Abstract
Cellobiose lipids (CL) are glycolipids secreted by many Ustilaginaceae species in aerobic fermentations characterised by excessive foaming. While increasing CL concentrations remains an aim for its industrial production, excessive foaming during fermentation presents a challenge even at laboratory scale. Foam fractionation (FF) provides a solution to the foaming problem and facilitates the proceeding purification of CL. Here, we present a first CL fermentation process applying FF. With our set-up, we manage to exploit the excessive foaming for continuous product separation. The set-up includes a foam collecting vessel (FCV) with inserts for CL accumulation and foamate recirculation to minimise biomass and nutrient loss. Integrating a foam column (FC) into the fermenter headspace enabled foam enrichment, resulting in the recovery of > 90% of the produced CL from the separated fractions consisting of foam depositions in the fermenter headspace and the FCV. We also increased the fermenter filling volume and thus achieved a higher fermentation capacity. The separated CL fraction was purified via ethanol extraction to obtain CL with purities > 90%. We further examined the effects of different culture media constituents, including biomass and CL, on foam generation and decay and assessed the effect of FC geometries on product enrichment and recovery. In this work, a FF set-up is presented that enables a stable CL fermentation without additional foam mitigation methods. At the same time, the application of FF separated a fraction that was highly enriched in CL during fermentation, resulting in highly pure CL after a simple ethanol extraction.
Similar content being viewed by others
1 Introduction
Many Ustilaginaceae species like Ustilago maydis and Sporisorium scitamineum secrete the glycolipids Cellobiose lipids (CL) when sugars are used as the only carbon source during aerobic fermentation [1, 2]. CL consists of a cellobiose disaccharide, glycosidically linked to a hydroxypalmitic acid [3, 4]. This secondary metabolite is secreted extracellularly and precipitates as needle-like crystals in the culture broth due to its low solubility in acidic pH values commonly applied during fermentations [5, 6].
Catabolic studies on the CL variant flocculosin suggest that it acts as a nutrient source when food limitation dominates [7]. Only a limited number of certain microorganisms can hydrolyse the glycosidic linkage in CL, which makes it a suitable nutrient storage molecule [4]. Moreover, it is thought that the amphiphilic character of CLs facilitates the solubilisation of hydrophobic nutrients [8].
Besides their biological function, CLs may be of great interest to the surfactants industry, providing a naturally synthesized alternative to synthetic surfactants. Several studies have examined the physicochemical characteristics and antimicrobial activity of different CL variants while suggesting potential application areas, such as in detergents, cosmetics, or pesticides [9,10,11,12]. Other research groups examined known producing microorganisms and their metabolic pathways to understand CL synthesis better [13]. Several fermentation media and parameters, as well as CL purification processes, were also presented in the literature [1, 14, 15]. Only a few studies examined CL fermentation in a bioreactor scale, with a maximum volume of 757 L [15]. An overview of all bioreactor fermentations of CL, among other glycolipids, was recently published by Zibek et al. [16]. Reported CL concentrations range from 1.5 g·L−1 up to 33 g·L−1 with differing degrees of purity [14, 17].
CL is usually secreted as a mixture of different structures by wild-type species, along with other glycolipids like mannosylerythritol lipids (MEL). Fatty acids in the substrates are predominantly metabolized to MEL [18,19,20]. Thus, an absence of oils in the substrate and the presence of a hydrophilic carbon source would shift the production toward CL. Furthermore, the absence of an additional hydrophobic carbon source prevents the occurence of emulsions, which largely facilitates proceeding purification and downstream processing (DSP). However, the benefit of hydrophobic carbon sources, such as vegetable oils, acting as antifoam agents, cannot be used here. Therefore, other solutions must be implemented to control excessive foam formation during CL fermentation. Excessive foam may result in blockage of exhaust air filters. Furthermore, the foam takes up a large volume of the fermenter, thus leading to a reduced working volume and utilisation of the fermenter's capacity. While mechanical, physical, or chemical methods for foam mitigation can be effective, they are associated with energy and material demand and may alter the composition of the culture broth. Chemical methods also make the downstream processing more complex because the added amount of antifoaming agent throughout the fermentation to destroy the foam needs to be separated.
Foam fractionation (FF) presents a solution to the foaming problem and facilitates the proceeding purification of CL. In general, amphiphilic molecules in aerated liquids adsorb at gas–liquid interfaces, resulting in an enriched layer of surface active molecules that stabilize the foam [21, 22]. The stable foam bubbles ascend to the liquid's surface due to density gradients. This results in separating the surface active molecules from the culture broth. This phenomenon is actively implemented during FF processes, where foam enriched with surface active molecules is collected and used to separate these molecules from the liquid [23]. When non-soluble and hydrophobic particles attached to gas bubbles are separated, the mechanism becomes foam flotation [24, 25].
FF has already been applied to several fermentations for the production of biosurfactants, primarily surfactin and rhamnolipid [26, 27]. However, to our knowledge, there is no published research or methodology for CL fermentation applying FF. Due to its amphiphilic character, CL proved to be a promising candidate for FF. Therefore, we aimed to establish a CL fermentation process applying FF to exploit the excessive foaming for continuous product separation and prevent downsides associated with overfoaming.
In a first step, to generate a broader understanding of the foaming behaviour of CL fermentation broths, we examined the effect of different culture media constituents, including biomass, CL, sugars, and other media components, on foam generation and decay. We then used different foam column geometries to determine their effect on product enrichment and recovery when foaming CL-containing culture broths in a shaking flask. Using foam columns in a foam fractionation set-up is an established method to enhance the enrichment of the target substance to be separated and, in some cases, even its recovery [24, 28].
Another relevant factor to foaming is the aeration rate in the fermenter and the size of introduced gas bubbles to the culture broth. If foam formation becomes too excessive, the aeration rate often needs to be reduced to avoid overfoaming. Another motivation for decreasing the aeration rate and/or power input for agitation is also the reduction of the electricity demand of the fermentation process and increasing the overall process economy [29]. This may, however, lead to undesired oxygen limitation and result in changes in metabolite formation [30] or even the death of the microorganisms if their oxygen demand is not covered. One approach to overcome this limitation is the usage of bubble-less membrane aeration if foaming is not desired, as was shown for surfactin fermentation, for instance [31].
On the other hand, if foaming is desired, the agitation and aeration rates must result in sufficient air bubbles supply for foam generation. Here, the size (affected by the agitation rate and the pore size of the aeration unit) and the number of gas bubbles (affected by the aeration rate) are relevant parameters. At the same time, the microorganisms' oxygen demand has to be covered at all times to avoid changes in their metabolism. Besides increasing the agitation and aeration rates, which are both associated with increased power consumption, using a porous ceramic aeration unit with a smaller pore size compared to the traditional aeration ring may lead to a larger amount of smaller gas bubbles. Therefore, the effect of a porous ceramic unit on oxygen transfer rates and their possible effect on foaming during fermentation was further compared to a traditional aeration ring.
Proceeding these preliminary examinations, a FF set-up with an integrated foam column (FC), including a foam collecting vessel (FCV) with inserts and foamate recirculation, was applied to the CL fermentation process with two different Ustilaginaceae species that show different foaming behaviour. The set-up was adapted to obtain the highest enrichment and recovery rates of CL while maintaining a minimal loss of both biomass and fermentation media. The separated CL fraction was then purified via ethanol extraction to obtain high purity CL, with purities > 90%.
2 Materials and methods
For fermentations in this study, two prominent CL-producing fungi were chosen, of which the mutant strain Ustilago maydis Δemt1 produces only CLs, while the wild-type strain Sporisorium scitamineum can produce both CL and MEL simultaneously. Both strains show similar growth behaviour. However, S. scitamineum tends to form more excessive foam during fermentation. All methods used for their cultivation and the examination of CL fermentation and foam fractionation are presented in the following sections.
