Abstract
The ethanol extract of an Indonesian marine sponge Lamellodysidea herbacea inhibited the activity of protein tyrosine phosphatase 1B (PTP1B), an important target enzyme for the treatment of type II diabetes. Bioassay-guided isolation yielded a known polybromodiphenyl ether (1) as a sole bioactive component. The structure of 1 was confirmed by spectroscopic data for 1 and its methyl ether derivative (2). Compound 1 markedly inhibited the PTP1B activity (IC50 = 0.85 μM) and showed a moderate cytotoxicity against two human cancer cell lines, HCT-15 (colon) and Jurkat (T-cell lymphoma) cells. On the other hand, compound 2 maintained potent inhibitory activity against PTP1B (IC50 = 1.7 μM) but did not show apparent cytotoxicity at 18 μM against these cancer cells. Four ester derivatives [acetyl (3), butyryl (4), hexanoyl (5), and benzoyl (6)] were prepared from 1 and their activities evaluated against PTP1B and two cancer cell lines to investigate the structure–activity relationships. Although compounds 3–6 exhibited potent inhibitory effects against PTP1B activity, cytotoxicity against HCT-15 and Jurkat cells was observed as a similar efficacy to that of 1. From these results, compound 2 was found to be the best inhibitor of PTP1B with no apparent cytotoxicity. Therefore, 2 may be a lead compound for making a new type of PTP1B inhibitor. Moreover, compound 2 did not inhibit the cell growth of Huh-7 cells (hepatoma). Hepatocytes are one of the locations of PTP1B, and Huh-7 cells are used to study the mechanism of action of compound 2.
Similar content being viewed by others

Introduction
Type-2 diabetes mellitus (T2DM) is a chronic metabolic disorder characterized by β-cell dysfunction and insulin resistance, and has emerged as a major health care burden around the world [1–3]. Protein tyrosine phosphatase 1B (PTP1B) is an enzyme found in the important insulin-targeted tissues such as liver, muscle, and fat cells. PTP1B plays a key role as a negative regulator in insulin signal transduction [4] by dephosphorylating activated insulin receptors (IR) or insulin receptor substrates (IRS) [5, 6]. An excess of PTP1B will impair insulin down-regulation [7–9], leading to type II diabetes mellitus. Based on the above research results, the inhibition of PTP1B has been sought as a novel therapeutic strategy, and much attention has been paid to PTP1B inhibitors using small molecules for the treatment of type II diabetes [10–12].
In our screening program to search for PTP1B inhibitors, we have tested the ethanol extracts of Indonesian marine organisms such as marine sponges and ascidians, and the extract of a marine sponge Lamellodysidea herbacea exhibited significant inhibitory activity against PTP1B. Bioassay-guided separation of the extract led to the isolation of a bioactive component, and the structure was assigned as 2-(3′,5′-dibromo-2′-methoxyphenoxy)-3,5-dibromophenol (1) [13]. We described herein the PTP1B inhibitory activity and cytotoxicity against two human cancer cell lines, HCT-15 (colon) and Jurkat (T-cell lymphoma), of compound 1 and its methyl ether (2) and ester derivatives (3–6).
Materials and methods
General experimental procedure
EI–MS was performed by a JMS-MS 700 mass spectrometer (JEOL, Tokyo, Japan). 1H- and 13C-NMR spectra were recorded on a JNM-AL-400 NMR spectrometer (JEOL) at 400 MHz for 1H and 100 MHz for 13C in CDCl3 (δH 7.26, δC 77.0). Preparative HPLC was carried out using the L-6200 system (Hitachi Ltd., Tokyo, Japan).
Materials
Fetal bovine serum (FBS) and other culture materials were purchased from Invitrogen (Carlsbad, CA, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals including organic solvent were purchased from Wako Pure Chemical Industries Ltd. (Osaka, Japan).
Marine sponge
The marine sponge was collected by scuba diving in the coral reef at Manado, Indonesia, in 2010 and identified as Lamellodysidea herbacea. The voucher specimen is deposited at the Faculty of Fisheries and Marine Science, Sam Ratulangi University and the Faculty of Pharmaceutical Sciences, Tohoku Pharmaceutical University as 10-09-16=2-6.
