Abstract
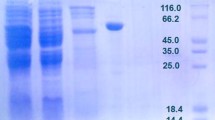
Pseudomonas aeruginosa PAO1 phosphoglucose isomerase was purified as an active soluble form by a single-step purification using Ni-NTA chromatography that showed homogeneity on SDS-PAGE with molecular mass ∼62 kDa. The optimum temperature and pH for the maximum isomerization activity with d-galactose were 60 °C and 7.0, respectively. Generally, sugar phosphate isomerases show metal-independent activity but PA-PGI exhibited metal-dependent isomerization activity with aldosugars and optimally catalyzed the d-galactose isomerization in the presence of 1.0 mM MnCl2. The apparent Km and Vmax for d-galactose under standardized conditions were calculated to be 1029 mM (±31.30 with S.E.) and 5.95 U/mg (±0.9 with S.E.), respectively. Equilibrium reached after 180 min with production of 567.51 μM d-tagatose from 1000 mM of d-galactose. Though, the bioconversion ratio is low but it can be increased by immobilization and enzyme engineering. Although various l-arabinose isomerases have been characterized for bioproduction of d-tagatose, P. aeruginosa glucose phosphate isomerase is distinguished from the other l-arabinose isomerases by its optimal temperature (60 °C) for d-tagatose production being mesophilic bacteria, making it an alternate choice for bulk production.






Similar content being viewed by others
References
EL, H., L, H., & JKN, J. (1949). Composition of the gum of Sterculia setigera: occurrence of d-tagatose in nature. Nature, 163, 177.
Adachi, S. (1958). Formation of lactulose and tagatose from lactose in strongly heated milk. Nature Group, 181, 840–841.
Livesey, G. (2001). Tolerance of low-digestible carbohydrates: a general view. British Journal of Nutrition, 85(Suppl. 1), S7–S16.
Livesey, G., & Brown, J. C. (1996). d-tagatose is a bulk sweetener with zero energy determined in rats. The Journal of Nutrition, 126(6), 1601–1609.
Levin, G. V. (2002). Tagatose, the new GRAS sweetener and health product. Journal of Medicinal Food, 5, 23–36.
Levin, G. V., Zelmer, L. R., Saunders, J. P., & Beadle, J. R. (1995). Sugar substitutes : their energy values, and potential health bulk characteristics. American Society for Clinical Nutrition, 62, 1161–1168.
Donner, T. W., Magder, L. S., & Zarbalian, K. (2010). Dietary supplementation with d-tagatose in subjects with type 2 diabetes leads to weight loss and raises high-density lipoprotein cholesterol ☆. Nutrition Research, 30(12), 801–806. doi:10.1016/j.nutres.2010.09.007.
Kim, P. (2004). Current studies on biological tagatose production using l-arabinose isomerase: a review and future perspective. Applied Microbiology and Biotechnology, 65(3), 243–249. doi:10.1007/s00253-004-1665-8.
Beadle, J.R., Saunder, J.P. & Wajada, T. J. (1992). Process for manufacturing tagatose. WO Patent 92/12263.
Izumori, K., Miyoshi, T., Tokuda, S., & Yamabe, K. (1984). Production of d-tagatose from dulcitol by Arthrobacter globiformis. Applied and Environmental Microbiology, 48(5), 1055–1057.
Izumori, K., & Tsuzaki, K. (1988). Production of d-tagatose from d-galactitol by Mycobacterium smegmatis. Journal of Fermentation Technology, 66(2), 225–227. doi:10.1016/0385-6380(88)90052-0.
Muniruzzaman, S., Tokunaga, H., & Izumori, K. (1994). Isolation of Enterobacter agglomerans strain 221e from soil, a potent d-tagatose producer from galactitol. Journal of Fermentation and Bioengineering, 78(2), 145–148. doi:10.1016/0922-338X(94)90253-4.
Manzoni, M., Rollini, M., & Bergomi, S. (2001). Biotransformation of d-galactitol to tagatose by acetic acid bacteria. Process Biochemistry, 36, 971–977.
Granström, T. B., Takata, G., Tokuda, M., & Izumori, K. (2004). Izumoring: a novel and complete strategy for bioproduction of rare sugars. Journal of Bioscience and Bioengineering, 97(2), 89–94. doi:10.1263/jbb.97.89.
