Abstract
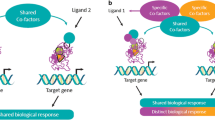
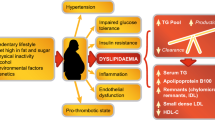
The hypolipidemic fibric acid drugs are peroxisome proliferator-activated receptor a (PPARα) ligands. PPARα activated by fibric acids form heterodimers with the 9-cis retinoic acid receptor (RXR). The PPAR/RXR heterodimers bind to peroxisome proliferator response elements (PPRE), which are located in numerous gene promoters and increase the level of the expression of mRNAs encoded by PPARα target genes. Fibric acids decrease triglyceride plasma levels through increases in the expression of genes involved in fatty acid-beta oxidation. Furthermore, they decrease triglycerides by increasing lipoprotein lipase gene expression and by decreasing apolipoprotein C-III gene expression. Fibric acids increase high-density lipoprotein (HDL) cholesterol partly by increasing apolipoprotein A-I and apolipoprotein A-II gene expression. Fibric acids also reduce vascular wall inflammation and the expression of genes involved in different vascular functions (ie, vasomotricity, thrombosis). Fibric acids are used to treat primary hypertriglyceridemia and mixed hyperlipidemia. Some fibric acid molecules are active in essential hypercholesterolemia. Clinical evidence shows that fibric acids reduce coronary atherosclerosis progression in dyslipidemic patients (eg, bezafibrate, gemfibrozil) and in type 2 diabetic patients (fenofibrate). Gemfibrozil decreases coronary morbidity and mortality in patients with low HDL cholesterol, normal triglycerides, and normal low-density lipoprotein (LDL) cholesterol plasma levels. Further clinical studies are necessary to investigate if fibric acids decrease cardiovascular mortality in type 2 diabetes and in primary prevention of hypertriglyceridemia and hypolipidemia.
Similar content being viewed by others
References and Recommended Reading
Braissant O, Foufelle F, Scotto C, et al.: Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha,-beta, and -gamma in the adult rat. Endocrinology 1996, 137:354–366.
Krey G, Braissant O, L’Horset F, et al.: Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol Endocrinol 1997, 11:779–791.
Issemann I, Green S: Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 1990, 347:645–650.
Lambe KG, Woodyatt NJ, Macdonald N, et al.: Species differences in sequence and activity of the peroxisome proliferator response element (PPRE) within the acyl CoA oxidase gene promoter. Toxicol Lett 1999, 110:119–127.
Willson TM, Brown PJ, Sternbach DD, Henke BR: The PPARs: from orphan receptors to drug discovery. J Med Chem 2000, 43:527–550.
Prevention of coronary heart disease: scientific background and new clinical guidelines. Recommendations of the European Atherosclerosis Society prepared by International Task Force for Prevention of Coronary Heart Disease. Nutr Metab Cardiovasc Dis 1992, 2:113–156.
Summary of the second report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II). JAMA 1993, 269:3015–3023.
Vu-Dac N, Schoonjans K, Kosykh V, et al.: Fibrates increase human apolipoprotein A-II expression through activation of the peroxisome proliferator-activated receptor. J Clin Invest 1995, 96:741–750.
Cattin L, Da Col PG, Feruglio FS, et al.: Efficacy of ciprofibrate in primary type II and IV hyperlipidemia: the Italian multicenter study. Clin Ther 1990, 12:482–488.
Tilly-Kiesi M, Tikkanen MJ: Low density lipoprotein density and composition in hypercholesterolaemic men treated with HMG CoA reductase inhibitors and gemfibrozil. J Intern Med 1991, 229:427–434.
Schoonjans K, Staels B, Auwerx J: The peroxisome proliferator activated receptors (PPARS) and their effects on lipid metabolism and adipocyte differentiation. Biochim Biophys Acta 1996, 1302:93–109.
Martin G, Schoonjans K, Lefebvre AM, et al.: Coordinate regulation of the expression of the fatty acid transport protein and acyl-CoA synthetase genes by PPARalpha and PPAR-gamma activators. J Biol Chem 1997, 272:28210–28217.
Mascaro C, Acosta E, Ortiz JA, et al.: Control of human muscle-type carnitine palmitoyltransferase I gene transcription by peroxisome proliferator-activated receptor. J Biol Chem 1998, 273:8560–8563.
Kelly LJ, Vicario PP, Thompson GM, et al.: Peroxisome proliferator-activated receptors gamma and alpha mediate in vivo regulation of uncoupling protein (UCP-1, UCP-2, UCP-3) gene expression. Endocrinology 1998, 139:4920–4927.
