Abstract
Bone is a unique tissue because of its mechanical properties, ability for self-repair, and enrollment in different metabolic processes such as calcium homeostasis and hematopoietic cell production. Bone barely tolerates deformation and tends to fail when overloaded. Fracture healing is a complex process that in particular cases is impaired. Osteoprogenitor cells proliferation, growth factors, and a sound tridimensional scaffold at fracture site are key elements for new bone formation and deposition. Mechanical stability and ample vascularity are also of great importance on providing a proper environment for bone healing. From mesenchymal stem cells delivery to custom-made synthetic scaffolds, many are the biological attempts to enhance bone healing. Impaired fracture healing represents a real burden to contemporary society. Sound basic science knowledge has contributed to newer approaches aimed to accelerate and improve the quality of bone healing.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Buckwalter JA, Glimcher MJ, Cooper RR, et al. Bone biology. Part I. Structure, blood supply, cells, matrix, and mineralization. J Bone Joint Surg. 1995;77A:1256–75.
Buckwalter JA, Glimcher MM, Cooper RR, et al. Bone biology. Part II. Formation form, modeling, and remodeling. J Bone Joint Surg. 1995;77A:1276–89.
McKibbin B. The biology of fracture healing in long bones. J Bone Joint Surg (Br). 1978;60-B:150–62.
Lyritis GP. The history of the walls of the Acropolis of Athens and the natural history of secondary fracture healing process. J Musculoskelet Neuronal Interact. 2000;1:1–3.
Carter DR, Beaupre GS, Giori NJ, Helms JA. Mechanobiology of skeletal regeneration. Clin Orthop Relat Res. 1998;355(Suppl):S41–55.
Olsen BR, Reginato AM, Wang W. Bone development. Annu Rev Cell Dev Biol. 2000;16:191–220.
Perren SM, Rahn BA. Biomechanics of fracture healing. Can J Surg. 1980;23:228–32.
Schenk RK. Biology of fracture repair. In Browner B, Jupipter J, Levine L, Trafton P, editors. Skeletal Trauma 3rd Edition. Philadelphia: Saunders; 2003. p. 29–73. ISBN-13:978-0721691756.
Giannoudis PV, Einhorn TA, Marsh D. Fracture healing: the diamond concept. Injury. 2007;38 Suppl 4:S3–6.
Matter P. History of the AO and its global effect on operative fracture treatment. Clin Orthop Relat Res. 1998;347:11–8.
Green E, Lubahn JD, Evans J. Risk factors, treatment, and outcomes associated with nonunion of the midshaft humerus fracture. J Surg Orthop Adv. 2005;14:64–72.
Marsell R, Einhorn TA. Emerging bone healing therapies. J Orthop Trauma. 2010;24 Suppl 1:S4–8. An overview on current therapies on bone healing is depicted in this article.
Giannoudis PV, Einhorn TA, Schmidmaier G, Marsh D. The diamond concept – open questions. Injury. 2008;39 Suppl 2:S5–8.
Hernigou P, Poignard A, Beaujean F, et al. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am. 2005;87:1430–7.
Kanczler JM, Oreffo ROC. Osteogenesis and angiogenesis: the potential for engineering bone. Eur Cell Mater. 2008;15:100–14.
Jones E, Yang X. Mesenchymal stem cells and bone regeneration: current status. Injury. 2011;42:562–8. This article provides a comprehensive review on the use of mesenchymal stem cells in fracture repair.
Santos MI, Reis RL. Vascularization in bone tissue engineering: physiology, current strategies, major hurdles and future challenges. Macromol Biosci. 2010;10:12–27.
Tao J, Sun Y, Wang QG, Liu CW. Induced endothelial cells enhance osteogenesis and vascularization of mesenchymal stem cells. Cells Tissues Organs. 2009;190:185–193.
Tortelli F, Tasso R, Loiacono F, Cancedda R. The development of tissue-engineered bone of different origin through endochondral and intramembranous ossification following the implantation of mesenchymal stem cells and osteoblasts in a murine model. Biomaterials. 2010;31:242–9.
Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–9.
Reddi AH. Bone morphogenetic proteins: from basic science to clinical applications. J Bone Joint Surg Am. 2001;83-A Suppl 1:S1–6.