2.1 Strains and seed culture
Ustilago maydis Δemt1 DSM 17,146 and Sporisorium scitamineum (Ustilago scitaminea) DSM 11,941 cultures obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ) were stored in glycerol cryo stocks at − 80 °C until used to streak agar plates. For U. maydis, a PD agar (24 g·L−1 potato-dextrose broth (Becton Dickinson, Le Pont de Claix, France) and 15 g·L−1 agar, pH 5.6) and for S. scitamineum, a YM agar (10 g·L−1 glucose, 5 g·L−1 peptone, 3 g·L−1 malt extract, 3 g·L−1 yeast extract and 15 g·L−1 agar, pH 6 [32]) were used. The pH was adjusted using 2 M H2SO4. The inoculated plates were incubated for 48 h at 30 °C and stored for a maximum of 30 days at 8 °C.
Strains were obtained from these agar plates to inoculate 20 mL of liquid PD-medium for U. maydis, or 20 mL of liquid YM-medium for S. scitamineum in 100 mL baffled shake flasks. After approx. 17 h at 30 °C and 120 rpm, this seed culture was used to inoculate a second seed culture (200 mL PD-medium for U. maydis or 200 mL of YM-medium for S.scitamineum in 1 L baffled shaking flasks) to an optical density (OD625) of 0.1 a.u.. This second seed culture was cultivated under the same conditions until the glucose concentration decreased to < 1 g·L−1, after which it was used to inoculate the fermenters used for CL fermentation.
2.2 Fermentation conditions during CL production
For the fermentation of CL, the same cultivation strategy was used for both strains U. maydis and S. scitamineum. The seed culture was transferred to the fermentation medium containing mineral salts, compounds supplying trace elements, and a vitamin solution, as previously described by our group [1]. For some fermentations presented in this paper, the same medium without a vitamin solution was used. However, since vitamins proved unnecessary for CL synthesis [1], and for clarity reasons, no differentiation based on the used cultivation medium is made in the results section. 0.6 g·L−1 urea and 100 g·L−1 sucrose were added as nitrogen and carbon sources, and the fermenter was inoculated to an OD625 of 0.3 a.u. After the initial glucose obtained from sucrose hydrolysis was consumed, a glucose or sucrose solution was fed to a concentration of 50 g·L−1. In the case of fermenter broth overflow, the liquid loss was compensated with water. All fermentations were conducted at 30 °C for 7–14 days. The termination criterion was the depletion of glucose from the culture medium or complete depletion of carbon source (fructose and glucose). 2 M H2SO4 and 2 M NaOH were used to adjust the pH to 2.5 if needed (adapted with modifications after [33]).
For bioreactor fermentations, a 10 L glass reactor (KGW; Germany) was filled with 6–9 L fermentation media, depending on the fermenter set-up with or without a foam column. DO, pH, and T were monitored online, while stirrer speed and gas flow rate were monitored and adjusted manually. The fermenter was aerated with 0.14–0.38 vvm and agitated with 160–500 rpm to maintain the DO level above 10% at a minimal power input. For aeration, either a traditional aeration ring or a porous ceramic membrane was used to introduce gas bubbles into the culture broth. kLa values of both aeration units were determined using the dynamic gassing out method [34] in the 10 L fermenter with 8 L filling volume at different aeration rates and stirrer speeds.
Shake flask cultivations in 1 L baffled shaking flasks were conducted in triplicates at 120 rpm and 30 °C to obtain complementary data on substrate uptake rates and CL formation rates during batch cultivation. Culture medium, carbon source, and nitrogen source concentrations were the same as used in the fermenter unless otherwise indicated.
Samples of 1 mL were obtained approx. every 24 h for analysis of OD625, CDW (cell dry weight), CL, sugar, and nitrogen concentrations, both from the fermenters and shaking flasks.
2.3 Foam generation and decay rate of different culture broth samples
To examine the foaming behaviour of different culture broth constituents using a small sample volume, an apparatus was adapted after [35] to determine foam generation ug and decay rates ud. The used apparatus consisted of a tube (d = 18 mm) with a scale and a frit at the bottom, through which 40 mL of air was sparged manually via a syringe (Fig. 1). A rotameter was used to enable a constant airflow. 2 mL samples were filled in the tube, and the time (tg, g: foam generation) needed for the foam to reach its maximum height after the air was sparged, the maximum height reached (hmax), as well as the time needed for the foam to collapse (td, d: foam decay) were recorded. ug and ud were calculated using the following equations:
The measurement was stopped if a sample needed a decay time td > 3 min. All samples were measured in triplicates. The standard deviation of this method was estimated at 14% for ug and 13% for ud based on 5 measurements of a sample of a culture broth.
2.4 Foaming in foam columns
In order to determine the effect of foam columns on CL enrichment and recovery, three columns with different heights/diameters (H/D [mm/mm] = 400/40; 300/30; 200/20) were examined in a foaming set-up (Fig. 2). Culture broth containing CL and biomass (feed) was sparged with air at 40–60 L h−1 for 13 min in a shake flask. The built foam rose from the top of the shake flask to the connected foam column and was collected in a beaker (foamate). At the end of the experiment, the volumes of the remaining broth in the shake flask (retentate) and the foamate were measured. CL and biomass concentrations in each fraction were determined and used to calculate CL enrichment and recovery.
The CL enrichment E [ − ] in a fraction is defined as the ratio of CL concentration in this fraction to the CL concentration in the culture broth. CL recovery R [%] is the percentage of CL obtained from a separated fraction out of the total produced or available CL amount. Biomass enrichment and recovery were calculated using biomass concentrations in each fraction.
2.5 Fermenter set-up for foam fractionation
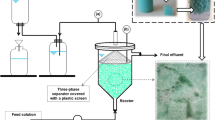
In order to continuously separate the produced foam during CL fermentation from the fermenter, a foam fractionation unit was set up in the glass reactor. In the initial approach, a set-up similar to [36] was built. The foam flowed from the reactor headspace due to pressure gradients through a silicone tube to a connected 2 L glass bottle (Schott AG, Germany) with inserts, the FCV. Different types of inserts were studied for their suitability as surfaces for CL accumulation in the foam collecting vessel (polypropylene particles (d ~ 3 mm); Luffa aegyptiaca sponge; polypropylene balls (d ~ 20 mm)). In order to decrease biomass and culture media loss, the foamate was recirculated back to the fermenter using a peristaltic pump (Watson-Marlow GmbH, Germany). Exhaust air was directed from the top of the foam collecting vessel. Exhaust gas composition was measured using a CO2 and O2 gas analyser from BlueSens gas sensor GmbH and used to calculate OUR and CER.
This set-up was further adjusted by adding a FC (d = 30 mm) to the reactor headspace to increase CL enrichment (Fig. 3). The column height was adjustable, and a H/D ratio of 10–20 was adapted during fermentation, depending on the foaming intensity. If needed, a second FCV was connected parallel to the first one.
Fermenter set-up for CL fermentation with foam fractionation. During fermentation, the foam flows through the foam column (FC) to a foam collecting vessel (FCV) with inserts, while the foamate is recirculated back to the fermenter. Exhaust air flows from the FCV through a foam overflow vessel (FOV) to the exhaust air cooler and filter
2.6 Analytical methods and calculations
Sample analysis was conducted as previously described in [1]. Biomass was quantified via OD625 and measurement of cell dry weight (CDW). Growth rates µ [h−1] were calculated using the OD625 values. Urea concentration was measured enzymatically with a urea/ammonia test kit (R-Biopharm; Germany), and sugar concentrations were measured via HPLC. Specific glucose and fructose uptake rates were calculated using the following equation:
where; \({q}_{s}\) substrate uptake rate [h−1]; \(t\) time [h], \({c}_{s}\) substrate concentration [g·L−1]; \({c}_{CDW}\) biomass concentration [g·L−1].
Sucrose is hydrolysed extracellularly by U. maydis to its constituents glucose and fructose [37]. For an accurate calculation of glucose and fructose uptake rates, the increase of glucose and fructose in the medium due to sucrose hydrolysis has to be considered. Therefore, the decrease of sucrose concentration in the fermentation medium during each calculation period was divided by two and considered as the concentration of produced glucose/fructose during this time span. This amount was then added to the measured glucose and fructose concentration and used for the calculation of the uptake rate according to equation (3).