Cheetham, P. S. J., & Wootton, A. N. (1993). Bioconversion of d-galactose into d-tagatose. Enzyme and Microbial Technology, 15(2), 105–108. doi:10.1016/0141-0229(93)90032-W.
Roh, H. J., Kim, P., Park, Y. C., & Choi, J. H. (2000). Bioconversion of d-galactose into d-tagatose by expression of l-arabinose isomerase. Biotechnology and applied biochemistry, 31 (Pt 1), 1–4. doi:10669396
Kim, P., Yoon, S. H., Roh, H. J., & Choi, J. H. (2001). High production of d-tagatose, a potential sugar substitute, using immobilized l-arabinose isomerase. Biotechnology Progress, 17(1), 208–210. doi:10.1021/bp000147u.
Yoon, S., Kim, P., & Oh, D. K. (2003). Properties of l-arabinose isomerase from Escherichia coli as a biocatalyst for d-tagatose production, 47–51.
Zhang, H., Jiang, B., & Pan, B. (2007). Purification and characterization of l-arabinose isomerase from Lactobacillus plantarum producing d-tagatose. World Journal of Microbiology and Biotechnology, 23(5), 641–646. doi:10.1007/s11274-006-9274-6.
Kim, H. J., & Oh, D. K. (2005). Purification and characterization of an l-arabinose isomerase from an isolated strain of Geobacillus thermodenitrificans producing d-tagatose. Journal of Biotechnology, 120(2), 162–173. doi:10.1016/j.jbiotec.2005.06.004.
Li, Y., Zhu, Y., Liu, A., & Sun, Y. (2011). Identification and characterization of a novel l-arabinose isomerase from Anoxybacillus flavithermus useful in d-tagatose production. Extremophiles, 15(3), 441–450. doi:10.1007/s00792-011-0375-2.
Kim, J. W., Kim, Y. W., Roh, H. J., Kim, H. Y., Cha, J. H., Park, K. H., & Park, C. S. (2003). Production of tagatose by a recombinant thermostable l-arabinose isomerase from Thermus sp. IM6501. Biotechnology Letters, 25(12), 963–967. doi:10.1023/A:1024069813839.
Jørgensen, F., Hansen, O. C., & Stougaard, P. (2004). Enzymatic conversion of d-galactose to d-tagatose: heterologous expression and characterisation of a thermostable l-arabinose isomerase from Thermoanaerobacter mathranii. Applied Microbiology and Biotechnology, 64(6), 816–822. doi:10.1007/s00253-004-1578-6.
Kim, B. C., Lee, Y. H., Lee, H. S., Lee, D. W., Choe, E. A., & Pyun, Y. R. (2002). Cloning, expression and characterization of l-arabinose isomerase from Thermotoga neapolitana: bioconversion of d-galactose to d-tagatose using the enzyme. FEMS Microbiology Letters, 212(1), 121–126. doi:10.1016/S0378-1097(02)00715-2.
Beerens, K., Desmet, T., & Soetaert, W. (2012). Enzymes for the biocatalytic production of rare sugars. Journal of Industrial Microbiology and Biotechnology, 39(6), 823–834. doi:10.1007/s10295-012-1089-x.
Yeom, S. J., Kim, Y. S., & Oh, D. K. (2013). Development of novel sugar isomerases by optimization of active sites in phosphosugar isomerases for monosaccharides. Applied and Environmental Microbiology, 79(3), 982–988. doi:10.1128/AEM.02539-12.
Yeom, S. J., Ji, J. H., Kim, N. H., Park, C. S., & Oh, D. K. (2009). Substrate specificity of a mannose-6-phosphate isomerase from bacillus subtilis and its application in the production of l-ribose. Applied and Environmental Microbiology, 75(14), 4705–4710. doi:10.1128/AEM.00310-09.
Park, C. S., Yeom, S. J., Kim, H. J., Lee, S. H., Lee, J. K., Kim, S. W., & Oh, D. K. (2007). Characterization of ribose-5-phosphate isomerase of Clostridium thermocellum producing d-allose from d-psicose. Biotechnology Letters, 29(9), 1387–1391. doi:10.1007/s10529-007-9393-7.
