Abstract
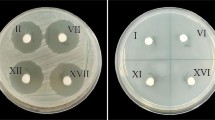
Although biofilms are formed on a variety of surfaces, of utmost significance are those formed on prosthetic devices used as implants. Such biofilms can lead to severe device-related infections that are difficult to treat. In a search for new antibiofilm agents that can be used as “active” implant coatings, purified fraction from a tannin-rich extract of Terminalia chebula was isolated and tested for its antibiofilm properties on a titanium implant material. The fraction, named as Fraction 7, was found to significantly reduce biofilm formation by hospital isolates of Staphylococcus aureus, at sub-inhibitory concentrations that were 64 times lower than the minimum inhibitory concentration (MIC). Simulated local delivery systems of the Fraction 7 set upon the surface of titanium alloy released the fraction in a controlled manner from a biodegradable carrier (PDLLA) and were found to significantly reduce biofilm formation by a methicillin-resistant hospital isolate of S. aureus in a load concentration dependent manner without preventing growth. This study therefore identifies a novel fraction from tannin-rich extract of T. chebula that has potential to be used as an antibiofilm coat on implant surfaces.








Similar content being viewed by others
References
Rochford, E. T. J., Richards, R. G., & Moriarty, T. F. (2012). Influence of material on the development of device-associated infections. Clinical Microbiology and Infection, 18(12), 1162–1167.
Aviv M, Berdicevsky I, Zilberman M. (2007). Gentamicin-loaded bioresorbable films for prevention of bacterial infections associated with orthopedic implants. Journal of Biomedical Materials Research Part A 10–19.
Bryers, J. D., & Ratner, B. D. (2004). Bioinspired implant materials befuddle bacteria. ASM New, 70(5), 232–237.
Navarro, M., Michiardi, A., Castaño, O., & Planell, J. A. (2008). Biomaterials in orthopaedics. Journal of the Royal Society Interface, 5(27), 1137–58.
Chen, C.-P., & Wickstrom, E. (2011). Self-protecting bactericidal titanium alloy surface formed by covalent bonding of daptomycin bisphosphonates. Bioconjugate Chemistry, 21(11), 1978–1986.
Harris, L. G., & Richards, R. G. (2004). Staphylococcus aureus adhesion to different treated titanium surfaces. Journal of Materials Science Materials in Medicine, 15(4), 311–4.
Hetrick, E. M., & Schoenfisch, M. H. (2006). Reducing implant-related infections: active release strategies. Chemical Society Reviews, 35(9), 780–9.
Darouiche, R. O. (2001). Device-associated infections: a macroproblem that starts with microadherence. Clinical Infectious Diseases : an Official Publication of the Infectious Diseases Society of America, 33(9), 1567–72.
Harris, L. G., Mead, L., Muller-Oberlander, E., & Richards, R. (2006). Bacteria and cell cytocompatibility studies on coated medical grade titanium surfaces. Journal of Biomedical Materials Research, Part A, 50–58.
Makadia, H. K., & Siegel, S. J. (2011). Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers, 3(3), 1377–1397.
Zilberman, M., & Elsner, J. J. (2008). Antibiotic-eluting medical devices for various applications. Journal of Controlled Release, 130(3), 202–15.
Bazaka, K., Jacob, M. V., Truong, V. K., et al. (2010). Plasma-enhanced synthesis of bioactive polymeric coatings from monoterpene alcohols: a combined experimental and theoretical study. Biomacromolecules, 11(8), 2016–26.
Khoo, X., & Grinstaff, M. W. (2011). Novel infection-resistant surface coatings: a bioengineering approach. MRS Bulletin, 36(05), 357–366.
Daniel.M. (2006) Medicinal Plants: Chemistry and Properties Science Publishers.
Gupta, A., Tandon, N., & Sharma, M. (Eds.). (2003). Quality Standards of Indian Medicinal Plants Volume 1.
The Ayurvedic Pharmacopoeia of India Part I Volume 1. (n.d.) (1st ed.). Government of India, Ministry of Health & Family Welfare, Dept of Indian System of Medicine and Homeopathy.
Ghosh, A., Das, B. K., Roy, A., Mandal, B., & Chandra, G. (2008). Antibacterial activity of some medicinal plant extracts. Journal of Natural Medicines, 62(2), 259–62.
Bag, A., Bhattacharyya, S. K., Chattopadhyay, R. R., & Rashid, R. A. (2013). The development of Terminalia chebula Retz. (Combretaceae) in clinical research. Asian Pacific Journal of Tropical Biomedicine, 3(3), 244–52.
Taganna, J. C., Quanico, J. P., Perono, R. M. G., Amor, E. C., & Rivera, W. L. (2011). Tannin-rich fraction from Terminalia catappa inhibits quorum sensing (QS) in Chromobacterium violaceum and the QS-controlled biofilm maturation and LasA staphylolytic activity in Pseudomonas aeruginosa. Journal of Ethnopharmacology, 134(3), 865–71.
Yarwood, J. M., Bartels, D. J., Volper, E. M., & Greenberg, E. P. (2004). Quorum Sensing in Staphylococcus aureus Biofilms. Journal of Bacteriology, 186(6), 1838–1850.
Roopashree, T., Dang, R., Shobha Rani, R., & Narendra, C. (2008). Antibacterial activity of antipsoriatic herbs : Cassia tora, Momordica charantia and Calendula officinalis. International Journal od Applied Research in Natural Products, 1(May), 20–28.