CL was quantified via TLC analysis using internal calibration standards [1]. The purity of the used standards was also determined densitometrically via TLC (n = 9) at 95.4 ± 1.6% for CL produced by U. maydis and at 91.6 ± 2.9% (n = 4) for CL produced by S. scitamineum, and used at concentrations of 10, 5, 2.5, and 1.25 g·L−1. CL formation rates were calculated only for shaking flask cultivations, using Eq. (4). The determination of CL concentrations in the culture broth during bioreactor fermentation was not representative of the formation rates due to CL transport with the foam. Therefore, a calculation of CL formation rates was not possible here.
where; \({r}_{CL}\) specific CL formation rate [h−1]; \(t\) time [h]; \({c}_{CL}\) CL concentration [g·L−1], \({c}_{CDW}\) biomass concentration [g·L−1].
The substrate-specific product yield coefficient YPS [g·g−1] and the biomass yield coefficient YXS [g·g−1] were calculated as the quotient of produced CL or biomass and the metabolised substrate.
2.7 Determination of CL solubility and CL purification via ethanol extraction
In order to optimise a purification method for CL extraction via ethanol with minimal solvent consumption, solubility of CL had to be determined in a first step. For solubility measurement, the saturation shake flask method was adapted after [38] as follows: 1 mL of different EtOH:H2O mixtures (w/w 100:0; 80:20; 60:40; 40:60; 20:80 and 0:100) were added to 220–250 mg of purified CL from U. maydis and incubated at 22—25 °C and 900 rpm for 72 – 138 h. The samples were then centrifuged at 1300 rpm and 22–25 °C for 10 min, and the supernatant was filtrated using a 2 µm cellulose acetate filter (Carl Roth GmbH). 300 µL of the filtered CL-saturated supernatant were added to a weighed vessel and dried at 65 °C until a constant weight was achieved. The dissolved CL was determined gravimetrically in triplicates and used to determine the solubility in different EtOH:H2O mixtures. The obtained values were used to adapt the amount needed for CL extraction during the purification process. After fermentation, the culture broth, foam depositions in the fermenter headspace, as well as in the FCV and FC, were purified separately using the same protocol. Each fraction was quantified to calculate the enrichment and recovery of CL.
In a first step, each of the fractions containing precipitated CL crystals and biomass was centrifuged to separate the solids from the aqueous phase containing remaining media components and sugars. The pellet was then washed with acidic water (pH 2, H2SO4) to further remove water-soluble impurities and centrifuged again. CL was extracted from the pellet with ethanol at room temperature, at a ratio of 2 gEtOH ·gPellet−1. This ratio was determined based on the results of the solubility tests and is explained in Sect. 3.6. After centrifugation, the extract containing CL was evaporated and dried. Finally, the dried CL was ground to a white powder. The extraction step was repeated until no CL crystals were visible in the pellet under the microscope. This purification method was described in [29].
3 Results and discussion
In order to design a foam fractionation unit that enables the maximum separation of CL while maintaining biomass and media components in the fermenter, it was necessary to obtain fundamental knowledge of the fermentation process and factors affecting foaming behavior. We here present the results of multiple fermentations in a 1 L shake flask and 10 L fermenter scale that show the time course of CL fermentation and foam generation over the different fermentation stages. We further show the effect of different media components, aeration strategies, as well as column and foam collecting vessel designs on foam generation and separability. Finally, we present our foam fractionation unit that enables complete in situ separation of the produced CL from the culture broth, as well as our optimized purification method.
3.1 The general course of CL fermentation in shake flasks
The general CL fermentation courses for U. maydis and S. scitamineum are very similar and vary mainly in the individual substrate uptake and product formation rates and their concentrations. To avoid repetition, general observations are presented and discussed in the following for U. maydis only but can also be transferred to S. scitamineum. Corresponding figures for S. scitamineum are presented in the Additional file (Additional file 1: Fig. S1).
Using 100 g·L−1 sucrose as a carbon source, the sugar is hydrolysed within the first 24–48 h of fermentation. Figure 4 shows the course of substrate uptake and CL production in 1 L shake flasks. The glucose fraction is metabolized by U. maydis within the first seven days, while fructose concentration in the medium remains almost at the same level. Depending on C-source availability and input, phase I and II describe the batch phase, where glucose is still available, whereas in phase III, glucose is depleted in batch fermentations, and only fructose is available. Substrate uptake showed for glucose a maximum qgluc,max of 0.33 h−1 opposed to qfruc,max of 0.11 h−1 for fructose (Fig. 4). This shows that glucose is the preferred substrate when both sugars are available in the medium and is explained by the suppression of other catabolite pathways when glucose, which provides the best energy gain, is present [39].
Course of substrate, biomass and CL concentrations, glucose and fructose uptake rates, and CL formation rates and yields during an U. maydis cultivation in 1 L shake flasks at 120 rpm and 30 °C. The error bars indicate the standard deviation of a biological triplicate. The numbers I–III indicate the different phases in relation to urea and glucose availability
Urea, with an initial concentration of 0.6 g·L−1, is completely metabolized within approx. the first 24–30 h (phase I). A shift in biomass composition before and after this exponential growth phase is evident, as previously reported [29]. After nitrogen depletion, the molar biomass composition shifts from C1H1.83O0.64N0.13 to C1H1.85O0.35N0.03 [29]. CL formation is usually first detected right before or after nitrogen depletion and decreases after glucose depletion if no additional sucrose or glucose is fed to the medium.
The highest CL formation rates are usually observed in phase I (0.13 ± 0.02 d−1) and II (0.09 ± 0.01 d−1), and the highest yields for a given timespan were observed in phase II during batch fermentation (Fig. 4). This shows that higher CL is yielded on the glucose fraction of sucrose compared to its fructose fraction. Together with the lower substrate uptake rate of fructose compared to glucose, these results suggest a termination of the fermentation after glucose depletion if shorter fermentation durations and higher space–time yields are aspired. Increasing the space–time yield of CL can largely enhance the process economy, as we showed in our previous publication [29]. Terminating the fermentation right after glucose depletion would, however, result in high concentrations of fructose remaining in the culture broth at the end of the fermentation. Although this means that almost half the amount of the added substrate is not metabolized for CL formation, the remaining fructose could be yielded as an additional by-product, as was suggested for other fermentation processes, e.g. [40, 41].
On the other hand, although both higher CL yields and formation rates were observed on the glucose fraction of sucrose compared to fructose, using only glucose as substrate did not lead to similar results. CL productivity was previously shown to be lower when using glucose and/or fructose as substrate compared to sucrose [14]. An explanation for this observation has yet to be found. However, due to the higher yields obtained with sucrose, we used it as the preferred carbon source for our further fermentations in the 10 L fermenter.
Overall, a similar fermentation course was observed in the 10 L fermenter. However, higher substrate uptake rates, as well as biomass and CL concentrations were usually achieved here due to the enhanced oxygen transfer compared to shake flask cultivations. Observations on biomass formation kinetics and foaming behaviour and the herewith associated CL concentrations in the fermenter are discussed in the following chapter.
3.2 The course of CL fermentation in a 10 L fermenter with an aeration ring
In bioreactor fermentations, glucose was usually metabolized faster, compared to shake flask cultivations, within the first 4–5 days (Additional file 1: Fig. S2). Here, additional glucose or sucrose was usually fed to the fermenter after glucose was depleted (phase III).
Fig. 5 A shows the time course of biomass formation of 6 fermentations in a 10 L fermenter. Biomass formation can be divided into three phases (I, II, and III), depending on substrate availability and uptake. Phase I, during the first 24–30 h, is characterized by N availability (Additional file 1: Fig. S3), and growth rates up to µmax = 0.16 h−1 are observed here. During phases II and III, where N is limited, the growth rate decreases gradually. The switch in biomass composition from phase I to phase II and III was previously explained by [42, 43] and attributed to both N accumulation as well as lipid formation and accumulation within the cells.