Kang Q, Sun MH, Cheng H, et al. Characterization of the distinct orthotopic bone-forming activity of 14 B.P. using recombinant adenovirus-mediated gene delivery. Gene Ther. 2004;11:1312–20.
Govender S, Csimma C, Genant HK, et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002;84:2123–34.
Friedlaender GE, Perry CR, Cole JD, et al. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am. 2001;83 Suppl 1:S151–8.
Jones AL, Bucholz RW, Bosse MJ, et al. Recombinant human BMP-2 and allograft compared with autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88:1431–41.
Ristiniemi J, Flinkkila T, Hyvonen P, et al. RhBMP-7 accelerates the healing in distal tibial fractures treated by external fixation. J Bone Joint Surg (Br). 2007;89:265–72.
Nauth A, Ristiniemi J, McKee MD, et al. Bone morphogenetic proteins in open fractures: past, present, and future. Injury. 2009;40 Suppl 3:S27–31.
Gautschi OP, Frey SP, Zellweger R. Bone morphogenetic proteins in clinical applications. ANZ J Surg. 2007;77:626–31.
Chrastil J, Low JB, Whang PG, Patel AA. Complications associated with the use of the recombinant human bone morphogenetic proteins for posterior interbody fusions of the lumbar spine. Spine. 2013;38:E1020–7.
Mroz TE, Wang JC, Hashimoto R, Norvell DC. Complications related to osteobiologics use in spine surgery: a systematic review. Spine. 2010;35(9 Suppl):S86–104.
Asahara T, Takahashi T, Masuda H, et al. VEGF contributes to postnatal neo- vascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–72.
Keramaris NC, Calori GM, Nikolaou VS, et al. Fracture vascularity and bone healing: a systematic review of the role of VEGF. Injury. 2008;39 Suppl 2:S45–57.
Kumar S, Wan C, Ramaswamy G, et al. Mesenchymal stem cells expressing osteogenic and angiogenic factors synergistically enhance bone formation in a mouse model of segmental bone defect. Mol Ther. 2010;18:1026–34.
Nauth A, Giannoudis PV, Einhorn TA, Hankenson KD, Friedlaender GE, Li R, et al. Growth factors: beyond bone morphogenetic proteins. J Orthop Trauma. 2010;24:543–6.
Hollinger JO, Hart CE, Hirsch SN, et al. Recombinant human platelet-derived growth factor: biology and clinical applications. J Bone Joint Surg Am. 2008;90 Suppl 1:48–54.
Graham S, Leonidou A, Lester M, et al. Investigating the role of PDGF as a potential drug therapy in bone formation and fracture healing. Expert Opin Investig Drugs. 2009;18:1633–54.
Alsousou J, Thompson M, Hulley P, et al. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: a review of the literature. J Bone Joint Surg (Br). 2009;91:987–96.
Calori GM, Tagliabue L, Gala L, d’Imporzano M, Peretti G, Albisetti W. Application of rhBMP-7 and platelet-rich plasma in the treatment of long bone non-unions: a prospective randomised clinical study on 120 patients. Injury. 2008;39:1391–402.
Dallari D, Savarino L, Stagni C, Cenni E, Cenacchi A, Fornasari PM, et al. Enhanced tibial osteotomy healing with use of bone grafts supplemented with platelet gel or platelet gel and bone marrow stromal cells. J Bone Joint Surg Am. 2007;89:2413–20.
Griffin XL, Smith CM, Costa ML. The clinical use of platelet-rich plasma in the promotion of bone healing: a systematic review. Injury. 2009;40:158–62.
Sheth U, Simunovic N, Klein G, Fu F, Einhorn TA, Schemitsch E, et al. Efficacy of autologous platelet-rich plasma use for orthopaedic indications: a meta-analysis. J Bone Joint Surg Am. 2012;94:298–307.
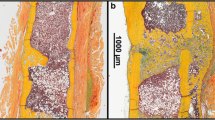
Lichte P, Pape HC, Pufe T, Kobbe P, Fischer H. Scaffolds for bone healing: Concepts, materials and evidence. Injury. 2011;42:569–73. Scaffolds are osteoconductive bases for bone regeneration. This article brings an overview on current strategies for developing new scaffolds for bone healing.
