) Shrinkage ratio of PDMS and its alignment method for the wafer level process. Microsystem Technologies 14(2):205–208. https://doi.org/10.1007/s00542-007-0417-y
de Lima AD, Merten MD, Voigt T (1997) Neuritic differentiation and synaptogenesis in serum-free neuronal cultures of the rat cerebral cortex. J Comp Neurol 382(2):230–246. https://doi.org/10.1002/(SICI)1096-9861(19970602)382:2<230::AID-CNE7>3.0.CO;2-4
Lein P, Johnson M, Guo X, Rueger D, Higgins D (1995) Osteogenic protein-1 induces dendritic growth in rat sympathetic neurons. Neuron 15(3):597–605. https://doi.org/10.1016/0896-6273(95)90148-5
Park JW, Vahidi B, Taylor AM, Rhee SW, Jeon NL (2006) Microfluidic culture platform for neuroscience research. Nat Protocols 1(4):2128–2136. https://doi.org/10.1038/nprot.2006.316
Bosse F (2012) Extrinsic cellular and molecular mediators of peripheral axonal regeneration. Cell Tissue Res 349(1):5–14. https://doi.org/10.1007/s00441-012-1389-5
Sofroniew MV, Howe CL, Mobley WC (2001) Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci 24:1217–1281. https://doi.org/10.1146/annurev.neuro.24.1.1217
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Science+Business Media LLC
About this protocol
Cite this protocol
Wanzenboeck, H.D., Scholze, P., Mika, J.K. (2020). Imaging and Electrophysiology of Individual Neurites Functionally Isolated in Microchannels. In: Pelc, R., Walz, W., Doucette, J.R. (eds) Neurohistology and Imaging Techniques. Neuromethods, vol 153. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-0428-1_12
Download citation
DOI: https://doi.org/10.1007/978-1-0716-0428-1_12
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-0426-7
Online ISBN: 978-1-0716-0428-1
eBook Packages: Springer Protocols