Park, H. Y., Park, C. S., Kim, H. J., & Oh, D. K. (2007). Substrate specificity of a galactose 6-phosphate isomerase from Lactococcus lactis that produces d-allose from d-psicose. Journal of Biotechnology, 132(1), 88–95. doi:10.1016/j.jbiotec.2007.08.022.
Swan, M. K., Hansen, T., Schönheit, P., & Davies, C. (2004). A novel phosphoglucose isomerase (PGI)/phosphomannose isomerase from the crenarchaeon Pyrobaculum aerophilum is a member of the PGI superfamily. Structural evidence at 1.16-Å resolution. Journal of Biological Chemistry, 279(38), 39838–39845. doi:10.1074/jbc.M406855200.
Sambrook, J. R. D. (2001). In Molecular cloning: a laboratory manual (3rd ed.). NY: Cold Spring Harbor Laboratory Press.
Bradford, M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Kulka, R. G. (1956). Colorimetric estimation of ketopentoses and ketohexoses. The Biochemical Journal, 63(4), 542–548.
Lutz*, S., Joseph, L., & L, L. (2008). Exploiting temperature-dependent substrate promiscuity for nucleoside analog activation by thymidine kinase from Thermotoga maritima. Journal of the American Chemical Society, 129(28), 8714–8715.
Staudigl, P., Haltrich, D., & Peterbauer, C. K. (2014). l-arabinose isomerase and l-arabinose isomerase and d-xylose isomerase from Lactobacillus reuteri: characterization, coexpression in the food grade host Lactobacillus plantarum, and application in the conversion of d-galactose and d-glucose. doi:10.1021/jf404785m
Kim, H., Ryu, S., Kim, P., & Oh, D. (2003). A feasible enzymatic process for d-tagatose production by an immobilized thermostable l-arabinose isomerase in a packed-bed bioreactor, 400–404. doi:10.1021/bp025675f
Oh, D. K. (2007). Tagatose: properties, applications, and biotechnological processes. Applied Microbiology and Biotechnology, 76(1), 1–8. doi:10.1007/s00253-007-0981-1.
Banerjee, S., Anderson, F., & Farber, G. K. (1995). The evolution of sugar isomerases. Protein Engineering, 8(12), 1189–1195. doi:10.1093/protein/8.12.1189.
Jogl, G., Rozovsky, S., McDermott, A. E., & Tong, L. (2003). Optimal alignment for enzymatic proton transfer: structure of the Michaelis complex of triosephosphate isomerase at 1.2-A resolution. Proceedings of the National Academy of Sciences of the United States of America, 100(1), 50–55. doi:10.1073/pnas.0233793100.
Zhang, R. G., Andersson, C. E., Savchenko, A., Skarina, T., Evdokimova, E., Beasley, S., & Mowbray, S. L. (2003). Structure of Escherichia coli ribose-5-phosphate isomerase: a ubiquitous enzyme of the pentose phosphate pathway and the Calvin cycle. Structure, 11(1), 31–42. doi:10.1016/S0969-2126(02)00933-4.
Hansen, T., Schlichting, B., Felgendreher, M., & Schönheit, P. (2005). Cupin-type phosphoglucose isomerases (Cupin-PGIs) constitute a novel metal-dependent PGI family representing a convergent line of PGI evolution. Journal of Bacteriology, 187(5), 1621–1631. doi:10.1128/JB.187.5.1621-1631.2005.
Teplyakov, A., Obmolova, G., Badet-Denisot, M. A., & Badet, B. (1999). The mechanism of sugar phosphate isomerization by glucosamine 6-phosphate synthase. Protein science : A publication of the Protein Society, 8(3), 596–602. doi:10.1110/ps.8.3.596.
Lee, D. W., Choe, E. A., Kim, S. B., Eom, S. H., Hong, Y. H., Lee, S. J., & Pyun, Y. R. (2005). Distinct metal dependence for catalytic and structural functions in the l-arabinose isomerases from the mesophilic Bacillus halodurans and the thermophilic Geobacillus stearothermophilus. Archives of Biochemistry and Biophysics, 434(2), 333–343. doi:10.1016/j.abb.2004.11.004.
Prabhu, P., Doan, T.-N.-T., Tiwari, M., Singh, R., Kim, S. C., Hong, M.-K., & Lee, J.-K. (2014). Structure-based studies on the metal binding of two-metal-dependent sugar isomerases. FEBS Journal, 281(15), 3446–3459. doi:10.1111/febs.12872.
