Abstract
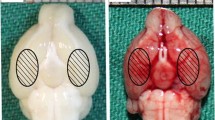
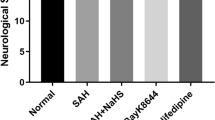
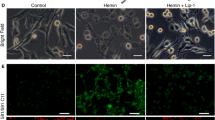
α-Lipoic acid-plus (LAP), an amine derivative of α-lipoic acid (LA), could protect cells against oxidant challenges via chelating intralysosomal iron. However, the application of LAP in experimental subarachnoid hemorrhage (SAH) is still not well known. This study was designed to evaluate the potential neuroprotection of LAP on the early brain injury (EBI) and the underlying mechanisms in a rat model of SAH. The SAH models were induced in Sprague–Dawley rats. LA and LAP were oral administration and lasted for 72 h once a day. The brain tissue samples were obtained for assay at 72 h after SAH. In experiment 1, we found that lysosome amounts in neurons decreased significantly in SAH group, and LAP (100 mg/kg) could stabilize lysosomal membrane markedly based on lysosomal-associated membrane protein-1 (LAMP-1) expression in neurons by immunofluorescence. Hence, the LAP dosages of 100 and 150 mg/kg were applied in experiment 2. Firstly, Western blot analysis showed that the protein levels of cathepsin B/D, caspase-3, Bax, ferritin, and heme-oxygenase-1 (HO-1) markedly increased after SAH, which were further confirmed by double immunofluorescence staining and reversed by LA and LAP treatments. In addition, LA and LAP also reduced oxidative stress and iron deposition in brain tissue. Furthermore, LA and LAP significantly ameliorated brain edema, blood–brain barrier injury, cortical apoptosis, and neurological behavior impairment induced by SAH. Finally, it is noteworthy that LAP exerted more significant effects than LA on these parameters as described above. LAP probably exerted neuroprotective effects via targeting lysosomes and chelating intralysosomal iron in EBI post-SAH in rats.










Similar content being viewed by others
References
Loftspring MC (2010) Iron and early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab 30:1791–1792. doi:10.1038/jcbfm.2010.139
Yuksel S, Tosun YB, Cahill J, Solaroglu I (2012) Early brain injury following aneurysmal subarachnoid hemorrhage: emphasis on cellular apoptosis. Turk Neurosurg 22:529–533. doi:10.5137/1019-5149.JTN.5731-12.1
Kusaka G, Ishikawa M, Nanda A, Granger DN, Zhang JH (2004) Signaling pathways for early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab 24:916–925
Ayer RE, Zhang JH (2008) Oxidative stress in subarachnoid haemorrhage: significance in acute brain injury and vasospasm. Acta Neurochir Suppl 104:33–41
He Y, Hua Y, Keep RF, Liu W, Wang MM, Xi G (2011) Hemoglobin expression in neurons and glia after intracerebral hemorrhage. Acta Neurochir Suppl 111:133–137. doi:10.1007/978-3-7091-0693-8_22
Lee JY, Keep RF, He Y, Sagher O, Hua Y, Xi G (2010) Hemoglobin and iron handling in brain after subarachnoid hemorrhage and the effect of deferoxamine on early brain injury. J Cereb Blood Flow Metab 30:1793–1803. doi:10.1038/jcbfm.2010.137
Nina P, Schisano G, Chiappetta F, Luisa Papa M, Maddaloni E, Brunori A, Capasso F, Corpetti MG, Demurtas F (2001) A study of blood coagulation and fibrinolytic system in spontaneous subarachnoid hemorrhage. Correlation with Hunt-Hess grade and outcome. Surg Neurol 55:197–203
Wagner KR, Sharp FR, Ardizzone TD, Lu A, Clark JF (2003) Heme and iron metabolism: role in cerebral hemorrhage. J Cereb Blood Flow Metab 23:629–652
Yu Z, Persson HL, Eaton JW, Brunk UT (2003) Intralysosomal iron: a major determinant of oxidant-induced cell death. Free Radic Biol Med 34:1243–1252