The course of biomass concentration during 6 cultivations A and CL concentration during 5 cultivations B of U. maydis in a 10 L fermenter using a standard aeration ring. Each cultivation is indicated with different data points’ shapes and colours. The numbers I–III indicate the different phases in relation to urea and glucose availability
Opposed to biomass formation, the determination of accurate CL concentrations in the fermenter was not possible due to foaming. Foaming usually started immediately after urea is depleted from the fermentation medium, transporting CL out of the broth to the fermenter headspace and, if available, through the foam column. Depending on the intensity of foam formation, CL concentrations measured within the culture broth do not reflect the actual synthesized amount of CL. This is reflected in high fluctuations of CL concentration (Fig. 5B). In some fermentations with moderate foam formation, up to 20 g·L−1 CL was measured in the broth, whereas in other fermentations with excessive foam formation, a maximum of below 1 g·L−1 CL could be measured in the fermentation broth throughout the whole fermentation process due to CL flotation with the foam. Other authors also observed a fluctuation in the foaming behaviour depending on the fermentation phase during the cultivation of various microorganisms, as summarized by Junker et al. [21].
The higher fluctuation in CL concentrations within the fermenter at similar fermentation conditions, compared to the relatively good reproducibility of biomass concentration results, indicates that CL transport by foam out of the culture broth is more pronounced compared to biomass transport. These are optimal conditions for an application of foam fractionation. In an optimal foam fractionation set-up, most of the product should be separated by the foam fraction (high recovery rates), while biomass should be retained in the fermenter. Otherwise, the presence of a biocatalyst in the fermenter would be limited by time, and no further product synthesis is possible.
During CL fermentation in fermenters, foaming behaviour in different phases of the fermentation is variable. Right after inoculation, moderate foam formation starts. However, this foam is unstable and thus does not reach the vessel's headspace. Approximately around the time when the nitrogen source is depleted from the fermentation media, foam formation increases significantly, and the foam fills up the whole fermenter head space and moves into the foam fractionation vessels. This was observed in multiple CL fermentations with U. maydis and S. scitamineum in a 10 L fermenter with a filling volume of 6–9 L. With time, foam stability decreases, and the headspace becomes emptier. After feeding carbohydrates, the second increase in foam formation and stability usually occurs. In order to gain a better understanding of the foaming behaviour during CL fermentations and design a suitable FF unit accordingly, the influence of different culture media constituents was examined.
3.3 Culture media constituents affecting the foaming behaviour
To identify optimal conditions for foam fractionation, we assessed a possible correlation between specific components in the culture broth and their foaming behaviour. For this purpose, different fractions of culture broth were analysed with our foaming apparatus. With this external experimental setup, the effect of different aeration and agitation rates are excluded.
Eight different samples were analysed, consisting of the cultivation medium from U. maydis, with or without biomass, CL, and other media components (Table 1). Both the cultivation medium (sample 1) and a sucrose solution (sample 2) show the highest foam generation rates ug above 0.1 cm·s−1 (Fig. 6). At the same time, they also show the highest foam decay rates ud above 0.07 cm·s−1. This indicates that although introducing air bubbles to the cultivation medium creates foam, this foam is not stable and collapses rapidly. These results correlate with the observed foaming pattern during fermentation, in which foaming is minimal initially. A sample taken after inoculation with biomass but before N-depletion (sample 3) showed an ug below the measurement threshold. This changed rapidly after N-depletion, where sample 4 showed a high ug and low ud, indicating the occurrence of foam stabilising components in the culture broth at this time point. This coincides with the observations made during CL fermentations, where increased foaming occurs around the time at which N is depleted from the medium. A similar behaviour was previously reported in the literature, where N-limitation also triggered foam formation in a Moniliella tomentosa var pollinis fermentation [30]. The excessive foaming there was attributed to the induction of polysaccharides and erythritol formation. CL formation increases after N-limitation, indicating that CL indeed has an essential role in foam stabilisation. Sample 5 containing higher CL and biomass concentrations further showed lower ud, indicating a positive correlation between CL and/or biomass concentration and foaming and foam stabilisation. To assess whether the enhanced foaming is solely due to contained CL and biomass or other water-soluble unknown components, the supernatant of sample 5 (sample 6) and its pellet resuspended in 0.9% NaCl (sample 7) were also examined. Both samples showed a high ug and a low ud, indicating that both biomass/CL and unknown water-soluble components positively affect foaming.
In general, low biosurfactant concentrations should serve as foam stabilizers, as previously shown for rhamnolipids, where low concentrations caused significant foam accumulation and higher concentrations resulted in enhanced foam stability [44]. These surface active components accumulate at the liquid–gas surface and thus stabilize foams. However, in the case of CL, the foam stabilizing effect may be due to two different mechanisms:
-
1.
Dissolved CL molecules are oriented with their hydrophilic moiety, the cellobiose disaccharide towards the liquid film of the foam lamellae, while the hydrophobic hydroxypalmitic acid is oriented towards the gas phase (Fig. 7A.
Fig. 7 Schematic illustration of the hypothesized mechanisms of the foam stabilising effect of CL. A CL adsorption of soluble CL molecules in the liquid film surrounding a foam bubble. The circles indicate the hydrophilic parts, and the lines indicate the hydrophobic parts of CL. B The natural flow of liquid in foam lamellae due to drainage. C CL particle in a foam lamella, hindering the liquid flow and thus stabilising the foam
-
2.
Precipitated CL crystals can act as particles and reduce drainage and coalescence. Particles in foam tend to act as small dams and block drainage in thin foam lamella, thus leading to foam stabilisation (Fig. 7B, C). This effect was described for large particles between 1 and 10 µm by Hudales et al. [45].
Considering the low solubility of CL in water [5], especially at the acidic pH value of fermentation, the first effect would be rather limited. Most of the produced CL precipitates as needle-like crystals [5] varying in size at pH 2.5. Microscopic images of CL culture broths reveal a heterogeneous size distribution of these crystals (own results, not shown), including crystals in the range of 1–10 µm, thus favoring the second foam stabilizing mechanism. Depending on the dominating effect, the mechanism of separating CL through foam during fermentations would be correctly termed foam fractionation or foam flotation. Since both effects would contribute to the separation process, in this paper for simplicity purposes, we only use the term foam fractionation.
Biomass can also serve as foam stabilizing agents due to their particulate character as wetted solids [46]. In a rhamnolipid fermentation, a more significant influence on foaming was attributed to Pseudomonas aeruginosa cells, compared to the effect of the biosurfactant [47]. However, comparing the effect of biomass vs. biosurfactant in the case of CL was not possible since extracting CL from the biomass pellet with ethanol for sample preparation would alter the cell surface and thus show different effects than the ones occurring during fermentation.
Further, cell hydrophobicity may vary along the different fermentation phases and affect the foaming behavior differently. For example, in a T. reesei fermentation, increased cell hydrophobicity during the late exponential phase was presented as a possible reason for increased foaming [48]. Whether a similar effect explains the increased foaming after N-depletion and the end of the exponential growth phase during CL fermentation needs to be examined in further work. Nevertheless, the obtained results show an overall stabilizing effect of both CL and biomass on foam. Additionally, unknown water-soluble components present in the supernatant showed an equally significant stabilizing effect on the foam. These may include other metabolites or extracellular proteins, present in the culture broth.
However, sucrose (sample 8) did not further enhance the foaming parameters, although a stabilizing effect on foam was previously attributed to carbohydrates [49], and increased foaming is usually observed during CL fermentation right after the feed of sugar.
Besides culture media, biomass, extracellular proteins, and secondary metabolites, pH was also described to significantly affect foam generation and stability by altering the charge of cells and thus changing electrostatic forces. In a rhamnolipid fermentation, foaming decreased at lower pH [47]. A change in pH further affects protein solubility, which can influence foam formation and stability [46]. However, in the case of CL fermentation, three different culture broth samples with adjusted pH values between 2.0 and 2.9 did not show a significant difference in ug or ud. This indicates that neither of the mentioned effects occurs in that pH range and shows stability in the foaming behaviour towards slight fluctuations in pH during the CL fermentation process.