Van Tilbeurgh, H., Jenkins, J., Chiadmi, M., Janin, J., Wodak, S. J., Mrabet, N. T., & Lambeir, A. M. (1992). Protein engineering of xylose (glucose) isomerase from Actinoplanes missouriensis. 3. Changing metal specificity and the pH profile by site- directed mutagenesis. Biochemistry, 31(24), 5467–5471. doi:10.1021/bi00139a007.
Roux, C., Lee, J. H., Jeffery, C. J., & Salmon, L. (2004). Inhibition of type I and type II phosphomannose isomerases by the reaction intermediate analogue 5-phospho-d-arabinonohydroxamic acid supports a catalytic role for the metal cofactor. Biochemistry, 43(10), 2926–2934. doi:10.1021/bi035688h.
Noltmann, A. E., & Gracy, W. R. (1968). A mechanism for catalysis and for the role of zinc in the enzymatic and the nonenzymatic isomerization*. The Journal of Biological Chemistry, 243(20), 5410–5419.
Yoon, R. Y., Yeom, S. J., Park, C. S., & Oh, D. K. (2009). Substrate specificity of a glucose-6-phosphate isomerase from Pyrococcus furiosus for monosaccharides. Applied Microbiology and Biotechnology, 83(2), 295–303. doi:10.1007/s00253-009-1859-1.
Wurth, C., Kessler, U., & Vogt, J. (2001). The effect of substrate binding on the conformation and structural stability of Herpes simplex virus type 1 thymidine kinase. Protein, 10(1), 63–73. doi:10.1110/ps.27401.Thymidine.
Yeom, S. J., Kim, B. N., Park, C. S., & Oh, D. K. (2010). Substrate specificity of ribose-5-phosphate isomerases from Clostridium difficile and Thermotoga maritima. Biotechnology Letters, 32(6), 829–835. doi:10.1007/s10529-010-0224-x.
Yoon, R. Y., Yeom, S. J., Kim, H. J., & Oh, D. K. (2009). Novel substrates of a ribose-5-phosphate isomerase from Clostridium thermocellum. Journal of Biotechnology, 139(1), 26–32. doi:10.1016/j.jbiotec.2008.09.012.
Prabhu, P., Kumar Tiwari, M., Jeya, M., Gunasekaran, P., Kim, I. W., & Lee, J. K. (2008). Cloning and characterization of a novel l-arabinose isomerase from Bacillus licheniformis. Applied Microbiology and Biotechnology, 81(2), 283–290. doi:10.1007/s00253-008-1652-6.
Kim, J. H., Prabhu, P., Jeya, M., Tiwari, M. K., Moon, H. J., Singh, R. K., & Lee, J. K. (2010). Characterization of an l-arabinose isomerase from Bacillus subtilis. Applied Microbiology and Biotechnology, 85(6), 1839–1847. doi:10.1007/s00253-009-2210-6.
Yoon, S. H., Kim, P., & Oh, D. K. (2003). Properties of l-arabinose isomerase from Escherichia coli as biocatalyst for tagatose production. World Journal of Microbiology and Biotechnology, 19(1), 47–51. doi:10.1023/A:1022575601492.
Ryu, S., Kim, C.-S., Kim, H.-J., Baek, D.-K., & Oh, D. (2003). Continuous d-tagatose production by an immobilized thermostable l-arabinose isomerase in a packed-bed bioreactor, 1643–1647. doi:10.1021/bp025675f
Leang, K., Takada, G., Fukai, Y., Morimoto, K., Granström, T. B., & Izumori, K. (2004). Novel reactions of l-rhamnose isomerase from Pseudomonas stutzeri and its relation with d-xylose isomerase via substrate specificity. Biochimica et Biophysica Acta - General Subjects, 1674(1), 68–77. doi:10.1016/j.bbagen.2004.06.003.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patel, M.J., Patel, A.T., Akhani, R. et al. Bioproduction of d-Tagatose from d-Galactose Using Phosphoglucose Isomerase from Pseudomonas aeruginosa PAO1. Appl Biochem Biotechnol 179, 715–727 (2016). https://doi.org/10.1007/s12010-016-2026-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2026-7