Gruskin E, Doll BA, Futrell FW, Schmitz JP, Hollinger JO. Demineralized bone matrix in bone repair: history and use. Adv Drug Deliv Rev. 2012;64:1063–77. This is a detailed review on the use of Demineralized bone matrix in bone repair.
Peterson B, Whang PG, Iglesias R, Wang JC, Lieberman JR. Osteoinductivity of commercially available demineralized bone matrix. Preparations in a spine fusion model. J Bone Joint Surg Am. 2004;86A:2243–50.
Wang JC, Alanay A, Mark D, Kanim LEA, Campbell PA, Dawson EG, et al. A comparison of commercially available demineralized bone matrix for spinal fusion. Eur Spine J. 2007;16:1233–40.
Acarturk TO, Hollinger JO. Commercially available demineralized bone matrix compositions to regenerate calvarial critical-sized bone defects. Plast Reconstr Surg. 2006;118:862–73.
Kuhne JH, Bartl R, Frisch B, et al. Bone formation in coralline hydroxyapatite. Effects of pore size studied in rabbits. Acta Orthop Scand. 1994;65:246–52.
Kokubo T, Kim HM, Kawashita M. Novel bioactive materials with different mechanical properties. Biomaterials. 2003;24:2161–75.
Laschke MW, Strohe A, Menger MD, Alini M, Eglin D. In vitro and in vivo evaluation of a novel nanosize hydroxyapatite particles/poly(ester-urethane) composite scaffold for bone tissue engineering. Acta Biomater. 2010;6:2020–7.
Dupont KM, Sharma K, Stevens HY, et al. Human stem cell delivery for treatment of large segmental bone defects. Proc Natl Acad Sci U S A. 2010;107:3305–10.
Evans C. Gene therapy for bone regeneration. Injury. 2011;42:599–604. This article focuses on principles of gene therapy for bone regeneration.
Kimelman N, Pelled G, Gazit Z, Gazit D. Applications of gene therapy and adult stem cells in bone bioengineering. Regen Med. 2006;1:549–61.
Bukata S. Systemic administration of pharmacological agents and bone repair: What can we expect. Injury. 2011;42:605–8. This is an extensive review on the use of systemic pharmacologic agents in bone repair.
Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–41.
Alkhiary YM, Gerstenfeld LC, Krall E, et al. Enhancement of experimental fracture-healing by systemic administration of recombinant human parathyroid hormone (PTH 1–34). J Bone Joint Surg Am. 2005;87:731–41.
Aspenberg P, Genant HK, Johansson T, et al. Teriparatide for acceleration of fracture repair in humans: a prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J Bone Miner Res. 2010;25:404–14. This article depicts the mechanism of action and clinical applications of Teriparatide.
Li J, Mori S, Kaji Y, et al. Effect of bisphosphonate (incadronate) in callus area and its effect on fracture healing in rats. J Bone Miner Res. 2000;15:2042–51.
Mc Donald MM, Dulai S, Godfrey C, et al. Bolus or weekly zoledronic acid administration does not delay endochondral fracture repair but weekly dosing enhances delays in hard callus remodeling. Bone. 2008;43:653–62.
Ozturan KE, Demir B, Yucel I, Cakici H, Yilmaz F, Haberal A. Effect of strontium ranelate on fracture healing in the osteoporotic rats. J Orthop Res. 2011;29:138–42.
Li YF, Luo E, Feng G, Zhu SS, Li JH, Hu J. Systemic treatment with strontium ranelate promotes tibial fracture healing in ovariectomized rats. Osteoporos Int. 2010;21:1889–97.
Komatsu DE, Mary MN, Schroeder RJ, et al. Modulation of Wnt signaling influences fracture repair. J Orthop Res. 2010;28:928–36.
Li J, Sarosi I, Cattley RC, Pretorius J, Asuncion F, Grisanti M, et al. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone. 2006;39:754–66.
Li X, Ominsky MS, Warnington KS, et al. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res. 2009;24:578–88.