Overall, although some culture components were identified as contributors to the foaming behaviour, no single component could be identified as the primary agent that significantly affects foaming. The foaming behaviour during CL fermentations remains a complex interplay of different parameters, as was previously shown for other fermentations, e.g., in [49]. In order to examine the effect of a foam column on CL enrichment and recovery, different foam columns were examined in a next step. Column design, including the column height and diameter can affect foam ripening effects and thus contribute to enrichment. A detailed overview on the effects of foam columns on enrichment and recovery is given in [28].
3.4 Effect of foam column on CL enrichment and recovery
The examination of three different foam columns with an U. maydis culture broth containing CL confirmed the hypothesized positive effect of the columns on CL enrichment in the foamate (Fig. 8). An increase in CL concentration in the foamate fraction from approximately 1.0 g∙L−1 up to 1.7–2.4 g∙L−1 was achieved with all three columns, resulting in an enrichment of 1.9–2.3 and recoveries of 41–54%, respectively. This shows that using a foam column increases CL enrichment in the separated foam. At the same time, CDW concentration decreased in the foamate fraction from 4.6, 5.8, and 3.0 g∙L−1 to 3.2, 2.5, and 2.8 g∙L−1, and increased in the retentate to 12.5, 11.3 and 11.0 g∙L−1, respectively. While biomass loss is still present, an enrichment of only 0.22–0.26 was achieved in the foamate, showing that the affinity of CL to the foam is higher than that of the biomass. Transferring these results to the bioreactor and implementing a foam column to the FF setup would thus enhance the separation efficiency of CL (recovery and enrichment) while maintaining biomass loss at a tolerable level. To further reduce biomass loss, a recirculation setup was implemented in the fermenter, as discussed in Sect. 3.7.
CL and biomass concentrations in the feed, retentate, and foamate fraction after foaming a CL-containing culture broth of U. maydis using the set-up described in Sect. 2.4. 200/20, 300/30, 400/40 indicate the length/diameter of each column in mm
No significant change in sugar concentration in either fraction was found, as the sugar is transported in solution with the liquid fraction. However, this highlights the necessity of foamate recirculation in order to minimize medium component loss.
3.5 Effect of different aeration units on kLa and foam formation
In order to assess the effect of aeration with a porous ceramic sparger on oxygen transfer rates and their possible effect on foaming during fermentation, kLa values of the unit were first measured and compared to the values obtained with a traditional aeration ring. Implementing the porous ceramic sparger instead of a regular aeration ring resulted in higher kLa values at the same power input and aeration rates (Fig. 9). This enabled covering the required oxygen supply to the microorganisms while reducing the power consumption needed for aeration and agitation.
In the case of U. maydis, an OURmax in the range of 1.8–3.0 mmol∙gCDW−1∙h−1 was measured, and an OURmax of 2.6 mmol∙gCDW−1∙h−1 was measured with S. scitamineum.
Another lower limit for the minimum kLa, or in that case for agitation and aeration rates, is the supply of sufficient air bubbles for foam generation when foaming is desired. The upper limit of kLa is a minimum excess above the required minimal values to avoid unnecessary energy demand. Several fermentations in the 1 L and 10 L scale, where kLa was adjusted to maintain a pO2 level above 10% to ensure sufficient oxygen supply to the microorganisms, showed that neither additional stirring nor gas bubble introduction to the liquid was necessary for foam generation using the traditional aeration ring. The minimum agitation rate necessary for foam generation was determined at different aeration rates for U. maydis and S. scitamineum in a 1 L bioreactor, as previously published in a project report by our group [51].
Using the ceramic sparger even increased foam formation during fermentation significantly. In fermentations, where the ceramic sparger was used, larger amounts of foam were produced (qualitative observation). This is explained by the smaller bubble sizes introduced to the fermenter by the ceramic sparger, resulting in more stable and dense foam, as previously described in [21, 52]. Therefore, the filling volume of the fermenter had to be decreased to have enough head space for the formed foam. However, an increase in the filling volume was possible when adding a foam column to the fermenter, as discussed in detail in Sect. 3.7.
3.6 Purification of CL and variation in CL purity through FF
For a FF setup to be efficient, it has to result in an increase in CL enrichment and recovery in the separated fractions. In order to calculate these values, a purification protocol with minimal ethanol consumption was optimized to obtain purified CL after fermentation. Furthermore, the effect of FF on the purity of the obtained CL fraction was analysed.
The low solubility of CL in water at acidic pH values is of great advantage for the purification process. CL is largely present as a solid in the culture broth and can thus be separated from the liquid phase containing the remaining culture media and other secondary metabolites. CL crystals usually arrange themselves as agglomerates and form larger particles that are easily separated together with the biomass. As previously described by us, the biosurfactant is then obtained via ethanol extraction from the biomass/CL pellet [29]. Since ethanol consumption is a considerable cost factor in the purification process, our goal was to determine the minimum amount of ethanol needed for CL extraction from the pellet.
Solubility curves of three CL samples from U. maydis and S. scitamineum in different EtOH:H2O mixtures showed a sudden decrease in solubility at EtOH contents below 60% (Fig. 10). This means that during purification, the highest extraction efficiency at RT can only be obtained at EtOH contents above 60% in the solvent. However, after separation, the CL/biomass pellet already contains about approx. > 80% liquid, depending on the separation efficiency. Therefore, an EtOH:pellet ratio of 2 g∙g−1 was used for purification to guarantee an EtOH content above 60% in the solvent. One or more extractions were necessary until the biomass pellet was CL-free, based on the CL amount in the pellet. For two fermentations of U. maydis and one of S. scitamineum, it was possible to extract more than 90% of the produced CL in the first extraction step from the culture broth, the depositions, and the FF fraction, respectively.
Solubility of three different purified CL samples obtained from U. maydis and S. scitamineum culture broths in different EtOH:H2O mixtures at room temperature. Solubility tests were performed in triplicates as described in Sect. 2.7. Error bars indicate the standard deviation of a triplicate
Initial CL samples extracted from the pellet of a culture broth showed a yellowish colouring (Fig. 11A). TLC examination showed this is due to sugar residues in the CL extract. After separation, the liquid content in the CL/biomass pellet contains dissolved sugar residues. Resuspending the pellet in acidic water results in a dilution of these sugars by the factor \(\frac{added \, amount \, of \, water}{water \, content \, in \, pellet}\). By adding equal amounts of acidic water to the pellet, as the initial volume of the broth, the sugar content in the pellet was reduced to < 0.3% in a single washing step with acidic water (pH 2.5, H2SO4) before extraction. Initial sugar residue concentrations in these samples varied between 15 and 43 g∙L−1. These results were obtained with four culture broths containing different sugar concentrations. The amount of acidic washing water used can be reduced depending on the concentration of sugar residues in the pellet. The added washing step resulted in white CL after drying (Fig. 11B). Washing CL with acidic water to obtain higher purity was previously suggested by [14]. However, in that protocol, the already extracted CL was washed after drying. Adding the washing step before ethanol extractions proved to be less time-consuming since an additional drying step could be omitted. Further, resuspending the wet pellet after the first centrifugation step was more practical compared to resuspending the already dried CL. The use of acidic water is crucial here, to prevent a loss of CL due to dissolving in water at higher pH values.
A better method to obtain CL with high purity was achieved by implementing FF. Extracts obtained from the FCV or even from the depositions in the fermenter headspace showed less impurities and fatty acids, and the dry CL did not have a yellowish colouring; it was "whiter" than the fraction obtained from the culture broth extract. This was reflected in the TLC analysis, where fatty acids and other impurities of extracts obtained from the foam fraction were less compared to the extracts obtained directly from the culture broth (Fig. 12A, B). The origin of the fatty acids and other non-polar impurities detected in the extracts could be from extracellular lipids present in the culture broth's liquid phase or lipids extracted from the biomass during purification. Having a smaller amount of biomass, due to FF (compare results in Sect. 3.4) would automatically result in a reduced amount of lipids that would be extracted. This will result in a higher purity of the extract obtained from depositions/foam, compared to the one obtained from the culture broth. Microscopic images of the depositions/foam fraction showed large amounts of CL crystals (Fig. 12C) with only few microbial cells. This may therefore be one reason for the increased purity of the CL extracts. Altogether, we managed to increase CL purity solely through FF without adding additional purification steps. Furthermore, using the same EtOH:pellet ratio during extraction, less ethanol was needed to extract CL from the foam depositions since the CL concentration in the foamate pellet is higher compared to pellets obtained from the culture broth.
