Abstract
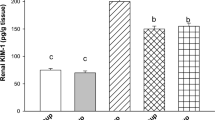
The protective effect of an ethanol extract of Curcuma comosa against cisplatin-induced renal toxicity in mice was studied. Adult male mice were pretreated for 4 days with the ethanol extract of C. comosa [100–200 mg/kg body weight (BW), orally (p.o.)] before injection of cisplatin (12.5 mg/kg BW, intraperitoneally (i.p.)). Five days later the mice were killed, and blood samples were collected to determine blood urea nitrogen (BUN) and plasma creatinine levels. Kidneys were examined histopathologically and levels of lipid peroxidation, gluthathione (GSH) content, and superoxide dismutase (SOD), gluthathione peroxidase (GPx), and catalase (CAT) activities were determined. Histological examinations revealed degenerative changes and tubular necrosis in mice treated with cisplatin, which were improved by pretreatment with C. comosa ethanol extract. Cisplatin raised BUN, creatinine, and kidney lipid peroxidation levels, and lowered kidney GSH content and levels of GPx, SOD, and CAT activities, all of which (except SOD and CAT) could be restored to normal values by pretreatment with 200 mg/kg BW of C. comosa ethanol extract. In addition, the ethanol extract of C. comosa and its isolated diarylheptanoid compound also exhibited radical scavenging activities. The results suggest that the ethanol extract of C. comosa exhibits effective protection against cisplatin-induced nephrotoxicity mediated through its antioxidant activity.




Similar content being viewed by others
References
Schrier RW (2002) Cancer therapy and renal injury. J Clin Invest 110:743–745
Meyer KB, Medias NE (1994) Cisplatin nephrotoxicity. Miner Electrolyte Metab 20:201–213
Chu G, Mantin R, Shen Y, Baskett G, Sussman H (1993) Massive cisplatin overdose by accidental substitution for carboplastin. Toxicity and management. Cancer 72:3707–3714
Badary OA, Abdel-Maksoud S, Ahmed WA, Owieda GH (2005) Naringenin attenuates cisplatin nephrotoxicity in rats. Life Sci 76:2125–2135
Ajith TA, Usha S, Nivitha V (2007) Ascorbic acid and alpha-tocopherol protect anticancer drug cisplatin induced nephrotoxicity in mice: a comparative study. Clin Chim Acta 375:82–86
Kuhad A, Pilkhwal S, Sharma S, Tirkey N, Chopra K (2007) Effect of curcumin on inflammation and oxidative stress in cisplatin-induced experimental nephrotoxicity. J Agric Food Chem 55:10150–10155
Menon VR, Sudheer AR (2007) Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol 595:105–125
Winuthayanon W, Suksen K, Boonchird C, Chuncharunee A, Ponglikitmongkol M, Suksamrarn A, Piyachaturawat P (2009) Estrogenic activity of diarylheptanoids from Curcuma comosa Roxb. requires metabolic activation. J Agric Food Chem 57:840–845
Jantaratnotai N, Utaisincharoen P, Piyachaturawat P, Chongthammakun S, Sanvarinda Y (2006) Inhibitory effect of Curcuma comosa on NO production and cytokine expression in LPS-activated microglia. Life Sci 78:571–577
Sodsai A, Piyachaturawat P, Sophasan S, Suksamrarn A, Vongsakul M (2007) Suppression by Curcuma comosa Roxb of pro-inflammatory cytokine secretion in phorbol-12-myristate-13-acetate stimulated human mononuclear cells. Int Immunopharmacol 7:524–531
Suksamrarn A, Ponglikitmonkol M, Wongkrajang K, Chindaduang A, Kittidanairak S, Jankam A, Yingyoungnarongkul B, Kittipanumat N, Chokchaisiri R, Khetkam P, Piyachaturawat P (2008) Diarylheptanoids, a new phytoestrogens from the rhizomes of Curcuma comosa: isolation, chemical modification and estrogenic activity evaluation. Bioorg Med Chem 16:6891–6902
Blois MS (1958) Antioxidant determination by the use of a stable free radical. Nature 181:1199–1200
