Abstract
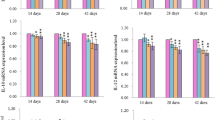
The purpose of this 42-day study was to examine the effect of dietary vanadium on the ileac T cells and contents of cytokines including interleukin-2 (IL-2), interleukin-6 (IL-6), and interferon-gamma (IFN-γ) in broilers by flow cytometry and enzyme-linked immunosorbent assay. A total of 420 one-day-old avian broilers were divided into six groups (seven replicates in each group and ten broilers in each replicate) and fed on control diet or the same diet supplemented with 5, 15, 30, 45, and 60 mg/kg vanadium in the form of ammonium metavanadate. The results showed that the percentages of CD3+, CD3+CD4+, and CD3+CD8+ T cells in both ileac lamina propria lymphocytes (LPLs) and intraepithelial lymphocytes (IELs) were significantly lower (P < 0.05 or P < 0.01) in the 45- and 60-mg/kg groups than in the control group from 14 to 42 days of age. The CD4+/CD8+ ratio was increased in ileac LPLs in the 60-mg/kg group at 28 days of age, and in ileac IELs in the 60-mg/kg group at 28 days of age and in the 45-mg/kg group at 42 days of age. Meanwhile, the ileac IL-2, IL-6 contents were decreased (P < 0.05 or P < 0.01) in the 60-mg/kg group from 14 to 42 days of age and in the 45-mg/kg group from 28 to 42 days of age in comparison with those of the control group. It was concluded that dietary vanadium in excess of 30 mg/kg reduced the ileac T cell population and percentages of T cell subsets, and IL-2, IL-6, and IFN-γ contents, implying that the immune function of local intestinal mucosa in broilers could be affected by the dietary vanadium.
Similar content being viewed by others
References
Domingo JL (1996) Vanadium: a review of the reproductive and developmental toxicity. Reprod Toxicol 10(3):175–182
Rehder D (2003) Biological and medicinal aspects of vanadium. Inorganic Chem Commun 6:604–617
Nielsen FH (1990) New essential trace elements for the life sciences. Biol Trace Elem Res 26–27(1):599–611
Duckworth WC, Solomon SS, Liepnieks J et al (1998) Insulin-like effects of vanadium in isolated rat adipocytes. Endoerinology 122:2285–2289
Madsen KL, Arisno D, Fedorak RN et al (1995) Vanadate treatment rapidly improves glucose transport and activates 6-phosphofructo-l-kinase in diabetic rat intestine. Diabetologia 38(4):403–412
Domingo JL (2002) Vanadium and tungsten derivatives as antidiabetic agents. Biol Trace Elem Res 88(2):97–112
Mukherjee B (2004) Vanadium—an element of atypical biological significance. Toxicol Lett 150:135–143
Beagley KW, Fujihashi K et al (1995) Differences in intraepithelial lymphocyte T cell subsets isolated from murine small versus large intestine. Immunology 154:5611–5619
Wijk F, Cheroutre H (2009) Intestinal T cells: facing the mucosal immune dilemma with synergy and diversity. Semin Immunol 21:130–138
Poucheret P, Verma S, Grynpas MD, McNeill JH (1998) Vanadium and diabetes. Mol Cell Biochem 188:73–80
Iji PA, Saki AA, Tivey DR (2001) Intestinal development and body growth of broiler chicks on diets supplemented with non-starch polysaccharides. Animal Feed Sci Technol 89(3–4):175–188
Katriina H, Shah E et al (2008) Systematic review of the association between circulating interleukin-6 (IL-6) and cancer. Eur J Cancer 44:937–945
Conrad A (2003) Interleukin-2: where are we going? J Assoc Nurses AIDS Care 14:83–88
Oliver D, Mercedes R (2009) The effects of IL-6 on CD4 T cell responses. Clin Immunol 130:27–33
Kate S, Paul JH (2004) Interferon-γ: an overview of signals, mechanisms and functions. J Leukoc Biol 75(2):163–189
Cui W, Cui H, Peng X et al (2011) Excess dietary vanadium induces the changes of subets and proliferation of splenic T cells in broilers. Biol Trace Elem Res 143:932–938
Cui W, Cui H, Peng X et al (2011) Effect of vanadium on the subset and proliferation of peripheral blood T-cell, and serum IL-2 content in broilers. Biol Trace Elem Res 141:192–199
Cui W, Cui H, Peng X et al (2011) Excess dietary vanadium induces lesions and changes of cell cycle of spleen in broilers. Biol Trace Elem Res 143:949–956
Cui W, Cui H, Peng X et al (2011) Changes of relative weight and cell cycle, and lesions of bursa of Fabricius induced by dietary excess vanadium in broilers. Biol Trace Elem Res 143:251–260