3.7 Evolution of a FF setup to obtain maximum enrichment and recovery
Based on the previously shown results, we optimised a FF setup to maximise both enrichment and recovery of CL, as well as the utilised fermenter volume, and to obtain CL with high purity. The final setup consisting of a FC and a FCV is illustrated in Fig. 3. By implementing the ceramic sparger (Sect. 3.5), a significant shift in CL accumulation was observed, compared to a fermentation with a FF setup using a traditional aeration ring (Table 2). The increased foam formation caused by the smaller air bubbles of the ceramic sparger, and the hereby associated CL transport out of the culture broth decreased the remaining CL in the culture broth to only 1%. 98.6% of the produced CL was recovered from the depositions in the reactor headspace and the FF unit, including the FCV and the tubes leading to it. However, due to the lower filling volume that had to be used because of the increased foaming, a larger fraction of the separated CL accumulated in the fermenter headspace and could not reach the FCV. A total enrichment of 386 was achieved. These results are highly important when considering the DSP as discussed in Sect. 3.6.
However, a downside associated with the use of ceramic spargers, and the therefrom resulting increase in foam formation, is the associated transport of biomass. Right after inoculation, biomass accumulation in the foam (foam flotation) was observed and reflected in the overall lower biomass concentrations in the culture broth when using the ceramic sparger, compared to the aeration ring (Additional file 1: Fig. S4). This problem of biomass stripping with the foam is a well-known problem, as discussed for instance by Noble et al., where 22% of the total biomass was stripped out with the foam fraction in less than 40 h of fermentation [49]. One method to decrease biomass loss was the recirculation of foamate. Another solution was to compensate for biomass loss with a urea feed, resulting in an additional growth phase of biomass. Interestingly though, a higher biomass-specific product yield compensated for the lower biomass concentration, resulting in higher CL yields (Table 2).
While the use of the ceramic sparger increased the recovery rate significantly, adding a FC contributed to an increased enrichment and complete recovery of the produced CL. The combination of the ceramic sparger and foam column resulted in the complete separation of CL from the culture broth, reflected in a recovery rate of 100% (Table 2). Furthermore, the flexible construction of the FC enabled us to adapt the H/D ratio of the column to the amount of generated foam during fermentation.
By adding a FC to the fermenter headspace, we were able to increase the working volume of the fermenter to 9 L because the column provided additional space for the foam. The empty fermenter headspace automatically acts as a foam column, providing the space and time for foam ripening effects to take place. While this would result in higher enrichment of CL, it also means a loss in potential working volume. Therefore, shifting the place of foam ripening from the fermenter headspace to the foam column increases available working volume while providing room for foam ripening. The higher working volume means a more efficient utilisation of equipment and thus a more economic fermentation process. Furthermore, the increase in the liquid column would provide gas bubbles with a longer residence time in the fermentation broth, resulting in a higher enrichment with amphiphilic molecules [53], like CL, to begin with.
Another advantage of the higher working volume was the shift in the amount of recovered CL from depositions in the fermenter headspace towards the fraction obtained from the FCV (Table 2). While CL obtained from the depositions in the fermenter headspace is already separated from the culture broth, it remains in the fermenter during fermentation. On the other hand, depositions from the foam entering the FCV are entirely separated from the fermenter during fermentation. This may be of enormous advantage for in situ product recovery. Chen et al. were able to continuously separate rhamnolipid during a P. aeruginosa fermentation by implementing a FF with a FCV. This resulted in doubling the obtained concentration compared to their batch process [54]. With our setup and a working volume of 9 L in the 10 L fermenter, 85% of the separated CL remains in the reactor headspace, and a maximum of 15% were completely separated in the FCV. In order to increase this amount, the reactor should be entirely filled until the top. This would result in even higher fermentation capacity utilization and a complete CL shift towards the FCV.
Looking at the low share of biomass in the FF fraction (1%) when implementing the FC, it becomes evident that biomass becomes de-enriched in the separated fraction, as indicated by the offline experiments in foam columns. Additionally, recirculating the foamate from the FCV enabled us to limit the potential loss of separated biomass and nutrients. CL accumulated on the inserts in the FCV, while nutrients and biomass were recirculated to the fermenter along with the liquid foamate. The inserts further helped collapse the foam in the FCV due to shear stress. Examination of different types of inserts proved the used polypropylene balls to be best suited. They provided an increased surface area for CL accumulation, and CL was easily recovered by simply washing the balls due to their smooth surface. In contrast, CL recovery from the examined natural sponge and smaller polypropylene particles (Additional file 1: Fig. S5) was difficult due to their small size.
Overall, the small amount of biomass in the FF would mean that solely washing this fraction from remaining sugars and drying it would result in purified CL. Depending on the aspired purity grade, this would save an additional separation step and, more importantly, the ethanol extraction. Linke et al. showed similar results for the recovery of laccase from a Pleurotus sapidus culture via foam fractionation. Equivalent purity was achieved compared to the purification from a filtrated supernatant [55]. However, depending on the area of application of the purified CL, a 100% cell-free product may be required.
The initial loss of biomass due to the implementation of the ceramic sparger was compensated by an additional urea feed during this fermentation. This resulted in final biomass concentrations in the same range as in the fermentation where the aeration unit was used (Table 2). However, looking at the biomass-specific CL yields, it becomes disputable whether or not an increase in biomass concentration is necessary for efficient CL fermentation. Higher biomass concentrations did not result in higher CL concentrations but rather lower biomass-specific CL yields.
Overall, our newly developed FF setup with foam column resulted in increased utilisation of the fermenter volume and complete recovery of CL from the culture broth. This resulted in a smaller fraction that needed to be purified to obtain CL. A similar fermenter setup with a foam column is currently scaled to a 42 L fermenter during an S. scitamineum cultivation.
4 Conclusion
This work presents a FF setup that enables a stable CL fermentation without using additional foam mitigation methods. Our preliminary results showed that a CL-enriched fraction can be obtained when separating the foam fraction through a foam column (FC) from the culture broth. This was shown using three different column geometries at a fixed H/D ratio of 10. At the same time, biomass was not enriched significantly in the foam fraction but was nevertheless present, however, at significantly reduced rates. Nevertheless, biomass and media recirculation during fermentation were necessary for CL fermentation with FF.
A FF setup with a foam collecting vessel (FCV) with inserts and foamate recirculation was thus designed. Aeration and agitation rates necessary to cover the microorganism's oxygen demand were sufficient to generate enough foam for foam fractionation. The fermenter headspace served as a column that enabled foam enrichment, and with this setup, we could recover > 90% of the produced CL from the separated fractions consisting of foam depositions in the fermenter headspace and the foam collecting vessel. Integrating an external FC into the fermenter headspace increased the fermenter filling volume, thus obtaining a higher fermentation capacity. At the same time, the foam column resulted in sufficient CL enrichment in the foam fraction, enabling us to recover 100% of the produced CL from the foam depositions in the remaining fermenter headspace and the FCV attached to the FC.
The separated CL fraction was then purified via ethanol extraction to obtain high purity CL, with purities > 90%, using only 2 gEtOH∙gPellet−1. Due to the high CL enrichment in the foam depositions, a lower amount of ethanol was needed for extraction compared to previous CL extraction from the culture broth. Furthermore, CL obtained from the foam depositions showed higher purity than CL obtained directly from the culture broth.