Extraction and isolation
The marine sponge (94 g wet weight) was thawed, cut into small pieces, and extracted three times with ethanol. The ethanol extract was evaporated to dryness (284.3 mg) and 20 mg of the crude extract was subjected to HPLC separation (90 % MeOH; detection, UV 210 nm; flow rate, 2.0 mL/min) using an ODS column (PEGASIL ODS, 10 mm × 250 mm, Senshu Scientific Co., Tokyo, Japan) to give 5.4 mg of 2-(3′,5′-dibromo-2′-methoxyphenoxy)-3,5-dibromophenol (1).
2-(3′,5′-Dibromo-2′-methoxyphenoxy)-3,5-dibromophenol (1)
Obtained as a viscous oil; 1H-NMR (CDCl3) δ 4.03 (s, 3H), 6.80 (d, 1H, J = 4.0), 7.18 (d, 1H, J = 4.0), 7.35 (d, 1H, J = 4.0), 7.45 (d, 1H, J = 4.0); 13C-NMR (CDCl3) δ 61.5, 117.3, 117.3, 118.7, 119.0, 119.9, 120.1, 127.4, 130.5, 139.0, 145.9, 150.5, 150.7; EI–MS m/z 528, 530 532, 534, and 526 [M+]; HREI–MS m/z 527.7180 (Δ −2.7 mmu, calcd for C13H 798 Br4O3: 527.7207), 529.7203 (Δ +1.7 mmu, calcd for C13H 798 Br 813 Br1O3: 529.7186), 531.7159 (Δ −0.7 mmu, calcd for C13H 798 Br 812 Br2O3: 531.7166), 533.7137 (Δ −0.9 mmu, calcd for C13H 798 Br 811 Br3O3: 533.7146), 535.7103 (Δ −2.2 mmu, calcd for C13H 818 Br4O3: 535.7125).
Preparation of methyl derivative (2)
TMS-diazomethane (73 μL, 0.064 mmol) was added to a MeOH solution of 1 (3.8 mg, 0.0071 mmol in 300 μL) and stirred at room temperature for 14 h. The reaction mixture was concentrated in vacuo to give a brown material, and a product was purified by preparative HPLC (90 % MeOH) using ODS column (PEGASIL ODS) to give 3,5-dibromo-2-(3′,5′-dibromo-2′-methoxyphenoxy)-1-methoxybenzene (2, 2.0 mg, 0.0037 mmol, 52 %).
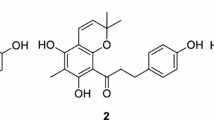
3,5-Dibromo-2-(3′,5′-dibromo-2′-methoxyphenoxy)-1-methoxybenzene (2)
Obtained as a viscous oil; 1H-NMR (CDCl3) δ 3.76 (s, 3H), 4.00 (s, 3H), 6.46 (d, 1H, J = 4.0), 7.09 (d, 1H, J = 4.0), 7.36 (d, 1H, J = 4.0), 7.42 (d, 1H, J = 4.0); 13C-NMR (CDCl3) δ 57.2, 61.7, 116.4, 117.2, 119.3, 119.4, 119.6, 128.2, 129.5, 137.2, 140.1, 146.2, 152.1, 154.2; EI–MS m/z 542, 544 546, 548, and 550 [M+]; HREI–MS m/z 541.7386 (Δ +2.2 mmu, calcd for C14H 7910 Br4O3: 541.7364), 543.7319 (Δ −2.4 mmu, calcd for C14H 7910 Br 813 Br1O3: 543.7343), 545.7318 (Δ −0.5 mmu, calcd for C14H 7910 Br 812 Br2O3: 545.7323), 547.7288 (Δ −1.4 mmu, calcd for C14H 7910 Br 811 Br3O3: 547.7302), 549.7262 (Δ −2.0 mmu, calcd for C14H 8110 Br4O3: 549.7282).
