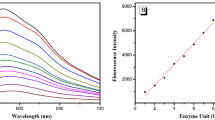
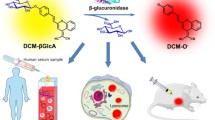
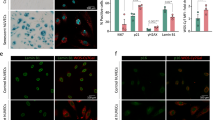
Abstract
β-Galactosidase (β-gal) is an enzyme commonly served as a reporter for the examination of transcription and transfection efficiencies. Due to its overexpression in primary and metastatic ovarian cancers, β-gal was also usually regarded as a molecular target for visualizing peritoneal metastases from ovarian cancers. Moreover, β-gal has been studied as a potential therapeutic target for lactose intolerance via gene replacement therapy in recent years. Interestingly, there were some reports that β-gal has been abnormally accumulated in senescent cells, which allowing this senescence-associated β-gal to be a significant biomarker for senescence. The great significance of β-gal has attracted many researchers’ attentions in developing highly selective and sensitive approaches to monitor the activity of this enzyme in vitro and in vivo. In this review, we reported the recent development of the various materials for β-gal detection and their application in disease progression monitoring, with a focus on fluorescent probe, nanomaterials, and biomolecules. Finally, the trends for the further development of the probe for fluorescence-guided diagnosis in clinical cases and its preclinical potential value were proposed.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Alam J, Cook JL (1990) Reporter genes: application to the study of mammalian gene transcription. Anal Biochem 188(2):245
Chatterjee SK, Bhattacharya M, Barlow JJ (1979) Glycosyltransferase and glycosidase activities in ovarian cancer patients. Cancer Res 39(6):1943
Salehi S, Eckley L, Sawyer GJ et al (2009) Intestinal lactase as an autologous beta-galactosidase reporter gene for in vivo gene expression studies. Hum Gene Ther 20(1):21
Kodibagkar VD, Yu J, Liu L et al (2006) Imaging β-galactosidase activity using 19F chemical shift imaging of LacZ gene-reporter molecule 2-fluoro-4-nitrophenol-β-d-galactopyranoside. Magn Reson Imaging 24(7):959
Van Dort ME, Lee KC, Hamilton CA et al (2008) Radiosynthesis and evaluation of 5-[125i]iodoindol-3-yl-β-d-galactopyranoside ([125i]IBDG) as a β-galactosidase imaging radioligand. Mol Imaging 7(4):187
Celen S, Deroose C, De Groot T et al (2008) Synthesis and evaluation of F-18- and C-11-labeled phenyl-galactopyranosides as potential probes for in vivo visualization of LacZ gene expression using positron emission tomography. Bioconjug Chem 19(2):441
Yamamoto A, Adachi S, Kawamura S et al (1974) Localized β-galactosidase deficiency. Arch Intern Med 134(4):627
Zhang YZ, Naleway JJ, Larison KD et al (1991) Detecting lacZ gene expression in living cells with new lipophilic, fluorogenic beta-galactosidase substrates. FASEB J 5(15):3108
Liu Y, Feng B, Cao X et al (2019) A novel “AIE + ESIPT” near-infrared nanoprobe for the imaging of γ-glutamyl transpeptidase in living cells and the application in precision medicine. Analyst 144:5136–5142. https://doi.org/10.1039/c9an00773c
Wehrman TS, Von DG, Krutzik PO et al (2006) Luminescent imaging of beta-galactosidase activity in living subjects using sequential reporter-enzyme luminescence. Nat Methods 3(4):295
Lee HW, Heo CH, Sen D et al (2014) Ratiometric two-photon fluorescent probe for quantitative detection of β-galactosidase activity in senescent cells. Anal Chem 86(20):10001
Hirabayashi K, Hanaoka K, Takayanagi T et al (2015) Analysis of chemical equilibrium of silicon-substituted fluorescein and its application to develop a scaffold for red fluorescent probes. Anal Chem 87(17):9061
Asanuma D, Sakabe M, Kamiya M et al (2015) Sensitive β-galactosidase-targeting fluorescence probe for visualizing small peritoneal metastatic tumours in vivo. Nat Commun 6:6463