Thedorus PM, Helmut S (1981) Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol 77:373–382
Winterbourn CC, Hawkins RE, Brian M, Carrell RW (1975) The estimation of red cell superoxide dismutase activity. J Lab Clin Med 85:337–341
Flohe L, Gunzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105:114–121
Beers RF, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–140
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Ito K, Sakakibara T, Handa J, Irie Y, Tsubosaki M, Matsuda A (1981) Safety test for cisplatin (NK801) (in Japanese). Kiso To Rinshou 15:5669–5686
Rao M, Praveen Rao PN, Kamath R, Rao MN (1999) Reduction of cisplatin-induced nephrotoxicity by cystone, a polyherbal ayurvedic preparation, in C57BL/6J mice bearing B16F1 melanoma without reducing its antitumor activity. J Ethnopharmacol 68:77–81
Lee S, Kim W, Moon SO, Sung MJ, Kim DH, Kang KP (2006) Rosiglitazone ameliorates cisplatin-induced renal injury in mice. Nephrol Dial Transplant 21:2096–2105
Atessahin A, Yilmaz S, Karahan I, Ceribasi AO, Karaoglu A (2005) Effects of lycopene against cisplatin-induced nephrotoxicity and oxidative stress in rats. Toxicology 212:116–123
Sueishi K, Mishima K, Makino K, Itoh Y, Tsuruya K, Hirakata H (2002) Protection by a radical scavenger edaravone against cisplatin-induced nephrotoxicity in rats. Eur J Pharmacol 451:203–208
Sadzuka Y, Shoji T, Takino Y (1992) Effect of cisplatin on the activities of enzymes which protect against lipid peroxidation. Biochem Pharmacol 43:1872–1875
Ramesh G, Reeves WB (2002) TNF-α mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest 110:743–745
Tarloff JB, Lash LH (2005) Toxicology of the kidney, 3rd edn. CRC, Florida, pp 784–785
Sinha M, Manna P, Sil PC (2006) Aqueous extract of the bark of Terminalia arjuna plays a protective role against sodium-fluoride-induced hepatic and renal oxidative stress. J Nat Med 61:251–260
Babu E, Gopalkrishnan VK, Sriganth NP, Gopalkrishnan R, Sakthisekharan D (1995) Cisplatin induced nephrotoxicity and the modulating effect of glutathione ester. Mol Cell Biochem 144:7–11
Dickey DT, Wu YJ, Muldoon LL, Neywelt EA (2005) Protection against cisplatin induced toxicities by N-acetylcysteine and sodium thiosulfate as assessed at the molecular, cellular, and in vivo levels. J Pharmacol Exp Ther 314:1052–1058
Luo J, Tsuji T, Yasuda H, Sun Y, Fujigaki Y, Hishida A (2008) The molecular mechanisms of the attenuation of cisplatin-induced acute renal failure by N-acetylcysteine in rats. Nephrol Dial Transplant 23:2198–2205
Pabla N, Dong Z (2008) Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int 73:994–1007
Ramesh G, Kimball SR, Jefferson LS, Reeves WB (2007) Endotoxin and cisplatin synergistically stimulate TNF-α production by renal epithelial cells. Am J Physiol Renal Physiol 292:F812–F819
Acknowledgments
This research was supported by National Research Council of Thailand, Post-graduate Education, Training and Research Program in Environmental Science and Technology, Thailand, and Research Team Strengthening Grant of BIOTEC, National Science and Technology Development Agency, Ministry of Science and Technology, Thailand. Authors thank Profs. Prapon Wilairat and Chumpol Pholpramool for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jariyawat, S., Kigpituck, P., Suksen, K. et al. Protection against cisplatin-induced nephrotoxicity in mice by Curcuma comosa Roxb. ethanol extract. J Nat Med 63, 430–436 (2009). https://doi.org/10.1007/s11418-009-0345-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-009-0345-5