Preparation of derivatives 3–6
Acetic anhydride (100 μL, 1.1 mmol) and 4-(dimethylamino)pyridine (1.0 mg, 0.0080 mmol) were added to a solution of 1 (3.0 mg, 0.056 mmol) in pyridine (100 μL), and the resulting solution was stirred at room temperature for 12 h. The reaction mixture was concentrated in vacuo to dryness, and a product was purified by preparative HPLC (column; PEGASIL ODS, 10 mm × 250 mm; solvent, 90 % MeOH; detection, UV at 220 nm; flow rate, 2.0 mL/min) to give 3,5-dibromo-2-(3′,5′-dibromo-2′-methoxyphenoxy)phenyl ethanoate (3, 1.2 mg, 0.0022 mmol, 30 %). The other derivatives (4–6) were prepared using the following regents instead of acetic anhydride: n-butyric anhydride (4, 1.4 mg, 0.0023 mmol, 32 %), n-hexanoic anhydride (5, 1.1 mg, 0.0018 mmol, 25 %), and benzoyl chloride (6, 1.5 mg, 0.0023 mmol, 33 %).
3,5-Dibromo-2-(3′,5′-dibromo-2′-methoxyphenoxy)phenyl ethanoate (3)
Obtained as a viscous oil; 1H-NMR (CDCl3) δ 2.08 (s, 3H), 3.95 (s, 3H), 6.78 (d, 1H, J = 2.4), 7.18 (d, 1H, J = 2.4), 7.34 (d, 1H, J = 2.4), 7.44 (d, 1H, J = 2.4); EI–MS m/z 570, 572 574, 576, and 578 [M+]; HREI–MS m/z 569.7316 (Δ +0.3 mmu, calcd for C15H 7910 Br4O4: 569.7313), 571.7296 (Δ +0.4 mmu, calcd for C15H 7910 Br 813 Br1O4: 571.7292), 573.7283 (Δ +1.1 mmu, calcd for C15H 7910 Br 812 Br2O4: 573.7272), 575.7247 (Δ −0.4 mmu, calcd for C15H 7910 Br 811 Br3O4: 575.7251), 577.7216 (Δ −1.5 mmu, calcd for C15H 8110 Br4O4: 577.7231).
3,5-Dibromo-2-(3′,5′-dibromo-2′-methoxyphenoxy)phenyl butanoate (4)
Obtained as a viscous oil; 1H-NMR (CDCl3) δ 0.89 (t, 3H, J = 7.2), 1.57 (m, 2H), 2.29 (t, 2H, J = 7.2), 3.95 (s, 3H), 6.58 (d, 1H, J = 1.9), 7.36 (d, 1H, J = 2.4), 7.40 (d, 1H, J = 1.9), 7.71 (d, 1H, J = 2.4); EI–MS m/z 598, 600 602, 604, and 606 [M+]; HREI–MS m/z 597.7625 (Δ −0.1 mmu, calcd for C17H 7914 Br4O4: 597.7626), 599.7580 (Δ −2.5 mmu, calcd for C17H 7914 Br 813 Br1O4: 599.7605), 601.7569 (Δ −1.6 mmu, calcd for C17H 7914 Br 812 Br2O4: 601.7585), 603.7591 (Δ +2.7 mmu, calcd for C17H 7914 Br 811 Br3O4: 603.7564), 605.7518 (Δ −2.6 mmu, calcd for C17H 8114 Br4O4: 605.7544).
3,5-Dibromo-2-(3′,5′-dibromo-2′-methoxyphenoxy)phenyl hexanoate (5)
Obtained as a viscous oil; 1H-NMR (CDCl3) δ 0.87 (t, 3H, J = 6.8), 1.25 (m, 4H), 1.51 (m, 2H), 2.30 (t, 2H, J = 7.8), 3.95 (s, 3H), 6.57 (d, 1H, J = 2.0), 7.36 (d, 1H, J = 2.0), 7.40 (d, 1H, J = 2.0), 7.71 (d, 1H, J = 2.4); EI–MS m/z 626, 628 630, 632, and 634 [M+]; HREI–MS m/z 625.7952 (Δ +1.3 mmu, calcd for C19H 7918 Br4O4: 625.7939), 627.7924 (Δ +0.6 mmu, calcd for C19H 7918 Br 813 Br1O4: 627.7918), 629.7881 (Δ −1.7 mmu, calcd for C19H 7918 Br 812 Br2O4: 629.7898), 631.7874 (Δ −0.4 mmu, calcd for C19H 7918 Br 811 Br3O4: 631.7878), 633.7856 (calcd for C19H 8118 Br4O4: 633.7856).