Zhang XX, Wu H, Li P et al (2016) A versatile two-photon fluorescent probe for ratiometric imaging E. coli β-galactosidase in live cells and in vivo. Chem Commun 52(53):8283
Redykeisar O, Kisinfinfer E, Ferber S et al (2014) Synthesis and use of QCy7-derived modular probes for the detection and imaging of biologically relevant analytes. Nat Protoc 9(1):27
Han J, Han MS, Tung CH (2013) A fluorogenic probe for β-galactosidase activity imaging in living cells. Mol BioSyst 9(12):3001
Gu K, Xu Y, Li H et al (2016) Real-time tracking and in vivo visualization of β-galactosidase activity in colorectal tumor with a ratiometric near-infrared fluorescent probe. J Am Chem Soc 138(16):5334
Berezin MY, Achilefu S (2010) Fluorescence lifetime measurements and biological imaging. Chem Rev 110(5):2641
Hsieh CC, Cheng YM, Hsu CJ et al (2008) Spectroscopy and femtosecond dynamics of excited-state proton transfer induced charge transfer reaction. J Phys Chem A 112(36):8323
Murale DP, Kim H, Choi WS et al (2013) Highly selective excited state intramolecular proton transfer (ESIPT)-based superoxide probing. Org Lett 15(15):3946
Otsubo T, Minami A, Fujii H et al (2013) 2-(Benzothiazol-2-yl)-phenyl-β-d-galactopyranoside derivatives as fluorescent pigment dyeing substrates and their application for the assay of β-d-galactosidase activities. Bioorg Med Chem Lett 23(7):2245
Cellier M, Fazackerley E, James AL, Orenga S, Perry JD, Turnbull G, Stanforth SP (2014) Synthesis of 2-arylbenzothiazole derivatives and their application in bacterial detection. Bioorg Med Chem 22(4):1250
Wei X, Wu Q, Zhang J et al (2016) Synthesis of precipitating chromogenic/fluorogenic β-glucosidase/β-galactosidase substrates by a new method and their application in the visual detection of foodborne pathogenic bacteria. Chem Commun 53(1):103
Mei J, Leung NLC, Kwok RTK et al (2015) Aggregation-induced emission: together we shine, united we soar! Chem Rev 115(21):11718
He XP, Zang Y, James TD et al (2016) Fluorescent glycoprobes: a sweet addition for improved sensing. Chem Commun 53(1):82
Dong Y, Wang W, Zhong C et al (2014) Investigating the effects of side chain length on the AIE properties of water-soluble TPE derivatives. Tetrahedron Lett 55(8):1496
Cao D, Yang L, Wang L (2014) Application of aggregation-induced emission (AIE) systems in sensing and bioimaging. Curr Org Chem 18(8):1028
Peng L, Gao M, Cai X et al (2015) A fluorescent light-up probe based on AIE and ESIPT processes for β-galactosidase activity detection and visualization in living cells. J Mater Chem B 3(47):9168
Jiang G, Zeng G, Zhu W et al (2017) A selective and light-up fluorescent probe for β-galactosidase activity detection and imaging in living cells based on an AIE tetraphenylethylene derivative. Chem Commun 53(32):4505
Huang Y, Feng H, Liu W et al (2017) Cation-driven luminescent self-assembled dots of copper nanoclusters with aggregation-induced emission for β-galactosidase activity monitoring. J Mater Chem B 5:5120
Jia X, Li J, Wang E (2013) Cu nanoclusters with aggregation induced emission enhancement. Small 9(22):3873
Jia X, Yang X, Li J et al (2014) Stable Cu nanoclusters: from an aggregation-induced emission mechanism to biosensing and catalytic applications. Chem Commun 50(2):237
Wu Z, Liu J, Gao Y et al (2015) Assembly-induced enhancement of Cu nanoclusters luminescence with mechanochromic property. J Am Chem Soc 137(40):12906
Chen PC, Li YC, Ma JY et al (2016) Size-tunable copper nanocluster aggregates and their application in hydrogen sulfide sensing on paper-based devices. Sci Rep 6:24882
Agostini A, Mondragón L, Bernardos A et al (2012) Targeted cargo delivery in senescent cells using capped mesoporous silica nanoparticles. Angew Chem Int Ed 51(42):10556