Examining different fermentation media constituents, including biomass and CL, showed that no single component could be identified as the primary agent that significantly affects foaming. Although it was shown that CL is a major contributor to foam generation, the foaming behaviour during CL fermentations remains a complex interplay of different parameters. However, since both foaming and purification were previously identified as the main hurdles for the economical production of biosurfactants [56], integrating FF in our CL fermentation process is even more beneficial.
Data availability
All data generated or analysed during this study are included in this published article [and its Additional information files].
Change history
20 April 2023
A Correction to this paper has been published: https://doi.org/10.1007/s43938-023-00026-5
References
Oraby A, Werner N, Sungur Z, et al. Factors affecting the synthesis of cellobiose lipids by sporisorium scitamineum. Front Bioeng Biotechnol. 2020;8: 555647. https://doi.org/10.3389/fbioe.2020.555647.
Teichmann B (2009) Das Cellobioselipid Ustilaginsäure aus Ustilago maydis: Biosynthese und transkriptionelle Regulation. PhD Thesis. UNiversität Marburg. Philipps
Eveleigh D, Dateo GP. Fungal metabolism of complex glycosides: ustilagic acid. J Biol Chem. 1964;239:839–824.
Lemieux RU. The biochemistry of the ustilaginales. III. The degradation products and proof of the chemical heterogeneity of ustilagic acid. Can J Chem. 1951;29:415–25. https://doi.org/10.1139/v51-050.
Lemieux RU, Thorn JA, Brice Carol, et al. Biochemistry of the ustilaginales: II Isolation and partial characterization of ustilagic acid. Can J Chem. 1951;5:409–14.
Bolker M, Basse CW, Schirawski J. Ustilago maydis secondary metabolism-from genomics to biochemistry. Fungal Genet Biol. 2008;45(Suppl 1):S88-93. https://doi.org/10.1016/j.fgb.2008.05.007.
Mimee B, Labbe C, Belanger RR. Catabolism of flocculosin, an antimicrobial metabolite produced by Pseudozyma flocculosa. Glycobiology. 2009;19:995–1001. https://doi.org/10.1093/glycob/cwp078.
Teichmann B, Linne U, Hewald S, et al. A biosynthetic gene cluster for a secreted cellobiose lipid with antifungal activity from Ustilago maydis. Mol Microbiol. 2007;66:525–33. https://doi.org/10.1111/j.1365-2958.2007.05941.x.
Imura T, Yamamoto S, Yamashita C, et al. aqueous gel formation from sodium salts of cellobiose lipids. J Oleo Sci. 2014;63:1005–10. https://doi.org/10.5650/jos.ess14104.
Puchkov EO, Zähringer U, Lindner B, et al. The mycocidal, membrane-active complex of Cryptococcus humicola is a new type of cellobiose lipid with detergent features. Biochimica et Biophysica Acta. 2002;2:161–70.
Imura T, Kawamura D, Ishibashi Y, et al. low molecular weight gelators based on biosurfactants, cellobiose lipids by Cryptococcus humicola. J Oleo Sci. 2012;61:659–64. https://doi.org/10.5650/jos.61.659.
Haskins RH, Thorn JA. Biochemistry of the ustilaginales: VII antibiotic activity of ustilagic acid. Can J Bot. 1951;29:585–92.
Teichmann B, Labbe C, Lefebvre F, et al. Identification of a biosynthesis gene cluster for flocculosin a cellobiose lipid produced by the biocontrol agent Pseudozyma flocculosa. Mol Microbiol. 2011;79:1483–95. https://doi.org/10.1111/j.1365-2958.2010.07533.x.
Günther M. Mikrobielle synthese modifizierung und tensideigenschaften von mannosylerythritollipiden und cellobioselipiden. Aufarbeitung: University of Stuttgart; 2014.
Roxburgh JM, Spencer J. Factors affecting the production of ustilagic acid by ustilago zeae. Agric Food Chem. 1954;2:1121–4.
Zibek S, Soberón-Chávez G. Overview on glycosylated lipids produced by bacteria and fungi: rhamno-, sophoro-, mannosylerythritol and cellobiose lipids. Adv Biochem Eng Biotechnol. 2022;181:73–122. https://doi.org/10.1007/978-3-031-07337-3.
Liu Y, Koh CMJ, Ji L. Bioconversion of crude glycerol to glycolipids in Ustilago maydis. Bioresour Technol. 2011;102:3927–33. https://doi.org/10.1016/j.biortech.2010.11.115.
Spoeckner S, Wray V, Nimtz M, et al. Glycolipids of the smut fungus Ustilago maydis from cultivation on renewable resources. Appl Microbiol Biotechnol. 1999;51:33–9. https://doi.org/10.1007/s002530051359.
Frautz B, Lang S, Wagner F. Formation of cellobiose lipids by growing and resting cells of Ustilago maydis. Biotechnol Lett. 1986;8:757–62. https://doi.org/10.1007/BF01020817.
Hewald S, Linne U, Scherer M, et al. Identification of a gene cluster for biosynthesis of mannosylerythritol lipids in the basidiomycetous fungus ustilago maydis. Appl Environ Microbiol. 2006;72:5469–77. https://doi.org/10.1128/AEM.00506-06.
Junker B. Foam and its mitigation in fermentation systems. Biotechnol Prog. 2007;23:767–84. https://doi.org/10.1021/bp070032r.
Walstra P, editor. Foam: physics, chemistry and structure: principles of foam formation and stability. Berlin: Spromger-Verlag; 1989.
Grieves RB. Foam separation: a review. Chem Eng J. 1975;2:93–106.
Lemlich R. Adsorptive bubble separation meethods—foam fractionation and allied techniques. Indust Eng Chem. 1968;60:17–29.
Stevenson P, Li X. Foam fractionation: principles and process design. Boca Raton: Taylor & Francis Group; CRC Press; 2014.
Díaz De Rienzo MA, Kamalanathan ID, Martin PJ. Comparative study of the production of rhamnolipid biosurfactants by B. thailandensis E264 and P. aeruginosa ATCC 9027 using foam fractionation. Process Biochem. 2016;51:820–7. https://doi.org/10.1016/j.procbio.2016.04.007.
Da Silva S, Taynara M, Soares CMF, Lima AS, et al. Integral production and concentration of surfactin from Bacillus sp. ITP-001 by semi-batch foam fractionation. Biochem Eng J. 2015;104:91–7. https://doi.org/10.1016/j.bej.2015.04.010.
Oraby A, Weickardt I, Zibek S. Foam fractionation methods in aerobic fermentation processes. Biotechnol Bioeng. 2022;119:1697–711. https://doi.org/10.1002/bit.28102.
Oraby A, Rupp S, Zibek S. Techno-economic analysis as a driver for optimisation of cellobiose lipid fermentation and purification. Front Bioeng Biotechnol. 2022. https://doi.org/10.3389/fbioe.2022.913351.
Burschäpersn J, Schustolla D, Schügerl K, et al. Engineering aspects of the production of sugar alcohols with the osmophilic yeast Moniliella tomentosa var pollinis: Part 1. Batch and fed-batch operation in bubble column and airlift tower loop if reactors. Process Biochem. 2002;38:559–70. https://doi.org/10.1016/S0032-9592(02)00179-6.
Coutte F, Lecouturier D, Yahia SA, et al. Production of surfactin and fengycin by Bacillus subtilis in a bubbleless membrane bioreactor. Appl Microbiol Biotechnol. 2010;87:499–507. https://doi.org/10.1007/s00253-010-2504-8.
DSMZ (2020) List of media for microorganisms. https://www.dsmz.de/collection/catalogue/microorganisms/culture-technology/list-of-media-for-microorganisms. Accessed 08 Jun 2020
Günther M, Zibek S, Hirth T, et al. Synthese und optimierung von cellobioselipiden und mannosylerythritollipiden. Chem Ing Tec. 2010;82:1215–21. https://doi.org/10.1002/cite.201000078.
Ws WISE. The measurement of the aeration of culture media. J Gen Microbiol. 1951;5:167–77. https://doi.org/10.1099/00221287-5-1-167.