Hasegawa Y, Suzuki H, Sozen T, Altay O, Zhang JH (2011) Apoptotic mechanisms for neuronal cells in early brain injury after subarachnoid hemorrhage. Acta Neurochir Suppl 110:43–48. doi:10.1007/978-3-7091-0353-1_8
Turk B, Turk V (2009) Lysosomes as “suicide bags” in cell death: myth or reality? J Biol Chem 284:21783–21787. doi:10.1074/jbc.R109.023820
Terman A, Kurz T (2013) Lysosomal iron, iron chelation, and cell death. Antioxid Redox Signal 18:888–898. doi:10.1089/ars
Lartigue L, Alloyeau D, Kolosnjaj-Tabi J, Javed Y, Guardia P, Riedinger A, Péchoux C, Pellegrino T, Wilhelm C, Gazeau F (2013) Biodegradation of iron oxide nanocubes: high-resolution in situ monitoring. ACS Nano 7:3939–3952. doi:10.1021/nn305719y
Repnik U, Stoka V, Turk V, Turk B (2012) Lysosomes and lysosomal cathepsins in cell death. Biochim Biophys Acta 1824:22–33. doi:10.1016/j.bbapap
Jensen SS, Aaberg-Jessen C, Christensen KG, Kristensen B (2013) Expression of the lysosomal-associated membrane protein-1 (LAMP-1) in astrocytomas. Int J Clin Exp Pathol 6:1294–1305
Patil SP, Tran N, Geekiyanage H, Liu L, Chan C (2013) Curcumin-induced upregulation of the anti-tau cochaperone BAG2 in primary rat cortical neurons. Neurosci Lett 554:121–125. doi:10.1016/j.neulet
Erşahin M, Toklu HZ, Cetinel S, Yüksel M, Erzik C, Berkman MZ, Yeğen BC, Sener G (2010) Alpha lipoic acid alleviates oxidative stress and preserves blood brain permeability in rats with subarachnoid hemorrhage. Neurochem Res 35:418–428. doi:10.1007/s11064-009-0072-z
Hardas SS, Sultana R, Clark AM, Beckett TL, Szweda LI, Murphy MP, Butterfield DA (2013) Oxidative modification of lipoic acid by HNE in Alzheimer disease brain. Redox Biology 1:80–85. doi:10.1016/j.redox
Tirosh O, Sen CK, Roy S, Kobayashi MS, Packer L (1999) Neuroprotective effects of alpha-lipoic acid and its positively charged amide analogue. Free Radic Biol Med 26:1418–1426
Persson HL, Yu Z, Tirosh O, Eaton JW, Brunk UT (2003) Prevention of oxidant-induced cell death by lysosomotropic iron chelators. Free Radic Biol Med 34:1295–1305
Wang Z, Ma C, Meng CJ, Zhu GQ, Sun XB, Huo L, Zhang J, Liu HX, He WC, Shen XM, Shu Z, Chen G (2012) Melatonin activates the Nrf2-ARE pathway when it protects against early brain injury in a subarachnoid hemorrhage model. J Pineal Res 53:129–137. doi:10.1111/j.1600-079X.2012.00978.x
Liu HD, Li W, Chen ZR, Zhou ML, Zhuang Z, Zhang DD, Zhu L, Hang CH (2013) Increased expression of ferritin in cerebral cortex after human traumatic brain injury. Neurol Sci 34:1173–1180. doi:10.1007/s10072-012-1214-7
Ayer RE, Sugawara T, Chen W, Tong W, Zhang JH (2008) Melatonin decreases mortality following severe subarachnoid hemorrhage. J Pineal Res 44:197–204. doi:10.1111/j.1600-079X
Jiang S, Xia R, Jiang Y, Wang L, Gao F (2014) Vascular endothelial growth factors enhance the permeability of the mouse blood–brain barrier. PLoS One 9:e86407. doi:10.1371/journal.pone.0086407. eCollection 2014
Kurz T, Terman A, Brunk UT (2007) Autophagy, ageing and apoptosis: the role of oxidative stress and lysosomal iron. Arch Biochem Biophys 462:220–230
Ghosh M, Carlsson F, Laskar A, Yuan XM, Li W (2011) Lysosomal membrane permeabilization causes oxidative stress and ferritin induction in macrophages. FEBS Lett 585:623–629. doi:10.1016/j.febslet.2010.12.043
Doeppner TR, Hermann DM (2010) Free radical scavengers and spin traps—therapeutic implications for ischemic stroke. Best Pract Res Clin Anaesthesiol 24:511–520. doi:10.1016/j.bpa.2010.10.003
Yang D, Han Y, Zhang J, Ding C, Anagli J, Seyfried DM (2011) Improvement in recovery after experimental intracerebral hemorrhage using a selective cathepsin B and L inhibitor. J Neurosurg 114:1110–1116. doi:10.3171/2010.6.JNS091856