Kesaniemi YA, Grundy SM: Influence of gemfibrozil and clofibrate on metabolism of cholesterol and plasma triglycerides in man. JAMA 1984, 251:2241–2246.
Hahn SE, Goldberg DM: Modulation of lipoprotein production in Hep G2 cells by fenofibrate and clofibrate. Biochem Pharmacol 1992, 43:625–633.
Desager JP, Horsmans Y, Vandenplas C, Harvengt C: Pharmacodynamic activity of lipoprotein lipase and hepatic lipase, and pharmacokinetic parameters measured in normolipidaemic subjects receiving ciprofibrate (100 or 200 mg/day) or micronised fenofibrate (200 mg/day) therapy for 23 days. Atherosclerosis 1996, 124(suppl):S65-S73.
Foger B, Drexel H, Hopferwieser T, et al.: Fenofibrate improves postprandial chylomicron clearance in II B hyperlipoproteinemia. Clin Invest 1994, 72:294–301.
Schoonjans K, Peinado-Onsurbe J, Lefebvre AM, et al.: PPARalpha and PPARgamma activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO J 1996, 15:5336–5348.
Quarfordt SH, Michalopoulos G, Schirmer B: The effect of human C apolipoproteins on the in vitro hepatic metabolism of triglyceride emulsions in the rat. J Biol Chem 1982, 257:14642–14647.
Windler E, Chao Y, Havel RJ: Regulation of the hepatic uptake of triglyceride-rich lipoproteins in the rat. Opposing effects of homologous apolipoprotein E and individual C apoproteins. J Biol Chem 1980, 255:8303–8307.
Clavey V, Lestavel-Delattre S, Copin C, et al.: Modulation of lipoprotein B binding to the LDL receptor by exogenous lipids and apolipoproteins CI, CII, CIII, and E. Arterioscler Thromb Vasc Biol 1995, 15:963–971.
Aalto-Setala K, Fisher EA, Chen X, et al.: Mechanism of hypertriglyceridemia in human apolipoprotein (apo) CIII transgenic mice. Diminished very low density lipoprotein fractional catabolic rate associated with increased apo CIII and reduced apo E on the particles. J Clin Invest 1992, 90:1889–1900.
Aalto-Setala K, Weinstock PH, Bisgaier CL, et al.: Further characterization of the metabolic properties of triglyceride-rich lipoproteins from human and mouse apoC-III transgenic mice. J Lipid Res 1996, 37:1802–1811.
Ebara T, Ramakrishnan R, Steiner G, Shachter NS: Chylomicronemia due to apolipoprotein CIII overexpression in apolipoprotein E-null mice. Apolipoprotein CIII-induced hypertriglyceridemia is not mediated by effects on apolipoprotein. Eur J Clin Invest 1997, 99:2672–2681.
Bard JM, Parra HJ, Camare R, et al.: A multicenter comparison of the effects of simvastatin and fenofibrate therapy in severe primary hypercholesterolemia, with particular emphasis on lipoproteins defined by their apolipoprotein composition. Metabolism 1992, 41:498–503.
Staels B, Koenig W, Habib A, et al.: Activation of human aortic smooth-muscle cells is inhibited by PPARα but not by PPARγ activators. Nature 1998, 393:790–793.
Haubenwallner S, Essenburg AD, Barnett BC, et al.: Hypolipidemic activity of select fibrates correlates to changes in hepatic apolipoprotein C-III expression: a potential physiologic basis for their mode of action. J Lipid Res 1995, 36:2541–2551.
Peters JM, Hennuyer N, Staels B, et al.: Alterations in lipoprotein metabolism in peroxisome proliferator-activated receptor alpha-deficient mice. J Biol Chem 1997, 272:27307–27312.
Ladias JA, Hadzopoulou-Cladaras M, Kardassis D, et al.: Transcriptional regulation of human apolipoprotein genes ApoB, ApoCIII, and ApoAII by members of the steroid hormone receptor superfamily HNF-4, ARP-1, EAR-2, and EAR-3. J Biol Chem 1992, 267:15849–15860.
Vu-Dac N, Gervois P, Pineda Torra IP, et al.: Retinoids increase human apo C-III expression at the transcriptional level via the retinoid X receptor. Contribution to the hypertriglyceridemic action of retinoids. J Clin Invest 1998, 102:625–632.
Kahri J, Sane T, Van Tol A, Taskinen MR: Effect of gemfibrozil on the regulation of HDL subfractions in hypertriglyceridaemic patients. J Intern Med 1995, 238:429–436.