3,5-Dibromo-2-(3′,5′-dibromo-2′-methoxyphenoxy)phenyl benzoate (6)
Obtained as a viscous oil; 1H-NMR (CDCl3) δ 3.80 (s, 3H), 6.68 (d, 1H, J = 2.4), 7.27 (d, 1H, J = 1.9), 7.41 (t, 2H, J = 7.7), 7.52 (d, 1H, J = 1.9), 7.59 (t, 1H, J = 7.3), 7.76 (d, 1H, J = 2.4), 7.83 (d, 2H, J = 7.2); EI–MS m/z 632, 634, 636, 638, and 640 [M+]; HREI–MS m/z 631.7455 (Δ −1.4 mmu, calcd for C20H 7912 Br4O4: 631.7469), 633.7468 (Δ +1.9 mmu, calcd for C20H 7912 Br 813 Br1O4: 633.7449), 635.7433 (Δ +0.5 mmu, calcd for C20H 7912 Br 812 Br2O4: 635.7428), 637.7430 (Δ +2.3 mmu, calcd for C20H 7912 Br 811 Br3O4: 637.7407), 639.7379 (Δ −0.9 mmu, calcd for C20H 8112 Br4O4: 639.7388).
PTP1B inhibitory assay
Protein tyrosine phosphatase 1B (PTP1B) inhibitory activity was determined by measuring the rate of hydrolysis of a substrate, p-nitrophenyl phosphate (pNPP, Sigma, St. Louis, MO, USA) according to the published method with a slight modification [14]. Briefly, PTP1B (100 μL of 0.5 μg/mL stock solution, Enzo Life Sciences, Farmingdale, NY, USA) in 50 mM citrate buffer (pH 6.0) containing 0.1 M NaCl, 1 mM dithiothreitol (DTT), and 1 mM N,N,N′,N′-ethylenediamine tetraacetate (EDTA) were added to each well of a 96-well plastic plate (Corning Inc., Corning, NY, USA). A sample (2.0 μL in MeOH) was added to each well to make the final concentrations from 0 to 4.7–5.6 μM and incubated for 10 min at 37 °C. The reaction was initiated by the addition of pNPP (100 μL of 4.0 mM stock solution) in the citrate buffer, incubated at 37 °C for 30 min, and terminated with the addition of 10 μL of a stop solution (10 M NaOH). The optical density of each well was measured at 405 nm using an MTP-500 microplate reader (Corona Electric Co., Ltd., Ibaraki, Japan). PTP1B inhibitory activity (%) is defined as [1 − (ABSsample − ABSblank)/(ABScontrol − ABSblank)] × 100, where ABSblank is the absorbance of wells containing only the buffer and pNPP, ABScontrol is the absorbance of p-nitrophenol liberated by the enzyme in the assay system without a test sample, and ABSsample is that with a test sample. The assays were performed in two duplicate experiments for all test samples. Oleanolic acid (Tokyo Chemical Industry, Tokyo, Japan), a known phosphatase inhibitor [15], was used as a positive control.
Cytotoxicity assay against HCT-15 and Jurkat cells
HCT-15 and Jurkat cells were obtained from the Center for Biomedical Research, Institute of Development, Aging, and Cancer, Tohoku University (Miyagi, Japan). The cell lines were cultured in RPMI-1640 medium. The medium was supplemented with 10 % fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. Exponentially growing cells, cultured in a humidified chamber at 37 °C containing 5.0 % CO2, were used for the experiments.