Hagerman AE. Tannin Chemistry. 2011. Available at: www.users.muohio.edu/hagermae/tannin.pdf.
Cortés, S., Pulgar, H., Sanhueza, V., et al. (2010). Identification of proanthocyanidins extracted from Pinus radiata D. Don bark. Ciencia e Investigacion Agreria., 37(2), 15–25.
Valgas C, de Souza S, Smânia E, Smânia A. (2007). Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. vol.38 no.2; 38(2).
Subbiahdoss, G., Pidhatika, B., Coullerez, G., Charnley, M., & Kuijer, R. (2010). Bacterial biofilm formation versus mamalian cell growth on titanium-based mono- and bi-functional coatings. European Cells and Materials., 19, 205–213.
Peng, Z., Tu, B., Shen, Y., et al. (2011). Quaternized chitosan inhibits icaA transcription and biofilm formation by Staphylococcus on a titanium surface. Antimicrobial Agents and Chemotherapy, 55(2), 860–866.
Chandra, J., Mukherjee, P. K., & Ghannoum, M. A. (2008). In vitro growth and analysis of Candida biofilms. Nature Protocols, 3(12), 1909–24.
Gollwitzer, H., Ibrahim, K., Meyer, H., Mittelmeier, W., Busch, R., & Stemberger, A. (2003). Antibacterial poly(D, L-lactic acid) coating of medical implants using a biodegradable drug delivery technology. Journal of Antimicrobial Chemotherapy, 51(3), 585–591.
Jurcisek J a, Dickson AC, Bruggeman ME, Bakaletz, L. O. (2011). In vitro biofilm formation in an 8-well chamber slide. Journal of visualized experiments : JoVE (47):9–10.
Sternberg C, Tolker-Nielsen T. (2006). Growing and analyzing biofilms in flow cells. Current protocols in microbiology; Unit 1B.2.
Nikolic, L., Ristic, I., Adnadjevic, B., Nikolic, V., Jovanovic, J., & Stankovic, M. (2010). Novel microwave-assisted synthesis of poly(D,L-lactide): the influence of monomer/initiator molar ratio on the product properties. Sensors (Basel, Switzerland), 10(5), 5063–73.
Quave, C. L., Plano, L. R. W., Pantuso, T., & Bennett, B. C. (2008). Effects of extracts from Italian medicinal plants on planktonic growth, biofilm formation and adherence of methicillin-resistant Staphylococcus aureus. Journal of Ethnopharmacology, 118(3), 418–28.
Richards, J. J., & Melander, C. (2009). Controlling Bacterial Biofilms. ChemBioChem, 10, 2287–2294.
Worthington, R. J., Richards, J. J., & Melander, C. (2012). Small molecule control of bacterial biofilms. Organic & Biomolecular Chemistry, 10(37), 7457–74.
Huber, B., Eberl, L., Feucht, W., & Polster, J. (2003). Influence of polyphenols on bacterial biofilm formation and quorum-sensing. Zeitschrift für Naturforschung. C. Journal of Biosciences, 58(11–12), 879–84.
Quave, C. L., Estévez-Carmona, M., Compadre, C. M., et al. (2012). Ellagic acid derivatives from Rubus ulmifolius inhibit Staphylococcus aureus biofilm formation and improve response to antibiotics. PloS One, 7(1), e28737.
Brackman, G., Cos, P., Maes, L., Nelis, H. J., & Coenye, T. (2011). Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrobial Agents and Chemotherapy, 55(6), 2655–61.
Ding, X., Yin, B., Qian, L., et al. (2011). Screening for novel quorum-sensing inhibitors to interfere with the formation of Pseudomonas aeruginosa biofilm. Journal of Medical Microbiology, 60(Pt 12), 1827–34.
Wu, J., & Yao, J. (2012). Fabrication and in vitro release behavior of a novel antibacterial coating containing halogenated furanone-loaded poly ( L-lactic acid ) nanoparticles on microarc-oxidized titanium. International Journal of Nanomedicine, 7, 5641–5652.
Otto, M. (2009). Staphylococcal biofilms. Current Topics in Microbiology and Immunology, 322, 207–228.
Park, S.-C., Park, Y., & Hahm, K.-S. (2011). The role of antimicrobial peptides in preventing multidrug-resistant bacterial infections and biofilm formation. International Journal of Molecular Sciences, 12(9), 5971–92.
Acknowledgements
Part of this work was done under the contingency grant allotted to the first author under the Faculty development program of University Grants Commission, Govt. of India vide letter F. No. 30-23/08(WRO). The authors also wish to thank Dr. Priti Mehta, Head, Department of Microbiology, KEM hospital, for providing the cultures, Mr. Ajay Pitre of Sushrut Surgicals for providing the titanium alloy pieces, and Dr. Arne Heydron, Denmark, for sending the COMSTAT 1 program.
Conflict of interest
None declared.
Ethical approval
Not required
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shukla, V., Bhathena, Z. Sustained Release of a Purified Tannin Component of Terminalia chebula from a Titanium Implant Surface Prevents Biofilm Formation by Staphylococcus aureus . Appl Biochem Biotechnol 175, 3542–3556 (2015). https://doi.org/10.1007/s12010-015-1525-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1525-2