Ozden H, Durmaz R, Kanbak G, Uzuner K, Aral E, Kartkaya K, Kabay SC, Atasoy MA (2011) Erythropoietin prevents nitric oxide and cathepsin-mediated neuronal death in focal brain ischemia. Brain Res 1370:185–193. doi:10.1016/j.brainres.2010.11.045
Xu J, Wang H, Ding K, Lu X, Li T, Wang J, Wang C, Wang J (2013) Inhibition of cathepsin S produces neuroprotective effects after traumatic brain injury in mice. Mediators Inflamm 2013:187873. doi:10.1155/2013/187873
Hook GR, Yu J, Sipes N, Pierschbacher MD, Hook V, Kindy MS (2014) The cysteine protease cathepsin B is a key drug target and cysteine protease inhibitors are potential therapeutics for traumatic brain injury. J Neurotrauma 31:515–529. doi:10.1089/neu.2013.2944
Friedrich V, Flores R, Sehba FA (2012) Cell death starts early after subarachnoid hemorrhage. Neurosci Lett 512:6–11. doi:10.1016/j.neulet.2012.01.036
Ostrowski RP, Colohan AR, Zhang JH (2006) Molecular mechanisms of early brain injury after subarachnoid hemorrhage. Neurol Res 28:399–414
Yu ZQ, Jia Y, Chen G (2014) Possible involvement of cathepsin B/D and caspase-3 in deferoxamine-related neuroprotection of early brain injury after subarachnoid haemorrhage in rats. Neuropathol Appl Neurobiol 40:270–283. doi:10.1111/nan.12091
Lloyd JB, Cable H, Rice-Evans C (1991) Evidence that desferrioxamine cannot enter cells by passive diffusion. Biochem Pharmacol 41:1361–1363
Cable H, Lloyd JB (1999) Cellular uptake and release of two contrasting iron chelators. J Pharm Pharmacol 51:131–134
Xi G, Keep RF, Hoff JT (2006) Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurology 5:53–63
Connor JR, Menzies SL, Burdo JR, Boyer PJ (2001) Iron and iron management proteins in neurobiology. Pediatr Neurol 25:118–129
Danielisova V, Gottlieb M, Burda J (2002) Iron deposition after transient forebrain ischemia in rat brain. Neurochem Res 27:237–242
Erdi MF, Guney O, Kiyici A, Esen H (2011) The effects of alpha lipoic acid on cerebral vasospasm following experimental subarachnoid hemorrhage in the rabbit. Turk Neurosurg 21:527–533. doi:10.5137/1019-5149
Bolognesi ML, Bergamini C, Fato R, Oiry J, Vasseur JJ, Smietana M (2014) Synthesis of new lipoic acid conjugates and evaluation of their free radical-scavenging and neuroprotective activities. Chem Biol Drug Des 83:688–696. doi:10.1111/cbdd.12282
Lee JY, Keep RF, Hua Y, Ernestus RI, Xi G (2011) Deferoxamine reduces early brain injury following subarachnoid hemorrhage. Acta Neurochir Suppl 112:101–106. doi:10.1007/978-3-7091-0661-7_18
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81171105, 81271300, and 81371279), Jiangsu Province’s Outstanding Medical Academic Leader program (No. LJ201139), the National Key Technology R&D program for the 12th Five-Year Plan of the People’s Republic of China (2011BAI08B05, 2011BAI08B06, and 2014BAZ04810), and the Scientific Department of Jiangsu Province (No. BL2014045) and Suzhou Government (Nos. LCZX201301, SZS201413, and SYS201332).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yang Wang and Anju Gao equally contribute to this work.
Rights and permissions
About this article
Cite this article
Wang, Y., Gao, A., Xu, X. et al. The Neuroprotection of Lysosomotropic Agents in Experimental Subarachnoid Hemorrhage Probably Involving the Apoptosis Pathway Triggering by Cathepsins via Chelating Intralysosomal Iron. Mol Neurobiol 52, 64–77 (2015). https://doi.org/10.1007/s12035-014-8846-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8846-y