Antropova YG, Bryantsev AL, Kalinina NI et al (2002) Proliferative activity and expression of cyclin-dependent kinase inhibitor p21WAF1 and p53 protein in endothelial cells of human aorta during replicative aging in vitro. Bull Exp Biol Med 134(1):81
Lee BY, Han JA, Im JS et al (2006) Senescence-associated β-galactosidase is lysosomal β-galactosidase. Aging Cell 5(2):187
Leckaczernik B, Moerman EJ, Shmookler Reis RJ et al (1997) Cellular and molecular biomarkers indicate precocious in vitro senescence in fibroblasts from SAMP6 mice. Evidence supporting a murine model of premature senescence and osteopenia. J Gerontol 52(6):B331
Gu Y, Wu Q, Zhu M et al (2011) Efficient pipeline configuration in distributed heterogeneous computing environments. Aging Cell 10(2):338
Chigira S, Sugita K, Kita K et al (2003) Increased expression of the Huntingtin interacting protein-1 gene in cells from Hutchinson Gilford Syndrome (Progeria) patients and aged donors. J Gerontol 58(10):B873
Cabrera S, Haskouri JE, Guillem C et al (2000) Generalised syntheses of ordered mesoporous oxides: the atrane route. Solid State Sci 2(4):405
Vallet-Regí M, Balas F, Arcos D (2007) Mesoporous materials for drug delivery. Angew Chem Int Ed 46(40):7548
Auvray X, Petipas C, Anthore R et al (1995) X-ray diffraction study of the ordered lyotropic phases formed by sugar-based surfactants. Langmuir 11(2):433
Tang C, Zhou J, Qian Z et al (2017) A universal fluorometric assay strategy for glycosidases based on functional carbon quantum dots: β-galactosidase activity detection in vitro and in living cells. J Mater Chem B 5(10):1971
Chen Y, Zhu C, Yang Z et al (2012) A new “turn-on” chemodosimeter for Hg2+: ICT fluorophore formation via Hg2+-induced carbaldehyde recovery from 1,3-dithiane. Chem Commun 48(42):5094
Zheng XT, Ananthanarayanan A, Luo KQ et al (2015) Glowing graphene quantum dots and carbon dots: properties, syntheses, and biological applications. Small 11(14):1620
Lim SY, Shen W, Gao Z (2015) Carbon quantum dots and their applications. Chem Soc Rev 44(1):362
Kim EJ, Kumar R, Sharma A et al (2017) In vivo imaging of β-galactosidase stimulated activity in hepatocellular carcinoma using ligand-targeted fluorescent probe. Biomaterials 122:83
Prost M, Hasserodt J (2014) “Double gating” – a concept for enzyme-responsive imaging probes aiming at high tissue specificity. Chem Commun 50(94):14896
Thorn-Seshold O, Vargas-Sanchez M, McKeon S et al (2012) A robust, high-sensitivity stealth probe for peptidases. Chem Commun 48(50):6253
Acknowledgments
We are grateful for the financial supports from the National Natural Science Foundation of China (81971678, 81671756), Key Research Project of Science and Technology Foundation of Hunan Province (2017SK2093 and 2019SK2211), Key Research Project of Science and Technology Foundation of Changsha (kq1801063), and Fundamental Research Funds for the Central Universities of Central South University (2018zzts041, 2018dcyj067).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Ethics declarations
Conflict of Interest: There are no conflicts to declare.
Ethical Approval: There is no ethical approval to declare.
Informed Consent: There is no informed consent to declare.
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bi, A. et al. (2019). Fluorescent Probes for Diagnostics of β-Galactosidase: From Micro to Macro. In: Cheng, Z. (eds) Fluorescent Imaging in Medicinal Chemistry . Topics in Medicinal Chemistry, vol 34. Springer, Cham. https://doi.org/10.1007/7355_2019_87
Download citation
DOI: https://doi.org/10.1007/7355_2019_87
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-46706-7
Online ISBN: 978-3-030-46707-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)