Zambon D, Ros E, Rodriguez-Villar C, et al.: Randomized crossover study of gemfibrozil versus lovastatin in familial combined hyperlipidemia: additive effects of combination treatment on lipid regulation. Metabolism 1999, 48:47–54.
Steinmetz A, Schwartz T, Hehnke U, Kaffarnik H: Multicenter comparison of micronized fenofibrate and simvastatin in patients with primary type IIA or IIB hyperlipoproteinemia. J Cardiovasc Pharmacol 1996, 27:563–570.
Schaefer EJ, Lamon-Fava S, Cole T, et al.: Effects of regular and extended-release gemfibrozil on plasma lipoproteins and apolipoproteins in hypercholesterolemic patients with decreased HDL cholesterol levels. Atherosclerosis 1996, 127:113–122.
Malmendier CL, Delcroix C: Effects of fenofibrate on high and low density lipoprotein metabolism in heterozygous familial hypercholesterolemia. Atherosclerosis 1985, 55:161–169.
Staels B, Van Tol A, Andreu T, Auwerx J: Fibrates influence the expression of genes involved in lipoprotein metabolism in a tissue-selective manner in the rat. Arterioscler Thromb 1992, 12:286–294.
Berthou L, Saladin R, Yaqoob P, et al.: Regulation of rat liver apolipoprotein A-I, apolipoprotein A-II and acyl-coenzyme A oxidase gene expression by fibrates and dietary fatty acids. Eur J Biochem 1995, 232:179–187.
Vu-Dac N, Schoonjans K, Laine B, et al.: Negative regulation of the human apolipoprotein A-I promoter by fibrates can be attenuated by the interaction of the peroxisome proliferator-activated receptor with its response element. J Biol Chem 1994, 269:31012–31018.
Berthou L, Duverger N, Emmanuel F, et al.: Opposite regulation of human versus mouse apolipoprotein A-I by fibrates in human apolipoprotein A-I transgenic mice. J Clin Invest 1996, 97:2408–2416.
Kockx M, Princen HM, Kooistra T: Fibrate-modulated expression of fibrinogen, plasminogen activator inhibitor-1 and apolipoprotein A-I in cultured cynomolgus monkey hepatocytes: role of the peroxisome proliferator-activated receptor-alpha. Thromb Haemost 1998, 80:942–948.
Hennuyer N, Poulain P, Madsen L, et al.: Beneficial effects of fibrates on apolipoprotein A-I metabolism occur independently of any peroxisome proliferative response. Circulation 1999, 99:2445–2451.
Guerre-Millo M, Gervois P, Raspe E, et al.: Peroxisome proliferator-activated receptor alpha activators improve insulin sensitivity and reduce adiposity. J Biol Chem 2000, 275:16638–16642.
Durrington PN, Mackness MI, Bhatnagar D, et al.: Effects of two different fibric acid derivatives on lipoproteins, cholesteryl ester transfer, fibrinogen, plasminogen activator inhibitor and paraoxonase activity in type IIb hyperlipoproteinaemia. Atherosclerosis 1998, 138:217–225.
Kockx M, de Maat MP, Knipscheer HC, et al.: Effects of gemfibrozil and ciprofibrate on plasma levels of tissue-type plasminogen activator, plasminogen activator inhibitor-1 and fibrinogen in hyperlipidaemic patients. Thromb Haemost 1997, 78:1167–1172.
Kockx M, Gervois P, Poulain P, et al.: Fibrates suppress fibrinogen gene expression in rodents via activation of the peroxisome proliferator-activated receptor-alpha. Blood 1999, 93:2991–2998.
Neve B, Corseaux D, Chinetti G, et al.: PPARalpha agonists inhibit tissue factor expression int THP-1 cells and human monocytes. Circulation 2000, in press.
Ross R: The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 1993, 362:801–809.
Inoue I, Shino K, Noji S, et al.: Expression of peroxisome proliferator-activated receptor alpha (PPARa) in primary cultures of human vascular endothelial cells. Biochem Biophys Res Commun 1998, 246:370–374.
Ricote M, Huang J, Fajas L, et al.: Expression of the peroxisome proliferator-activated receptor gamma (PPARg) in human atherosclerosis and regulation in macrophages by colony stimulating factors and oxidized low density lipoprotein. Proc Natl Acad Sci U S A 1998, 95:7614–7619.
Chinetti G, Griglio S, Antonucci M, et al.: Activation of proliferator-activated receptors alpha and gamma induces apoptosis of human monocyte-derived macrophages. J Biol Chem 1998, 273:25573–25580.