Cytotoxic activity was evaluated using the colorimetric MTT assay [16]. HCT-15 (1.0 × 104 cells in 100 μL) or Jurkat cells (2.0 × 104 cells in 100 μL) were added to each well of a 96-well plastic plate. A sample (1.0 μL in MeOH) was added to each well to make the final concentrations from 0 to 39–47 μM, and the cells were incubated for 48 h at 37 °C. MTT (10 μL of 5.5 mg/mL stock solution) and a cell lysate solution (90 μL, 40 % N,N-dimethylformamide, 20 % sodium dodecyl sulfate, 2.0 % CH3COOH and 0.030 % HCl) were added to each well, and the plate was shaken thoroughly by agitation at room temperature for overnight. The optical density of each well was measured at 570 nm using an MTP-500 microplate reader.
Cytotoxicity assay against Huh-7 cells
Cytotoxic activity against Huh-7 cells was assessed by the MTT assay, a modification of our previously described method [17]. Following the treatment of cells with test samples, 10 μL of MTT (5.0 mg/mL saline) was added to each well, the samples were incubated for 90 min at 37 °C and centrifuged (300g for 5 min), and the supernatant was aspirated off. The cells were lysed and solubilized by the addition of 100 μL of 0.040 N HCl in 2-propanol. The absorbance of each well was determined at 590 nm using an Inter-med model NJ-2300 Microplate Reader (Cosmo Bio Co., Ltd., Tokyo, Japan). Survival (%) was calculated relative to the control.
Results and discussion
Among the ethanol extracts of about 90 marine sponges and ascidians collected in the coral reefs at North Sulawesi, Indonesia, the extract of a marine sponge Lamellodysidea herbacea showed potent inhibitory activity (IC50 = 0.58 μg/mL) against PTP1B in the screening bioassay. Bioassay-guided isolation by HPLC yielded compound 1 as an inhibitor of PTP1B. The other fractions obtained after separation of 1 did not show an inhibitory activity against PTP1B.
The EI–MS spectrum of 1 showed the presence of four Br atoms, and the molecular formula C13H8Br4O3 was deduced from HREI–MS data. The 13C NMR spectrum of 1 revealed 13 carbon signals, and the signals due to two sets of meta-coupled aromatic protons (δ 6.80, 7.18, 7.35, and 7.45) and OMe protons (δ 4.03) were detected in the 1H NMR spectrum. The positions of an OMe, OH, and four Br atoms were assigned by the analysis of 2D NMR (1H–1H COSY, HMQC, and HMBC) data for 1 and confirmed by the NOE experiments on the methyl derivative (2). The NMR data for 1 were identical with those of the reported values for 2-(3′,5′-dibromo-2′-methoxyphenoxy)-3,5-dibromophenol (Fig. 1) [13].
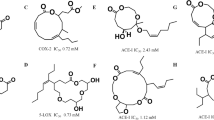
Compounds 1 and 2 inhibited the PTP1B activity (Fig. 2) with IC50 values of 0.85 and 1.7 μM, respectively, which were almost the same efficacy as that of oleanolic acid (1.1 μM), a positive control (Table 1). Oleanolic acid is a ubiquitous triterpene detected in various plants, most of which are used as crude Asian drugs for the treatments of inflammation, cancers, hepatitis, and diabetes [15, 18, 19], and has recently been reported to have a significant inhibitory activity against PTP1B [20]. Oleanolic acid derivatives were demonstrated to promote cellular insulin signaling by increasing the level of insulin receptor phosphorylation [20]. The highest concentration of compound 2 did not show a dose-dependent effect (Fig. 2). This will be due to a solubility problem of 2 at higher concentration in this bioassay system.
Interestingly, the methylation of a phenol in 1 reduced the cytotoxicity against HCT-15 and Jurkat cells (Fig. 2; Table 1). Compound 1 had a moderate cytotoxicity against HCT-15 and Jurkat cells with IC50 values of 12 and 9.5 μM, respectively. On the other hand, 2 did not show an apparent cytotoxicity at 18 μM.
Therefore, the ester derivatives (3–6) were prepared from 1 (Scheme 1) and tested for their activity against PTP1B and two cancer cell lines (Table 1). Compound 3–6 revealed comparable to stronger inhibitory activity against PTP1B than that of 1, but cytotoxicity against HCT-15 and Jurkat cells were observed. From these results, 2 is found to be the most interesting compound among these compounds as it possessed potent inhibitory activity against PTP1B and showed much reduced cytotoxicity.