Deng Y, Cui H, Peng X et al (2011) Effect of dietary vanadium on cecal tonsil T cell subsets and IL-2 contents in broilers. Biol Trace Elem Res. doi:10.1007/s12011-010-9018-9
Montufar-Solis D, Klein JR (2006) An improved method for isolating intraepithelial lymphocytes (IELs) from the murine small intestine with consistently high purity. J Immunol Methods 308:251–254
Todd D, Singh AJ (1999) A new isolation method for rat intraepithelial lymphocytes. J Immunol Methods 224:111–127
Resèndiz-Albor AA, Esquivel R et al (2005) Striking phenotypic and functional differences in lamina propria lymphocytes from the large and small intestine of mice. Life Sci 76:2783–2803
Gaca MD, Pickering JA et al (1999) Human and rat hepatic stellate cells produce stem cell factor: a possible mechanism for mast cell recruitment in liver fibrosis. J Hepatol 30:850–860
Acheson DWK, Luccioli S (2004) Mucosal immune responses. Best Pract Res Clin Gastroenterol 18(2):387–404
Lillehoj HS, Chung KS (1992) Postnatal development of tlymphocyte subpopulations in the intestinal intraepithelium and lamina propria in chickens. Vet Immunol Immunopathol 31:347–360
Lillehoj HS, Trout JM (1996) Avian gut-associated lymphoid tissues and intestinal immune responses to Eimeria parasites. Clin Microbiol Rev 9:349–360
Chen T, Cui Y, Bai C, Gong T, Peng X, Cui H (2009) Decreased percentages of the peripheral blood T-cell subsets and the serum IL-2 contents in chickens fed on diets excess in fluorine. Biol Trace Elem Res 132:122–128
Kannangai R, Prakash KJ et al (2000) Peripheral CD4+/CD8+ T-lymphocyte counts estimated by an immunocapture method in the normal healthy south Indian adults and HIV seropositive individuals. J Clin Virol 17(2):101–108
Jesús H, Yonathan G (2001) Comparative evaluation of the CD4+CD8+ and CD4+CD8− lymphocytes in the immune response to porcine rubulavirus. Vet Immunol Immunopathol 79(3–4):249–259
Czesnikiewicz-Guzik M, Lee W et al (2008) T cell subset-specific susceptibility to aging. Clin Immunol 127:107–118
Magd AK, Ahmed EH et al (2005) Immune-mediated liver injury: prognostic value of CD4+, CD8+, and CD68+ in infants with extrahepatic biliary atresia. J Pediatr Surg 40(8):1252–1257
Ulrike H, Hananske AR, Martha HM et al (1987) Biphasic effect of vanadium salts on in vitro tumor colony growth. Int J Cell Cloning 5(2):170–178
Bonham M, O’Connor JM, Hannigan BM, Strain JJ (2002) The immune system as a physiological indicator of marginal copper status. Br J Nutr 87:383–403
Lan RY, Selmi C (2008) The regulatory, inflammatory, and T cell programming roles of interleukin-2 (IL-2). J Autoimmun 31:7–12
Matsuda T, Suematsu S, Kawano M et al (1989) IL-6yBSF2 in normal and abnormal regulation of immune responses. Ann N Y Acad Sci 557:466–476
Kishimoto T (2006) Interleukin-6: discovery of a pleiotropic cytokine. Arthritis Res Ther 8(suppl 2):S2
Rochman I, Paul WE, Ben-Sasson SZ (2005) IL-6 increases primed cell expansion and survival. J Immunol 174:4761–4767
Longhi MP, Wright K, Lauder SN, Nowell MA, Jones GW, Godkin AJ, Jones SA, Gallimore AM (2008) Interleukin-6 is crucial for recall of influenza-specific memory CD4 T cells. PLoS Pathog 4:e1000006
Esteves I, Walravens K et al (2004) Protective killed Ehrlichia ruminantium vaccine elicits IFN-γ responses by CD4+ and CD8+ T lymphocytes in goats. Vet Immunol Immunopathol 98:49–57
Acknowledgments
The study was supported by the program for Changjiang Scholars and Innovative Research Team in University (IRT 0848) and the Education Department and Scientific Department of Sichuan Province (09ZZ017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Kangping Wang and Yuanxin Deng contributed equally to this work and are considered as first authors.
Rights and permissions
About this article
Cite this article
Wang, K., Cui, H., Deng, Y. et al. Effect of Dietary Vanadium on the Ileac T Cells and Contents of Cytokines in Broilers. Biol Trace Elem Res 147, 113–119 (2012). https://doi.org/10.1007/s12011-011-9274-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-011-9274-8