Devchand PR, Keller H, Peters JM, Vazquez M, Gonzalez FJ, Wahli W. The PPARa-leukotriene B4 pathway to inflammation control. Nature 1996;384:39–43.
Colville-Nash PR, Qureshi SS, Willis D, Willoughby DA: Inhibition of inducible nitric oxide synthase by peroxisome proliferator-activated receptor agonists: correlation with induction of heme oxygenase 1. J Immunol 1998, 161:978–984.
Delerive P, Martin-Nizard F, Chinetti G, et al.: PPAR activators inhibit thrombin-induced endothelin-1 production in human vascular endothelial cells. Circulation 1998, 17:I-406-2137.
Delerive P, De Bosscher K, Besnard S, et al.: Peroxisome proliferator-activated receptor alpha negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-kappaB and AP-1. J Biol Chem 1999, 274:32048–32054.
Kannel WB, Castelli WP, Gordon T, McNamara PM: Serum cholesterol, lipoproteins, and the risk of coronary heart disease. The Framingham study. Ann Intern Med 1971, 74:1–12.
Stamler J, Wentworth D, Neaton JD: Is relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? Findings in 356,222 primary screenees of the Multiple Risk Factor Intervention Trial (MRFIT). JAMA 1986, 256:2823–2828.
Stampfer MJ, Colditz GA, Willett WC, et al.: Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses’ health study. N Engl J Med 1991, 325:756–762.
Smith GD, Shipley MJ, Marmot MG, Rose G: Plasma cholesterol concentration and mortality. The Whitehall Study. JAMA 1992, 267:70–76.
Gordon DJ, Probstfield JL, Garrison RJ, et al.: High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 1989, 79:8–15.
Hodis HN, Mack WJ, Azen SP, et al.: Triglyceride-and cholesterol-rich lipoproteins have a differential effect on mild/moderate and severe lesion progression as assessed by quantitative coronary angiography in a controlled trial of lovastatin. Circulation 1994, 90:42–49.
Assmann G, Schulte H: Role of triglycerides in coronary artery disease: lessons from the Prospective Cardiovascular Munster Study. Am J Cardiol 1992, 70:10H-13H.
Manninen V, Tenkanen L, Koskinen P, et al.: Joint effects of serum triglyceride and LDL cholesterol and HDL cholesterol concentrations on coronary heart disease risk in the Helsinki Heart Study. Implications for treatment. Circulation 1992, 85:37–45.
Austin MA: Plasma triglyceride and coronary heart disease. Arterioscler Thromb 1991, 11:2–14.
Hokanson JE, Austin MA: Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk 1996, 3:213–219.
Frick MH, Syvanne M, Nieminen MS, et al.: Prevention of the angiographic progression of coronary and vein-graft atherosclerosis by gemfibrozil after coronary bypass surgery in men with low levels of HDL cholesterol. Lopid Coronary Angiography Trial (LOCAT) Study Group. Circulation 1997, 96:2137–2143.
Rubins HB: Veterans Affairs-High Density Lipoprotein Cholesterol Intervention Trial (VA-HIT). Scientific Sessions “American Heart Association”, Dallas, November 11th 1998. Plenary Session XII “Late breaking clinical trials 1998.
Ericsson CG, Wilson J, Grop L, et al.: Effect of bezafibrate treatment over five years on coronary plaques causing 20% to 50% diameter narrowing. The Bezafibrate Coronary Atherosclerosis Intervention Trial. Am J Cardiol 1997, 80:1125–1129.
Goldbourt U, Brunner D, Behar S, Reicher-Reiss H: Baseline characteristics of patients participating in the Bezafibrate Infarction Prevention (BIP) Study. Eur Heart J 1998, 19(suppl H):H42-H47.
Ericsson CG, Hamsten A, Nilsson J, et al.: Angiographic assessment of effects of bezafibrate on progression of coronary artery disease in young male postinfarction patients. Lancet 1996, 347:849–853.
Kaplinsky E, Brunner D: The bezafibrate infarction prevention (BIP) study results. XXth Congress of the European Society of Cardiology Vienne, August 22–August 26. 1998.
Steiner G. The Diabetes Atherosclerosis Intervention Study (DAIS): a study conducted in cooperation with the World Health Organization. The DAIS Project Group. Diabetologia 1996, 39:1655–1661.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fruchart, JC., Staels, B. & Duriez, P. The role of fibric acids in atherosclerosis. Curr Atheroscler Rep 3, 83–92 (2001). https://doi.org/10.1007/s11883-001-0015-x
Issue Date:
DOI: https://doi.org/10.1007/s11883-001-0015-x