The inhibitory activity of 1 and 2 on cell proliferation of human hepatoma Huh-7 cells was therefore examined. Since PTP1B is located in the insulin-targeted tissues such as liver, muscle, and fat cells, Huh-7 cells are used for cell-based experiments to investigate the mechanism of action of PTP1B inhibitors. Compound 2 showed weaker cytotoxicity (IC50 = 48 μM) than 1 (32 μM) (Table 1). Cell-based experiments are now in progress using Huh-7 cells and compound 2.
Polybrominated diphenyl ethers have been isolated from marine organisms, such as sponges, ascidians, and algae, and are reported to exhibit a variety of biological activities: antibacterial and antifungal activities [21–24], brine shrimp toxicity [23], antimicroalgal activity [25], anti-inflammatory activity [26], maturation of starfish oocytes [27], and inhibitory activities against several enzymes [27–29]. In this study, we demonstrated that a known bromodiphenyl ether (1) was a potent inhibitor of PTP1B, an important target enzyme for the treatment of type II diabetes, and that the methoxy derivative (2) is more useful than the original phenol and the ester derivatives. Compound 2 will be a new lead compound for PTP1B inhibitors.
References
Bugianesi E, Moscatiello S, Ciaravella MF, Marchesini G (2010) Insulin resistance in nonalcoholic fatty liver disease. Curr Pharm Des 16:1941–1951
Romero AP, Mendez MI, Baget BM, Fernandez BJ, Santos BE (2010) Review of the relationship between renal and retinal microangiopathy in diabetes mellitus patients. Curr Diabetes Rev 6:88–101
Hummasti S, Hotamisligil GS (2010) Endoplasmic reticulum stress and inflammation in obesity and diabetes. Circ Res 107:579–591
Byon JCH, Kusari AB, Kusari J (1998) Protein-tyrosine phosphatase-1B acts as a negative regulator of insulin signal transduction. Mol Cell Biochem 182:101–108
Tonks NK, Diltz CD, Fischer EH (1988) Characterization of the major protein-tyrosine-phosphatases of human placenta. J Biol Chem 263:6731–6737
Gonzalez-Rodrıguez A, Gutierrez JAM, Sanz-Gonzalez S, Ros M, Burks DJ, Valverde AM (2010) Inhibition of PTP1B restores IRS1-mediated hepatic insulin signaling in IRS2-deficient mice. Diabetes 59:588–599
Ahmad F, Li P-M, Meyerovitch J, Goldstein BJ (1995) Osmotic loading of neutralizing antibodies demonstrates a role for protein tyrosine phosphatase 1B in negative regulation of the insulin action pathway. J Biol Chem 270:20503–20508
Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, Ramachandran C, Gresser MJ, Tremblay ML, Kennedy BP (1999) Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 283:1544–1548
Haj FG, Zabolotny JM, Kim Y-B, Kahn BB, Neel BG (2005) Liver-specific protein-tyrosine phosphatase 1B (PTP1B) re-expression alters glucose homeostasis of PTP1B−/− mice. J Biol Chem 280:15038–15046
Barr AJ (2010) Protein tyrosine phosphatases as drug targets: strategies and challenges of inhibitor development. Future Med Chem 2:1563–1576
Zhang S, Zhang ZY (2007) PTP1B as a drug target: recent developments in PTP1B inhibitor discovery. Drug Discov Today 12:373–381
Lee S, Wang Q (2007) Recent development of small molecular specific inhibitor of protein tyrosine phosphatase 1B. Med Res Rev 27:553–573
Raymond SN, Kevin DC, Robert JW (1981) Polybrominated oxydiphenol derivatives from the sponge Dysidea herbacea: structure determination by analysis of 13C spin-lattice relaxation data for quaternary carbons and 13C–1H coupling constants. Tetrahedron 37:2341–2349
Cui L, Na MK, Oh H, Bae EY, Jeong DG, Ryu SE, Kim S, Kim BY, Oh WK, Ahn JS (2006) Protein tyrosine phosphatase 1B inhibitors from Morus root bark. Bioorg Med Chem Lett 16:1426–1429
