Abstract
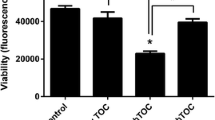
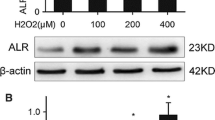
Oxidative stress plays an important role in various renal and hepatic pathologies, and reduction of oxidative stress-induced processes is an important protective strategy in tissues of diverse origins against harmful stimuli. Pituitary adenylate cyclase activating polypeptide (PACAP) is a well-known cytotrophic and cytoprotective peptide. PACAP promotes cell survival in numerous cells and tissues exposed to various stimuli. Protective effects of PACAP have been shown in the kidney, but it is not known whether PACAP is protective against oxidative stress in renal cells. Little is known about the effects of PACAP in the liver. The aim of the present study was to investigate whether PACAP is protective against oxidative stress in primary rat kidney cell culture and whether PACAP has any effect on cell survival in human WRL-68 hepatocytes and HEP-G2 hepatocellular carcinoma cells subjected to oxidative stress. Cells were exposed to various concentrations of H2O2 with or without PACAP co-treatment and cell viability was evaluated with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide test (MTT). We found that oxidative stress induced a significant decrease in cell viability in both cell lines. PACAP could dose-dependently increase the percentage of living cells in kidney cells, but it failed to do so in hepatocytes. Given the survival-promoting effects of PACAP against oxidative stress in rat kidney, we conducted a further experiment to determine whether PACAP influences the markers of oxidative stress in vivo. We have proven earlier that PACAP was effective in kidney ischemia/reperfusion injury in vivo. In the present study, we determined the levels of the oxidative stress marker malondialdehyde and the activity of the scavenger molecules glutathione (GSH) and superoxide dismutase (SOD) following kidney ischemia/reperfusion in rats. We found that PACAP significantly increased the level of GSH and counteracted the marked reduction of SOD activity after ischemia/reperfusion in vivo. In summary, the present study showed that while PACAP was able to significantly increase the cell survival in primary kidney cell cultures exposed to oxidative stress, possibly involving interaction with the endogenous scavenger system, it failed to influence the viability of normal or cancerous hepatocytes.



Similar content being viewed by others
References
Arimura A, Somogyvari-Vigh A, Weill C et al (1994) PACAP functions as a neurotrophic factor. Ann NY Acad Sci 739:228–243
Arimura A, Li M, Batuman V (2006) Treatment of renal failure associated with multiple myeloma and other diseases by PACAP-38. Ann NY Acad Sci 1070:1–4
Aubert N, Vaudry D, Falluel-Morel A et al (2008) PACAP prevents toxicity induced by cisplatin in rat and primate neurons but not in proliferating ovary cells: involvement of the mitochondrial apoptotic pathway. Neurobiol Dis 32:66–80
Boronkai A, Brubel R, Racz B (2009) Effects of pituitary adenylate cyclase activating polypeptide (PACAP) on the survival and signal transduction pathways in human choriocarcinoma cells. Ann NY Acad Sci 1163:353–357
Botia B, Seyer D, Ravni A et al (2008) Peroxiredoxin 2 is involved in the neuroprotective effects of PACAP in cultured cerebellar granule neurons. J Mol Neurosci 36:61–72
Canonico PL, Copani A, D’Agata V et al (1996) Activation of pituitary adenylate cyclase activating polypeptide receptors prevents apoptotic cell death in cultured cerebellar granule cells. Ann NY Acad Sci 805:470–472
Chignard N, Mergey M, Barbu V et al (2005) VPAC1 expression is regulated by FXR agonists in the human gallbladder epithelium. Hepatology 42:549–557
Comporti M, Arezzini B, Signorini C, Vecchio D, Gardi C (2009) Oxidative stress, isoprostanes and hepatic fibrosis. Histol Histopathol 24:893–900
Delgado M, Ganea D (2000) VIP and PACAP inhibit activation induced apoptosis in T lymphocytes. Ann NY Acad Sci 921:55–67
El Fahime E, Lutz-Bucher B, Felix JM, Koch B (1996) Pituitary adenylate cyclase activating polypeptide induces expression of corticosteroid-binding globulin in cultured fetal hepatocytes: synergy with tri-iodothyronine. Biochem J 315:643–649