Lunkenheimer K, Malysa K. Simple and generally applicable method of determination and evaluation of foam properties. J of Surf Deterg. 2003;1:69–74.
Anic I, Apolonia I, Franco P, et al. Production of rhamnolipids by integrated foam adsorption in a bioreactor system. AMB Express. 2018;8:122. https://doi.org/10.1186/s13568-018-0651-y.
Wahl R, Wippel K, Goos S, et al. A novel high-affinity sucrose transporter is required for virulence of the plant pathogen Ustilago maydis. PLoS Biol. 2010;8: e1000303. https://doi.org/10.1371/journal.pbio.1000303.
Baka E, Comer JE, Takacs-Novak K. Study of equilibrium solubility measurement by saturation shake-flask method using hydrochlorothiazide as model compound. J Pharm Biomed Anal. 2008;2:335–41.
Goulet KM, Saville BJ. Carbon acquisition and metabolism changes during fungal biotrophic plant pathogenesis: insights from Ustilago maydis. Can J Plant Path. 2017;39:247–66. https://doi.org/10.1080/07060661.2017.1354330.
Atiyeh H, Duvnjak Z. Production of fructose and ethanol from media with high sucrose concentrations by a mutant ofSaccharomyces cerevisiae. J Chem Technol Biotechnol. 2001;76:1017–22. https://doi.org/10.1002/jctb.474.
Patil SS, Kadam SR, Bastawde KB, et al. Production of lactic acid and fructose from media with cane sugar using mutant of Lactobacillus delbrueckii NCIM 2365. Lett Appl Microbiol. 2006;43:53–7. https://doi.org/10.1111/j.1472-765X.2006.01907.x.
Ageitos JM, Vallejo JA, Veiga-Crespo P, et al. Oily yeasts as oleaginous cell factories. Appl Microbiol Biotechnol. 2011;90:1219–27. https://doi.org/10.1007/s00253-011-3200-z.
Klement T, Milker S, Jäger G, et al. Biomass pretreatment affects Ustilago maydis in producing itaconic acid. Microb Cell Fact. 2012;11:43. https://doi.org/10.1186/1475-2859-11-43.
Jiang J, Zu Y, Li X, et al. Recent progress towards industrial rhamnolipids fermentation: process optimization and foam control. Bioresour Technol. 2020;298: 122394. https://doi.org/10.1016/j.biortech.2019.122394.
Hudales J, Stein H. The influence of solid particles on foam and film drainage. J Colloid Interface Sci. 1990;140:307–13. https://doi.org/10.1016/0021-9797(90)90351-N.
Vardar-Sukan F. Foaming: consequences, prevention and destruction. Biotechnol Adv. 1998;16:913–48. https://doi.org/10.1016/S0734-9750(98)00010-X.
Sodagari M, Ju L-K. Cells were a more important foaming factor than free rhamnolipids in fermentation of Pseudomonas aeruginosa E03–40 for high rhamnolipid production. J Surfact Deterg. 2014;17:573–82. https://doi.org/10.1007/s11743-013-1535-4.
Zhang Q, Lo C-M, Ju L-K. Factors affecting foaming behavior in cellulase fermentation by Trichoderma reesei Rut C-30. Bioresour Technol. 2007;98:753–60. https://doi.org/10.1016/j.biortech.2006.04.006.
Noble I, Collins M, Porter N, et al. An investigation of the physico-chemical basis of foaming in fungal fermentations. Biotechnol Bioeng. 1994;44:801–7. https://doi.org/10.1002/bit.260440705.
Zibek S, Beck A (2021) Allianz Biotenside: Funktionsoptimierte Biotenside auf Basis von regional verfügbaren Rohstoffen durch optimierte biotechnologische Verfahren: Teilprojekt 2 Optimierung und Etablierung der mikrobiellen Herstellung und Aufarbeitung von Cellobiose- und Mannosylerythritollipiden. [Fraunhofer-Institut für Grenzflächen- und Bioverfahrenstechnik IGB]. https://doi.org/10.2314/KXP:1806160609
Oraby A, Baron-Nunez FW (2021) Allianz Biotenside: Funktionsoptimierte Biotenside auf Basis von regional verfügbaren Rohstoffen durch optimierte biotechnologische Verfahren: Teilprojekt 16: Grundlagenforschung zur mikrobiellen Herstellung von Mannosylerythritol- und Cellobioselipiden. [Universität Stuttgart, Institut für Grenzflächenverfahrenstechnik und Plasmatechnologie (IGVP)]. https://doi.org/10.2314/KXP:1806163152
Long X, Sha R, Meng Q, et al. Mechanism study on the severe foaming of rhamnolipid in fermentation. J Surfact Deterg. 2016;19:833–40. https://doi.org/10.1007/s11743-016-1829-4.
Linke D, Berger RG. Foaming of proteins: new prospects for enzyme purification processes. J Biotechnol. 2011;152:125–31. https://doi.org/10.1016/j.jbiotec.2010.07.022.
Chen C, Li D, Li R, et al. Enhanced biosurfactant production in a continuous fermentation coupled with in situ foam separation. Chem Eng ProcesS Process Intensif. 2021;159: 108206. https://doi.org/10.1016/j.cep.2020.108206.
Linke D, Zorn H, Gerken B, et al. Laccase isolation by foam fractionation—new prospects of an old process. Enzyme Microb Technol. 2007;40:273–7. https://doi.org/10.1016/j.enzmictec.2006.04.010.
Blesken CC, Strümpfler T, Tiso T, et al. Uncoupling foam fractionation and foam adsorption for enhanced biosurfactant synthesis and recovery. Microorganisms. 2020. https://doi.org/10.3390/microorganisms8122029.
Acknowledgements
The authors would like to acknowledge the effort of Alexander Beck invested in commenting and discussing this work. Thank you for your fruitful input to this manuscript. We would also like to thank Oliver Oßwald and his colleagues from Festo SE & Co.KG for providing us with the ceramic aeration unit used in our experiments.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was partly funded by a Phd-scholarship from the German Federal Environmental Foundation (DBU) AZ: 80017/333 and a project fund from the Federal Ministry of Education and Research (BMBF) Innovation Alliance Biosurfactants FKZ: 031B0469M and 031B1059P.
Author information
Authors and Affiliations
Contributions
AO contributed conception and design of the study, analysis and interpretation of the data and wrote the first draft of the manuscript; DH, IW, LM, SN and JK contributed with the experimental work and analysis; SR and GT contributed to manuscript revision, SZ acquired grants and supervised AO as group leader in her work, and contributed to design and revision of the manuscript. All authors read and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Competing of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised to swap the legends of Figures 6 and 7, that had inadvertently been interchanged.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file 1: Fig S1.
Course of substrate and CL concentrations during a S. scitamineum cultivation in 1 L shake flasks at 120 rpm and 30 °C. The error bars indicate the standard deviation of a biological triplicate. The numbers I–III indicate the different phases in relation to urea and glucose availability. Fig S2. Course of substrate concentrations during an U. maydis fermentation in a 10 L fermenter. After glucose depletion, glucose was fed to a concentration of 20 g∙L−1. The numbers I–III indicate the different phases in relation to urea and glucose availability. Fig S3. Course of urea concentration during 8 fermentations of U. maydis in a 10 L fermenter. The numbers I-III indicate the different phases in relation to urea and glucose availability. Fig S4. The course of biomass concentration during 6 cultivations of U. maydis in a 10 L fermenter using a standard aeration ring and the ceramic aeration unit. The numbers I–III indicate the different phases in relation to urea and glucose availability. Fig S5. Inserts used for the foam collecting vessel with adsorbed CL agglomerates. A polypropylene particles d ~ 3 mm B Luffa aegyptiaca sponge, C polypropylene balls d ~ 20 mm.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Oraby, A., Hug, D., Weickardt, I. et al. Fermentation and recovery of cellobiose lipids using foam fractionation. Discov Chem Eng 3, 3 (2023). https://doi.org/10.1007/s43938-022-00015-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43938-022-00015-0