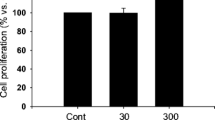
Summary
This study examined the effect of tanshinone II A (TSN II A) on the cardiac fibrosis induced by transforming growth factor β1 (TGF-β1) and the possible mechanisms. Cardiac fibroblasts were isolated from cardiac tissues of neonatal Sprague-Dawley (SD) rats by the trypsin digestion and differential adhesion method. The cells were treated with 5 ng/mL TGF-β1 alone or pretreated with TSN II A at different concentrations (10−5 mol/L, 10−4 mol/L). Immunocytochemistry was used for cell identification, RT-PCR for detection of the mRNA expression of connective tissue growth factor (CTGF) and collagen type I (COL I), Western blotting for detection of the protein expression of Smad7 and Smad3, and immunohistochemistry and immunofluorescence staining for detection of the protein expression of phosphorylated Smad3 (p-Smad3), CTGF and COLI. The results showed that TGF-β1 induced the expression of CTGF, COL I, p-Smad3 and Smad7 in a time-dependent manner. The mRNA expression of CTGF and COL I was significantly increased 24 h after TGF-β1 stimulation (P<0.01 for all). The protein expression of p-Smad3 and Smad7 reached a peak 1 h after TGF-β1 stimulation, much higher than the baseline level (P<0.01 for all). Pretreatment with high concentration of TSN A resulted in a decrease in the expression of p-Smad3, CTGF and COL I (P<0.01). The protein expression of Smad7 was substantially upregulated after pretreatment with two concentrations of TSN II A as compared with that at 2 h post TGF-β1 stimulation (P<0.05 for low concentration of TSN I IA; P<0.01 for high concentration of TSN II A). It was concluded that TSN II A may exert an inhibitory effect on cardiac fibrosis by upregulating the expression of Smad7, suppressing the TGF-β1-induced phosphorylation of Smad3 and partially blocking the TGF-β1-Smads signaling pathway.
Similar content being viewed by others
References
Baudino TA, Carver W, Giles W, et al. Cardiac fibroblasts: friend or foe?. Am J Physiol Heart Circ Physiol, 2006,291(3):H1015–H1026
Drobic V, Cunnington RH, Bedosky KM, et al. Differential and combined effects of cardiotrophin-1 and TGF-β1 on cardiac myofibroblast proliferation and contraction. Am J Physiol Heart Circ Physiol, 2007,293(2): H1053–H1064
Frank V, Alian M. Transforming growth factor-beta and fibrosis. World J Gastroenterol, 2007,13(22):3056–3062
Kania G, Blyszczuk P, Stein S, et al. Heart-infiltrating prominin-1(+)/CD133(+) progenitor cells represent the cellular source of transforming growth factor beta-mediated cardiac fibrosis in experimental autoimmune myocarditis. Circ Res, 2009,105(5):462–470
Zhang DM, Qin Y, Niu FL, et al. Effects of tanshinones II A on proliferation and type I collagen synthesis of rat cardiac fibroblasts. Liaoning Zhongyi Zazhi (Chinese), 2008,35(12):1934–1936
Rosenkranz S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res, 2004,63(3):423–432
Sun XY, Cao L, Yu GH, et al. Effect of sodium tanshinone II A sulfonate on proliferation and cyclin E expression in human renal interstitial fibroblasts in vitro. Acta Academiae Medicinae Militaris Tertiae (Chinese), 2009,29(7):585–587
Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res, 2007,74(2):184–195
Moustakas A, Souchelnytskyi S, Heldin CK. Smad regulation in TGF-β signal transduction. Cell Science, 2001,114(6):4359–4369
Cunnington RH, Nazari M, Dixon MC. C-Ski, Smurf2, and Arkadia as regulators of TGF-β signaling: new targets for managing myofibroblast function and cardiac fibrosis. Canadian J Physiol Pharmacol, 2009,87(10):764–772
Wang B, Hao J, Jones SC, et al. Decreased Smad7 expression contributes to cardiac fibrosis in the infarcted rat heart. Heart Circ Physiol, 2002,282(5): H1685–H1696
He JH, Chen YL, Chen BL, et al. B-type natriuretic peptide attenuates cardiac hypertrophy via the transforming growth factor-beta1/smad7 pathway in vivo and in vitro. Clin Exp Pharmacol Physiol, 2010,37(3):283–289
Author information
Authors and Affiliations
Corresponding author
Additional information
This project was supported by a grant from the Natural Science Foundation of Hubei Province of China (Nos. 2009CDB092, 2007ABA272).
Rights and permissions
About this article
Cite this article
Zhou, D., Li, Z., Zhang, L. et al. Inhibitory effect of tanshinone II A on TGF II-β1-induced cardiac fibrosis. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 32, 829–833 (2012). https://doi.org/10.1007/s11596-012-1042-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-012-1042-2