Ferencz A, Szanto Z, Kalmar-Nagy K et al (2004) Mitigation of oxidative injury by classic and delayed ischemic preconditioning prior to small bowel autotransplantation. Transplant Proc 36:286–288
Ferencz A, Racz B, Tamas A et al (2009) Influence of PACAP on oxidative stress and tissue injury following small bowel autotransplantation. J Mol Neurosci 37:168–176
Ferencz A, Kiss P, Weber G et al (2010a) Comparison of intestinal warm ischemic injury in PACAP knock-out and wild-type mice. J Mol Neurosci. doi:10.1007/s12031-010-9357-6
Ferencz A, Weber G, Helyes Z, Hashimoto H, Baba A, Reglodi D (2010b) Presence of endogenous PACAP-38 ameliorated intestinal cold preservation tissue injury. J Mol Neurosci. doi:10.1007/s12031-010-9352-y
Gagnon AW, Aiyar N, Elshourbagy NA (1994) Molecular cloning and functional characterization of a human liver vasoactive intestinal peptide receptor. Cell Signal 6:321–333
Glad H, Ainsworth MA, Svendsen P, Fahrenkrug J, Schaffalitzky de Muckadell OB (2003) Effect of vasoactive intestinal peptide and pituitary adenylate cyclase activating polypeptide on pancreatic, hepatic and duodenal mucosal bicarbonate secretion in the pig. Digestion 67:56–66
Gonzalez BJ, Basille M, Vaudry D, Fournier A, Vaudry H (1997) Pituitary adenylate cyclase activating polypeptide promotes cell survival and neurite outgrowth in rat cerebellar neuroblasts. Neuroscience 78:419–430
Guijarro LG, Rodriguez-Henche N, Garcia-Lopez E et al (1995) Receptors for pituitary adenylate cyclase activating peptide in human liver. J Clin Endocrinol Metab 80:2451–2457
Gutierrez-Canas I, Rodriguez-Henche N, Bolanos O, Carmena MJ, Prieto JC, Juarranz MG (2003) VIP and PACAP are autocrine factors that protect the androgen-independent prostate cancer cell line PC-3 from apoptosis induced by serum withdrawal. Br J Pharmacol 139:1050–1058
Hayez N, Harfi I, Lema-Kisoka R, Svoboda M, Corazza F, Sariban E (2004) The neuropeptides vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase activating polypeptide (PACAP) modulate several biochemical pathways in human leukemic myeloid cells. J Neuroimmunol 149:167–181
Horvath G, Mark L, Brubel R et al (2010a) Mice deficient in pitutiary adenylate cyclase activating polypeptide display increased sensitivity to renal oxidative stress in vitro. Neurosci Lett 469:70–74
Horvath G, Racz B, Reglodi D et al (2010b) Effects of PACAP on mitochondrial apoptotic pathways and cytokine expression in rats subjected to renal ischemia–reperfusion. J Mol Neurosci. doi:10.1007/s12031-010-9342-0
Huang WT, Li CJ, Wu PJ, Chang YS, Lee TL, Weng CF (2009) Expression and in vitro regulation of pituitary adenylate cyclase activating polypeptide (pacap38) and its type I receptor (pac1-r) in the gonads of tilapia (Oreochromis mossambicus). Reproduction 137:449–467
Kiso T, Ito S, Ohta T, Asano T, Nakazato Y (1994) Characterization of vasoactive intestinal peptide receptors in canine liver membranes. Biochem Pharmacol 47:241–245
Le SV, Yamaguchi DJ, McArdle CA, Tachiki K, Pisegna JR, Germano P (2002) PAC1 and PACAP expression, signaling, and effect on the growth of HCT8, human colonic tumor cells. Regul Pept 109:115–125
Lemberg A, Fernandez MA (2009) Hepatic encephalopathy, ammonia, glutamate, glutamine and oxidative stress. Ann Hepatol 8:95–102
Li M, Maderdrut JL, Lertora JJ, Batuman V (2007) Intravenous infusion of pituitary adenylate cyclase activating polypeptide (PACAP) in a patient with multiple myeloma and myeloma kidney: a case study. Peptides 28:1891–1895
Li M, Maderdrut JL, Lertora JJ, Arimura A, Batuman V (2008) Renoprotection by pituitary adenylate cyclase activating polypeptide in multiple myeloma and other kidney diseases. Regul Pept 145:24–32
Li M, Balamuthusamy S, Khan AM, Maderdrut JL, Simon EE, Batuman V (2010a) Pituitary adenylate cyclase activating polypeptide ameliorates cisplatin-induced acute kidney injury. Peptides 31:592–602
Li M, Balamuthusamy S, Khan AM, Maderdrut JL, Simon EE, Batuman V (2010b) Pituitary adenylate cyclase activating polypeptide prevents cisplatin-induced renal failure. J Mol Neurosci. doi:10.1007/s12031-010-9394-1
Mathieu M, Girosi L, Vallarino M, Tagliafierro G (2005) PACAP in developing sensory and peripheral organs of the zebrafish, Danio rerio. Eur J Histochem 49:167–178