Nelson FR, Brighton CT, Ryaby J, Simon BJ, Nielson JH, Lorich DG, et al. Use of physical forces in bone healing. J Am Acad Orthop Surg. 2003;11:344–54.
Pounder NM, Harrison AJ. Low intensity pulsed ultrasound for fracture healing: a review of the clinical evidence and the associated biological mechanism of action. Ultrasonics. 2008;48:330–8.
Griffin XL, Costello I, Costa ML. The role of low intensity pulsed ultrasound therapy in the management of acute fractures: a systematic review. J Trauma. 2008;65:1446–52.
Fukada E, Yasuda I. On the piezoelectric effect of bone. J Phys Soc Japan. 1957;12:1158–69.
Mollon B, da Silva V, Busse JW, Einhorn TA, Bhandari M. Electrical stimulation for long-bone fracture-healing: a meta-analysis of randomized controlled trials. J Bone Joint Surg Am. 2008;90:2322–30.
Keating JF, Hajducka CL, Harper J. Minimal internal fixation and calcium- phosphate cement in the treatment of fractures of the tibial plateau. A pilot study. J Bone Joint Surg (Br). 2003;85:68–73.
Russell TA, Leighton RK. Comparison of autogenous bone graft and endothermic calcium phosphate cement for defect augmentation in tibial plateau fractures. A multicenter, prospective, randomized study. J Bone Joint Surg Am. 2008;90:2057–61.
Goff T, Kanakaris NK, Giannoudis PV. Use of bone graft substitutes in the management of tibial plateau fractures. Injury. 2013;44 Suppl 1:S86–94. This is an interesting review on the use of bone substitutes in the management of tibial plateau fractures.
Aryee S, Imhoff AB, Rose T, Tischer T. Do we need synthetic osteotomy augmentation materials for opening-wedge high tibial osteotomy. Biomaterials. 2008;29:3497–502.
Nathan ST, Fisher BE, Roberts CS, Giannoudis PV. The management of nonunion and delayed union of patella fractures: a systematic review of the literature. Int Orthop. 2011;35:791–5.
Cox G, Jones E, Mcgonagle D, Giannoudis PV. Reamer-irrigator-aspirator indications and clinical results: a systematic review. Int Orthop. 2011;35:951–6. This article reviews the clinical applications of reamer-irrigator-aspirator as a source of bone autograft in fracture repair.
Gross AE, Shasha N, Aubin P. Long-term follow-up of the use of fresh osteochondral allografts for posttraumatic knee defects. Clin Orthop Relat Res. 2005;435:79–87.
Williams III RJ, Ranawat AS, Potter HG, Carter T, Warren RF. Fresh stored allografts for the treatment of osteochondral defects of the knee. J Bone Joint Surg Am. 2007;89:718–26.
Ghazavi MT, Pritzker KP, Davis AM, Gross AE. Fresh osteochondral allografts for post-traumatic osteochondral defects of the knee. J Bone Joint Surg (Br). 1997;79:1008–13.
Sherman SL, Garrity J, Bauer K, Cook J, Stannard J, Bugbee W. Fresh osteochondral allograft transplantation for the knee: current concepts. J Am Acad Orthop Surg. 2014;22:121–33. This is a current concepts article on the use of fresh osteochondral allografts for the knee.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
M. Kfuri Jr, R. Lara de Freitas, B. B. Batista, R. Salim, M. T. Castiglia, R. A. Tavares, and P. H. Araújo declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kfuri, M., de Freitas, R.L., Batista, B.B. et al. Updates in biological therapies for knee injuries: bone. Curr Rev Musculoskelet Med 7, 220–227 (2014). https://doi.org/10.1007/s12178-014-9225-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12178-014-9225-z
Keywords
- Bone fracture
- Fracture healing
- Angiogenesis
- Callus
- Endochondral
- Intramembranous
- Periosteal
- Bone morphogenetic protein
- Cancellous voids
- Bone defects
- Allograft
- Bone substitutes
- Scaffolds
- Bone graft
- Growth factors
- Bone regeneration
- Gene therapy
- Physical stimulation
- Ultrasound
- Osteochondral graft
- Knee injuries
- Biological therapies