Liu J (1995) Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol 49:57–68
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Kanno S, Hiura T, Ohtake T, Koiwai K, Suzuki H, Ujibe M, Ishikawa M (2007) Characterization of resistance to cytosine arabinoside (Ara-C) in NALM-6 human B leukemia cells. Clin Chim Acta 377:144–149
Nishino H, Nishino A, Takayasu J, Hasegawa T, Iwashima A, Hirabayashi K, Iwata S, Shibata S (1988) Inhibition of the tumor-promoting action of 12-O-tetradecanoylphorbol-13-acetate by some oleanane-type triterpenoid compounds. Cancer Res 48:5210–5215
Sato H, Genet C, Strehle A, Thomas C, Lobstein A, Wagner A, Mioskowski C, Auwerx J, Saladin R (2007) Anti-hyperglycemic activity of a TGR5 agonist isolated from Olea europaea. Biochem Biophys Res Commun 362:793–798
Zhang YN, Zhang W, Hong D, Shi L, Shen Q, Li JY, Li J, Hu LH (2008) Oleanolic acid and its derivatives: new inhibitor of protein tyrosine phosphatase 1B with cellular activities. Bioorg Med Chem 16:8697–8705
Sharma GM, Vig B (1972) Studies on the antimicrobial substances of sponges. VI. Structures of two antibacterial substances isolated from the marine sponge Dysidea herbacea. Tetrahedron Lett 17:1715–1718
Salva J, Faulkner DJ (1990) A new brominated diphenyl ether from a Philippine Dysidea species. J Nat Prod 53:757–760
Handayani D, Edrada RA, Proksch P, Wray V, Witte L, van Soest RW, Kunzmann A, Soedarsono (1997) Four new bioactive polybrominated diphenyl ethers of the sponge Dysidea herbacea from West Sumatra, Indonesia. J Nat Prod 60:1313–1316
Sionov E, Roth D, Losica HS, Kashman Y, Rudi A, Chill L, Berdicevsky I, Segal EJ (2005) Antifungal effect and possible mode of activity of a compound from the marine sponge Dysidea herbacea. J Infect 50:453–460
Hattori T, Konno A, Adachi K, Shizuri Y (2001) Four new bioactive bromophenols from the palauan sponge Phyllospongia dendyi. Fish Sci 67:899–903
Kuniyoshi M, Yamada K, Higa T (1985) A biologically active diphenyl ether from the green alga Cladophora fascicularis. Experientia 41:523–524
Liu H, Namikoshi M, Meguro S, Nagai H, Kobayashi H, Yao X (2004) Isolation and characterization of polybrominated diphenyl ethers as inhibitors of microtubule assembly from the marine sponge Phyllospongia dendyi collected at Palau. J Nat Prod 67:472–474
Fu X, Schmitz FJ, Govindan M, Abbas SA, Hanson KM, Horton PA, Crews P, Laney M, Schatzman RC (1995) Enzyme inhibitors: new and known polybrominated phenols and diphenyl ethers from four Indo-Pacific Dysidea sponges. J Nat Prod 58:1384–1391
Xu Y, Johnson RK, Hecht SM (2005) Polybrominated diphenyl ethers from a sponge of the Dysidea genus that inhibit Tie2 kinase. Bioorg Med Chem 13:657–659
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research (21603012) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan to M.N. and by a Colab. Res. & Int. Pub. Project No. 492/SP 2H/PL/2011 from DGHE, Ministry of National Education of Indonesia to R.E.P.M. We are grateful to the Center for Biomedical Research, Institute of Development, Aging and Cancer, Tohoku University for kindly providing human cancer cell lines. We express our thanks to Dr. K. Ogawa of Z. Nakai Laboratory for identification of the marine sponge and Mr. T. Matsuki and S. Sato for mass spectra.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Yamazaki, H., Sumilat, D.A., Kanno, Si. et al. A polybromodiphenyl ether from an Indonesian marine sponge Lamellodysidea herbacea and its chemical derivatives inhibit protein tyrosine phosphatase 1B, an important target for diabetes treatment. J Nat Med 67, 730–735 (2013). https://doi.org/10.1007/s11418-012-0735-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-012-0735-y