Nguyen TD, Heintz GG, Wolfe MS (1993) Structural characterization of PACAP receptors on rat liver plasma membranes. Am J Physiol 265:G811–818
Pineau N, Lelievre V, Goursaud S et al (2001) The polypeptide PHI discriminates a GTP-insensitive form of VIP receptor in liver membranes. Neuropeptides 35:117–126
Racz B, Gasz B, Borsiczky B et al (2007) Protective effects of pituitary adenylate cyclase activating polypeptide in endothelial cells against oxidative stress-induced apoptosis. Gen Comp Endocrinol 153:115–123
Racz B, Gasz B, Gallyas F Jr et al (2008) PKA-Bad-14-3-3 and Akt-Bad-14-3-3 signaling pathways are involved in the protective effects of PACAP against ischemia/reperfusion-induced cardiomyocyte apoptosis. Regul Pept 145:105–115
Racz B, Reglodi D, Horvath G et al (2010). Protective effect of PACAP against doxorubicin-induced cell death in cardiomyocyte culture. J Mol Neurosci. doi:10.1007/s12031-010-9349-6
Reglodi D, Zs F, Tamas A et al (2004) Effects of PACAP on in vitro and in vivo neuronal cell death, platelet aggregation and production of reactive oxygen radicals. Regul Pept 123:51–59
Reubi JC, Zimmermann A, Jonas S et al (1999) Regulatory peptide receptors in human hepatocellular carcinomas. Gut 45:766–774
Reubi JC, Laderach U, Waser B, Gebbers JO, Robberecht P, Laissue JA (2000) Vasoactive intestinal peptide/pituitary adenylate cyclase activating peptide receptor subtypes in human tumors and their tissues of origin. Cancer Res 60:3105–3112
Robberecht P, Gourlet P, Cauvin A et al (1991) PACAP and VIP receptors in rat liver cell membranes. Am J Physiol 260:G97–G102
Rodriguez-Henche N, Rodriguez-Pena MS, Guijarro LG, Prieto JC (1994) Characterization of vasoactive intestinal peptide receptors in human liver. Biochim Biophys Acta 1221:193–198
Sandgren K, Lin Z, Ekblad E (2003) Differential effects of VIP and PACAP on survival of cultured adult rat myenteric neurons. Regul Pept 111:211–217
Sekiguchi Y, Kasai K, Hasegawa K, Suzuki Y, Shimoda S (1994) Glycogenolytic activity of pituitary adenylate cyclase activating polypeptide (PACAP) in vivo and in vitro. Life Sci 55:1219–1228
Somogyvari-Vigh A, Reglodi D (2004) Pituitary adenylate cyclase activating polypeptide: a potential neuroprotective peptide—review. Curr Pharm Des 10:2861–2889
Szakaly P, Kiss P, Lubics A et al (2008) Effects of PACAP on survival and renal morphology in rats subjected to renal ischemia/reperfusion. J Mol Neurosci 36:89–96
Vaccari S, Latini S, Barberi M, Teti A, Stefanini M, Canipari R (2006) Characterization and expression of different pituitary adenylate cyclase activating polypeptide/vasoactive intestinal polypeptide receptors in rat ovarian follicles. J Endocrinol 191:287–299
Valiante S, Prisco M, De Falco M et al (2009) Distribution and molecular evolution of the neuropeptide pituitary adenylate cyclase activating polypeptide (PACAP) and its receptors in the lizard Podarcis sicula (Squamata, Lacertidae). J Mol Neurosci 39:144–156
Vaudry D, Hamelink C, Damadzic R et al (2005) Endogenous PACAP acts as a stress response peptide to protect cerebellar neurons from ethanol or oxidative insult. Peptides 26:2518–2524
Vaudry D, Falluel-Morel A, Bourgault A et al (2009) Pituitary adenylate cyclase activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev 61:283–357
Wei Y, Mojsov S (1996) Tissue specific expression of different human receptor types for pituitary adenylate cyclase activating polypeptide and vasoactive intestinal polypeptide: implications for their role in human physiology. J Neuroendocrinol 8:811–817
Yokota C, Kawai K, Ohashi S, Watanabe Y, Yamashita K (1995) PACAP stimulates glucose output from the perfused rat liver. Peptides 16:55–60
Acknowledgments
This work was supported by the following grants: OTKA K72592, F67830, CNK 78480, PD77474, Bolyai Scholarship, ETT278-04/2009, University of Pécs Medical School Research Grant 2009, and Richter Gedeon Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Horvath, G., Brubel, R., Kovacs, K. et al. Effects of PACAP on Oxidative Stress-Induced Cell Death in Rat Kidney and Human Hepatocyte Cells. J Mol Neurosci 43, 67–75 (2011). https://doi.org/10.1007/s12031-010-9428-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-010-9428-8